Abstract
Rare renal epithelial neoplasms have been recognized to have an angioleiomyoma or leiomyoma-like proliferation of stromal smooth muscle; however, the nature of these tumors and their relationships to other renal cell carcinomas are poorly understood. We analyzed 23 such tumors for their clinicopathological, immunohistochemical, and cytogenetic features using fluorescence in situ hybridization. Twelve showed a homogeneous combination of features and were reclassified as renal cell carcinoma with angioleiomyoma-like stroma. These were composed of neoplastic glandular structures lined by cells with mixed clear, pale, and eosinophilic cytoplasm forming occasional papillary tufts. The stroma resembled smooth muscle and often extended away from the epithelial component, entrapping perinephric fat or non-neoplastic renal elements. Immunohistochemistry showed the epithelium to have reactivity for: carbonic anhydrase IX, CD10, vimentin, cytokeratin 7, cytokeratin 34βE12, and PAX8 but not α-methylacyl-coA-racemase. The stroma labeled for smooth muscle (smooth muscle actin 3+, desmin 1+, caldesmon 3+) but not epithelial antigens. Neither component showed substantial reactivity for HMB45, melan-A, cathepsin K, or TFE3 protein. An interrupted, conspicuous layer of CD34-positive endothelial cells rimmed the epithelium, imparting a two-cell layer pattern resembling myoepithelial or basal cells. Chromosome 3p deletion and trisomy 7 and 17 were uniformly absent. Follow-up was available for three patients, none of whom experienced malignant behavior. Eleven tumors were excluded from this category and considered to be clear cell renal cell carcinoma with a reactive proliferation of smooth muscle (n=4) or tangential sectioning of the pseudocapsule (n=2), renal cell carcinoma unclassified (n=4), or clear cell papillary renal cell carcinoma (n=1). In summary, renal cell carcinoma with angioleiomyoma-like stroma is a distinct neoplasm with characteristic morphological, immunohistochemical, and molecular features, unrelated to clear cell renal cell carcinoma. The immunoprofile overlaps partly with that of clear cell papillary renal cell carcinoma, though morphology and reactivity for CD10 are points of contrast.
Similar content being viewed by others
Main
Recently, a small subgroup of renal epithelial neoplasms has been recognized to contain a stromal proliferation of smooth muscle resembling leiomyoma or angioleiomyoma.1, 2, 3, 4 Several names have been used for such tumors, including renal angiomyoadenomatous tumor,3 renal cell carcinoma associated with prominent angioleiomyoma-like proliferation,2 and clear cell renal cell carcinoma with smooth muscle stroma.4 As only a small number of tumors with these features have been reported and their clinicopathological, immunohistochemical, and molecular features are incompletely understood, it is unclear whether these lesions comprise a single distinct type of renal neoplasm or whether these reports encompass multiple entities. The relationships of these tumors to clear cell renal cell carcinoma4 and clear cell papillary renal cell carcinoma5, 6, 7, 8, 9, 10, 11 (also known as clear cell tubulopapillary renal cell carcinoma)6, 12 are also poorly understood.13 To elucidate the characteristics of these tumors and assess whether they represent a distinct subcategory of renal neoplasia, we studied the clinicopathological, immunohistochemical, and molecular features of renal epithelial neoplasms noted to have a smooth muscle stromal component from our pathology archives.
Materials and methods
Pathology and Light Microscopy
Renal epithelial neoplasms with a stromal component resembling smooth muscle were retrieved via an electronic database search of the pathology archives and the contributing authors’ consultation files and via retrospective review of a consecutive series of 341 clear cell renal cell carcinomas (a 3-year period) with special attention to the presence of stromal smooth muscle. Tumors that demonstrated one or more tissue blocks with stroma resembling smooth muscle were selected for analysis of their morphological features, immunohistochemical staining characteristics, and abnormalities of chromosomes 3p, 7, and 17 by fluorescence in situ hybridization (FISH). Other tumors with a mixture of epithelial and stromal components were not studied, such as mixed epithelial and stromal tumor of the kidney, cystic nephroma, angiomyolipoma with epithelial cysts, or synovial sarcoma of the kidney. Reclassification of the tumors was attempted based on their constellation of morphological, immunohistochemical, and molecular features.
Clinicopathological characteristics were assessed, including those listed in Table 1. Hematoxylin and eosin and Masson trichrome-stained sections were prepared from the available paraffin blocks. The volume of the tumor comprised of cysts was estimated as a percentage of the entire tumor volume. Other architectural features were assessed, including the presence of branched tubular structures, papillary structures, ‘secretory cells’ with nuclei aligned above the basement membrane,10 entrapment of non-neoplastic renal tubules or glomeruli within the smooth muscle proliferation, extension of the smooth muscle proliferation into adipose tissue, extension of the epithelial component into adipose tissue, vascular invasion by either component, and a dual cell layer pattern with the epithelial elements rimmed by a conspicuous layer of capillaries. Cytological features were assessed, including nuclear grade,14 presence of ill-defined apical cytoplasm,3 and cytoplasmic staining characteristics. Other features studied included the light microscopic appearance of the stromal component (smooth muscle-like, hyalinized, or edematous, with or without hemangioma-like areas), ossification, and other forms of calcification. Morphological features were compared with a control group of 55 clear cell papillary renal cell carcinomas from 34 patients, the features of which were previously published,10 with particular emphasis on the presence of well-formed bundles of smooth muscle within the tumor stroma, extension of smooth muscle into adipose tissue, prominence of blood vessels within the stroma, and entrapment or dilation of non-neoplastic renal tubules within the tumor stroma.
Immunohistochemistry
Antibodies directed against α-methylacyl-coA-racemase (AMACR/P504S, 13H4; Dako Corp), carbonic anhydrase IX (polyclonal rabbit; Abcam Inc.), cathepsin K (3F9; Abcam), CD10 (56C6; Dako Corp), CD34 (QBEnd; Dako Corp), cytokeratin (CK) 34βE12 (34βE12; Dako Corp), CK7 (OV-TL 12/30; Dako Corp), desmin (D33; Dako Corp), caldesmon (h-CD; Dako Corp), human melanosome (HMB45; Dako Corp), melan-A (A103; Dako Corp), PAX8 (polyclonal; Cell Marque), smooth muscle actin (1A4 clone; Dako Corp), TFE3 (MRQ-37; Cell Marque), and vimentin (V9; Dako Corp) were utilized in a Dako automated instrument. Positive and negative controls gave appropriate results for each procedure.
In the epithelial component, the extent of immunohistochemical staining was estimated microscopically as a percentage of all neoplastic cells showing a positive staining reaction. Particular patterns of labeling, such as the ‘cup-shaped’ staining pattern of carbonic anhydrase IX seen in clear cell papillary renal cell carcinoma,8, 15 were additionally noted. For antibodies with a nuclear labeling pattern (PAX8 and TFE3) and for evaluation of the stromal compartment, the staining reaction was considered 3+ if it yielded diffusely and intensely positive labeling in essentially all cells from low magnification ( × 4 objective). The staining reaction was considered 2+ if labeling was easily recognized from low magnification but not diffuse or 1+ if labeling was focal or weak, requiring high magnification to be identified. Immunohistochemical staining results were compared with those previously published for clear cell papillary renal cell carcinoma6, 8, 10 and clear cell renal cell carcinoma.16, 17 Staining reactions for caldesmon, CD34, and cytokeratin 34βE12 were also studied in a control group of 10 tumors: 5 clear cell papillary renal cell carcinomas and 5 clear cell renal cell carcinomas.
FISH
FISH evaluation of chromosomes 3p, 7, and 17 was performed as described previously.18, 19 Briefly, multiple tissue sections of 4-μm thickness were prepared from formalin-fixed paraffin-embedded tissue blocks. A hematoxylin and eosin-stained slide from each block was examined to identify the neoplastic cells. The slides were deparaffinized with two washes of xylene, 15 min each, and subsequently washed twice with absolute ethanol, 10 min each, and then air-dried in a fume hood. Next, the slides were treated with 0.1 mM citric acid (pH 6.0) (Zymed, South San Francisco, CA, USA) at 95 °C for 10 min and rinsed in distilled water for 3 min followed by a wash of 2xSSC (standard saline citrate) for 5 min. Digestion of the tissue was performed by applying 0.4 ml of pepsin (5 mg/ml in 0.1 N HCl/0.9 NaCl) (Sigma, St Louis, MO, USA) at 37 °C for 40 min. The slides were rinsed with distilled water for 3 min, washed with 2xSSC for 5 min, and air-dried. Centromeric chromosome enumeration probes (CEP) were utilized for chromosome 3 (CEP3, Orange), chromosome 7 (CEP7, Green), and chromosome 17 (CEP17, Orange) from Vysis (Downers Grove, IL, USA). FISH for chromosomes 7 and 17 was performed with centromeric α-satellite DNA probe cocktail containing CEP7 and CEP17. Deletion of chromosome 3p was assessed using a probe cocktail containing probe to chromosome 3p25 (3pTel25, Green, Vysis) and CEP3. Probes CEP7–CEP17 were diluted with tDenHyb1 and probes CEP3–3p25 were diluted with tDenHyb 2 (Insitus, Alburquerque, NM, USA) in a ratio of 1:100.
The hybridized slides were examined using a Zeiss Axioplan 2 microscope (ZEISS, Gottingen, Germany) with the following filters from Chroma (Chroma, Brattleboro, VT, USA): SP-100 for 40, 6-diamidino-2-phenylindole, FITC MF-101 for Spectrum Green (3pTel25, CEP7), and Gold 31003 for Spectrum Orange (CEP3, CEP17). The images were acquired with a charged coupled device camera and analyzed with the MetaSystem Isis Software (MetaSystem, Belmont, MA, USA). Four sequential focus stacks with 0.4-μm intervals were acquired and then integrated into a single image to reduce thickness-related artifacts.
The method of analysis was described previously.20, 21 In brief, for each slide, 100–150 nuclei from tumor tissue were scored for signals from probes under the fluorescence microscope with × 1000 magnification. Non-neoplastic renal cortex was examined as an internal control. The method of analysis for 3p25 deletion was based upon previous studies of deletion of chromosomes 1p and 19q in oligodendrogliomas. The cutoff value for 3p deletion was defined as a 3p25/CEP3 ratio of ≤0.7, as previously described.7, 18, 22, 23, 24 Definitions of chromosomal trisomy of chromosomes 7 and 17 were based on the Gaussian model and related to the non-neoplastic controls. The cutoff values for each probe were set at mean plus three standard deviations (mean+3 s.d.) of the control values.25, 26
Results
Clinicopathological Characteristics of Renal Cell Carcinomas with Angioleiomyoma-Like Stroma
Twelve tumors from 11 patients were reclassified as renal cell carcinoma with angioleiomyoma-like stroma, based on a unique combination of morphological, immunophenotypical, and molecular findings (Table 1). None of the tumors that were selected for analysis based on retrospective review of 341 consecutive clear cell renal cell carcinomas was ultimately reclassified into this category. Tumors ranged in size from 1.0 to 3.5 cm (median 2.3). The patients’ ages ranged from 41 to 78 years (median 66). Six patients were men, and five were women. The nuclear grade was 2 (8 of 12, 67%) or 3 (4 of 12, 33%). One tumor occurred in an allograft kidney. Follow-up of >1 year was available for three patients, ranging in duration from 26 to 58 months, of whom all were alive without evidence of residual, recurrent, or metastatic disease. One patient (patient 4) was noted at the time of initial resection to have a contralateral renal tumor (tumor 5) that was resected 4 months after the first, although no additional follow-up was available after the second resection. Both tumors demonstrated similar morphological and immunohistochemical features.
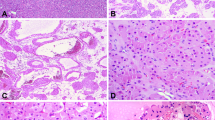
Grossly, these tumors formed pink-tan or white fibrous-appearing nodules and lacked the golden-yellow, heterogeneous and hemorrhagic cut surface that is typical of clear cell renal cell carcinoma (Figure 1a). Microscopically, a cystic component was most often absent (n=7). The stromal component not only microscopically contained areas with a smooth muscle-like appearance in all cases but also commonly contained areas of hyalinization or edema. Three tumors contained hemangioma-like foci within the stroma, one prominently and two focally. At least a minor vascular component formed by slit-like vascular spaces dispersed in the smooth muscle stroma was present in all tumors, similar to the solid pattern of angioleiomyoma.27 In some tumors (8/12), the stroma also contained vascular channels with thicker, muscular walls, similar to the venous pattern of angioleiomyoma.27 The smooth muscle proliferation commonly extended into the adjacent adipose tissue (n=9, 75%, Figures 1b and c) and often entrapped adjacent non-neoplastic renal tubules (n=9, 75%), some of which were dilated, imparting a cystic nephroma-like appearance (Figure 1c).
Grossly (a), renal cell carcinomas with angioleiomyoma-like stroma formed a solid, white-tan, fibrous-appearing nodule without the golden-yellow heterogeneous cut surface typical of clear cell renal cell carcinoma. Microscopically (b), the tumors were composed of a neoplastic proliferation of glands in a smooth muscle stroma. The smooth muscle proliferation often extended into and entrapped lobules of adjacent adipose tissue (black arrows) and entrapped non-neoplastic tubules that were often dilated (white arrow). Higher magnification (c) demonstrates a cystic nephroma-like area of entrapped non-neoplastic tubules (right) and the smooth muscle proliferation encasing lobules of adipose tissue (left). The epithelial component (d) formed glandular structures distributed in the smooth muscle proliferation, characteristically rimmed by a prominent layer of endothelial cells mimicking a basal cell or myoepithelial cell layer. Cytoplasm of the epithelial cells often varied from clear (e) to pale, flocculent and eosinophilic (f), even within the same tumor.
The epithelial component was composed of clusters of glandular structures dispersed in the muscular stroma (Figure 1d). These were lined by cells with cytoplasm that varied from optically clear to pale with a flocculent appearance and eosinophilic within the same tumor (Figures 1e and f). In contrast to the stromal component, epithelial elements did not extend into adipose tissue in any tumor. Glands with a branched configuration were present in seven tumors and a ‘secretory cell’ pattern with nuclei aligned at the apical aspect of the cells was present only focally in one tumor. Small papillary tufts were present in three tumors (Figure 2a), occasional foci of branching papillae in four tumors (Figure 2b), and areas composed entirely of papillary structures were present in a single tumor. All showed a dual-cell layer pattern at least focally (Figure 2c), composed of epithelial nests or glands tightly rimmed by a prominent layer of CD34-positive endothelial cells (Figure 2d, see results of immunohistochemical staining below), imparting a resemblance to a basal cell or myoepithelial cell layer. Similarly, hemangioma-like vascular proliferations (Figure 2e) were highlighted by immunohistochemical staining for CD34 (Figure 2f). The apical aspect of the cytoplasm lining the glandular structures had an ill-defined appearance in some areas of all tumors, in three focally and in nine more diffusely. Two tumors had dystrophic calcification in the hyaline stroma and four had areas of osseous metaplasia. In contrast, only a single tumor from the control group of 55 clear cell papillary renal cell carcinomas10 morphologically demonstrated bundles of smooth muscle within the central tumor stroma, and none exhibited bundles of smooth muscle radiating away from the epithelial component into the adjacent adipose tissue.
In renal cell carcinomas with angioleiomyoma-like stroma, small papillary tufts often protruded into the glandular structures (a). Some exhibited more complex branching papillary structures (b). A prominent layer of endothelial cells rimmed the tubules and nests, imparting a two-cell layer appearance (c). The endothelial nature of these cells was confirmed by immunohistochemical staining for CD34 (d). Similarly, a subset of tumors contained hemangioma-like proliferations of capillaries (e), highlighted by immunostaining for CD34 (f).
Histochemical and Immunohistochemical Staining Characteristics of Renal Cell Carcinomas with Angioleiomyoma-Like Stroma
Histochemical staining with the Masson trichrome technique showed a mixture of smooth muscle and collagen staining patterns in the stroma of all of the renal cell carcinomas with angioleiomyoma-like stroma, even in areas where the light microscopic appearance was fibrous or edematous. Results of immunohistochemical staining are detailed in Table 2: Antibodies to carbonic anhydrase IX (Figures 3a and b), CK7 (Figure 3c), CD10 (Figure 3d), and cytokeratin 34βE12 (Figure 3e) labeled the epithelial cells and not the stromal cells in all tumors (median 95, 90, 40, and 55% of tumor cells, respectively). There was a membranous pattern of labeling for carbonic anhydrase IX in all tumors, and eight showed the ‘cup-shaped’ staining pattern characteristic of clear cell papillary renal cell carcinoma,8 five focally and three more diffusely. Entrapped non-neoplastic renal tubules when present showed a negative reaction for carbonic anhydrase IX. Staining for CD10 was not restricted to a cystic component as has been reported in clear cell papillary renal cell carcinoma.10 Staining for AMACR was typically absent in both epithelium and stroma but often highlighted the entrapped non-neoplastic renal tubules. The five control cases of clear cell papillary renal cell carcinoma also showed positive staining for cytokeratin 34βE12, ranging from 35 to 75% of tumor cells. In contrast, only one of five clear cell renal cell carcinomas showed focal labeling for cytokeratin 34βE12 in ⩽5% of tumor cells.
Immunohistochemical staining of renal cell carcinomas with angioleiomyoma-like stroma (a) characteristically yielded positive labeling of the epithelial component for carbonic anhydrase IX (b), typically with a diffuse, strong membranous pattern. Cytokeratin 7 (c) was also positive in the epithelium, although often <100% of cells. CD10 typically yielded apical and lateral membranous staining (d), more diffusely than is expected for clear cell papillary renal cell carcinoma. Cytokeratin 34βE12 (e) also showed a positive reaction in the epithelial component in a variable percentage of the tumor cells. Antibody to vimentin (f) variably labeled both the epithelial and stromal cells.
Antibody to vimentin usually labeled both the epithelial and stromal components of the renal cell carcinomas with angioleiomyoma-like stroma (Figure 3f, 9/10). All renal cell carcinomas with angioleiomyoma-like stroma showed intense, typically diffuse nuclear labeling with antibody to PAX8 in the epithelium only. Antibody to cathepsin K labeled macrophages within glandular lumina and in seven of the ten stained tumors labeled the stromal cells minimally (1+), sometimes including the capillary network surrounding the tumor cells. Staining for CD34 showed an interrupted or ‘dotted’ pattern of capillary endothelial cells tightly rimming the neoplastic epithelial component in all tumors (Figure 2d). In the control group of clear cell papillary renal cell carcinomas, one tumor demonstrated this pattern diffusely, whereas three showed a similar pattern mainly around the cells lining cysts. In clear cell renal cell carcinomas, the cystic component of only one tumor showed this pattern. Other areas of all three types of tumor (renal cell carcinoma with angioleiomyoma-like stroma, clear cell papillary renal cell carcinoma, and clear cell renal cell carcinoma) demonstrated a more continuous reticular network of labeling for CD34 around the tumor cell nests, particularly in areas composed of compact architecture. No tumor showed epithelial labeling for cathepsin K or any labeling for HMB45, melan-A, or TFE3 protein.
Immunohistochemistry for smooth muscle actin was usually diffusely and strongly positive in the stromal component (Figure 4a), whereas desmin showed a more variable staining reaction, often showing limited staining, most commonly 1+ (Figure 4b). The stromal component showed intense and diffuse positive reactivity for caldesmon (Figure 4c, all 2+ to 3+), which highlighted the extension of smooth muscle into the perinephric fat, when present. Areas that were edematous with a less conspicuous smooth muscle component often still demonstrated a diffuse and strong staining reaction for caldesmon, similar to the findings using the trichrome stain (Figure 4d). In the control group of five clear cell papillary renal cell carcinomas, staining for caldesmon also often showed a positive reaction, although more limited in extent (absent in one tumor, 1+ in two tumors, and 2+ in two tumors). The control group of five clear cell renal cell carcinomas showed a negative reaction in the tumor stroma, although there was labeling of the tumor pseudocapsule and the walls of intratumoral blood vessels in both clear cell papillary renal cell carcinoma and clear cell renal cell carcinoma. Other immunohistochemical markers were used as part of the diagnostic evaluation of a subset of cases and available for review, as detailed in Table 2.
The stroma of renal cell carcinomas with angioleiomyoma-like stroma characteristically showed diffuse, strong labeling with antibody to smooth muscle actin (a), although reactivity for desmin was more variable and often focal (b). Labeling for caldesmon (c) was typically strong and diffuse, supporting the morphological impression of smooth muscle differentiation. Trichrome stain similarly showed a mixture of collagen and smooth muscle in the stroma (d). Fluorescence in situ hybridization uniformly showed no deletion of chromosome 3p in either the epithelial (e) or stromal (f) component.
FISH Results in Renal Cell Carcinomas with Angioleiomyoma-Like Stroma
Material was available (paraffin blocks or unstained slides) in 11 of the 12 renal cell carcinomas with angioleiomyoma-like stroma for assessment of chromosome 3p status by FISH and in 10 for assessment of chromosomes 7 and 17. None showed deletion of chromosome 3p in either the epithelial component (Figure 4e) or the stromal component (Figure 4f), evidenced by two pairs of centromeric chromosome 3 probe signals and chromosome 3p probe signals, and none showed trisomy of chromosome 7 or 17.
Excluded Tumors
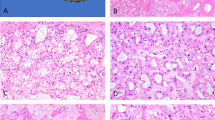
After reclassification, 11 tumors were excluded from the category of renal cell carcinoma with angioleiomyoma-like stroma, as summarized in Table 3. In two of these excluded tumors, a smooth muscle component was present in only one tissue block and interpreted as tangential sectioning of the tumor pseudocapsule in a clear cell renal cell carcinoma (Figures 5a and b). In this situation, morphological and immunohistochemical (carbonic anhydrase IX+, CD10+, CK7−, cytokeratin 34βE12−) features were otherwise typical of clear cell renal cell carcinoma, although the pseudocapsule labeled for markers of smooth muscle differentiation by immunohistochemistry (smooth muscle actin 3+, desmin 1+, caldesmon 3+). In another four tumors, smooth muscle bundles dissected into the tumor in more than one tissue block, although morphological and immunohistochemical features were again similar to those of clear cell renal cell carcinoma (Figure 5c). Chromosome 3p deletion was present in the epithelial component of two of the three of these tumors evaluated by FISH, and three of these four tumors invaded the renal capsule or renal sinus, suggesting that the presence of smooth muscle bundles was a reactive phenomenon.
Tumors excluded from the category of renal cell carcinoma with angioleiomyoma-like stroma: Two tumors had a prominent component of smooth muscle-like stroma containing numerous blood vessels in only one of the tumor sections (a), whereas the remainder of the tumor had a typical morphological appearance (b) and immunohistochemical profile of clear cell renal cell carcinoma. This was interpreted as tangential sectioning of the tumor pseudocapsule in one tissue block. Four clear cell renal cell carcinomas contained bundles of smooth muscle in multiple tumor sections (c). The smooth muscle demonstrated strong positive labeling for caldesmon (inset); however, otherwise the immunoprofile was typical of clear cell renal cell carcinoma and chromosome 3p deletion was often present. This situation was interpreted as the result of invasion of adjacent structures. One unclassified renal cell carcinoma (d) was thought morphologically to have smooth muscle stroma; however, immunohistochemical staining for smooth muscle actin (inset) and caldesmon (not pictured) highlighted only the intratumoral blood vessels and not the spindle-shaped cells. The spindle-shaped cells also labeled for some epithelial antigens, supporting exclusion from this category. Two unclassified renal cell carcinomas (e) were composed of nests of cells with clear cytoplasm in a prominent stroma. Although immunohistochemical features overlapped with renal cell carcinoma with angioleiomyoma-like stroma, extent of labeling for carbonic anhydrase IX was less and chromosome 3p deletion was present. One unclassified renal cell carcinoma (f) was composed entirely of complex papillary architecture. Immunohistochemical and molecular features were similar to those of renal cell carcinoma with angioleiomyoma-like stroma; however, due to the contrasting exclusively papillary morphology, this tumor could not be confidently placed in that category.
Three excluded tumors were originally considered renal cell carcinomas with angioleiomyoma-like stroma based on their morphological features but were recategorized as renal cell carcinoma, unclassified, based on the results of immunohistochemical and molecular analysis. In one (patient 18, Figure 5d), immunohistochemical staining did not reveal smooth muscle differentiation in the spindle cell component (smooth muscle actin 1+, desmin−, caldesmon−). In contrast to the renal cell carcinomas with angioleiomyoma-like stroma, the spindle cell component of this tumor labeled for carbonic anhydrase IX in a patchy distribution, CD10 diffusely, and focally PAX8. Chromosome 3p deletion was not detected. The patient had a lymph node metastasis at the time of nephrectomy and additionally developed a distant metastasis to the soft tissue of the orbit 12 months after nephrectomy. The latter was composed predominantly of spindle-shaped cells that were similarly positive by immunohistochemistry for CD10 and PAX8. Based on these features, this tumor was recategorized as renal cell carcinoma, unclassified, although an unusual pattern of sarcomatoid clear cell renal cell carcinoma could not be entirely excluded. Two tumors (from patients 19 and 20, Figure 5e) were composed entirely of a nested arrangement of cells with clear cytoplasm resembling clear cell renal cell carcinoma but with a prominent expanded stromal compartment (smooth muscle actin 3+, desmin 1+, caldesmon 2+). The immunophenotype of the epithelial component was similar to that of the renal cell carcinomas with angioleiomyoma-like stroma (carbonic anhydrase IX+, CD10+, CK7+, cytokeratin 34βE12+). However, labeling for carbonic anhydrase IX was not diffuse (5 and 35% of cells), and chromosome 3p deletion was present in the epithelial component (70% of cells for both tumors).
One additional tumor originally considered renal cell carcinoma, unclassified, (patient 21, Figure 5f) had a papillary architecture lined by cells with clear to eosinophilic, flocculent cytoplasm. Immunohistochemical and molecular results were similar to those of the renal cell carcinomas with angioleiomyoma-like stroma (carbonic anhydrase IX+, CD10+, CK7+, cytokeratin 34βE12+, 3p deletion absent); however, due to the contrasting morphology (exclusively papillary), this tumor could not be confidently placed in the same category. One tumor was originally considered a clear cell papillary renal cell carcinoma. Morphology and immunoprofile in two of the sections resembled the other renal cell carcinomas with angioleiomyoma-like stroma; however, features in the remaining sections resembled clear cell papillary renal cell carcinoma. No single slide showed a transition between these morphological patterns. Due to the uncertainty of distinguishing between these two entities and the inability to exclude the possibility of two juxtaposed tumors, a diagnosis of renal cell carcinoma with angioleiomyoma-like stroma could not be established with certainty in this case.
Discussion
Recently, it has been recognized that a small group of renal epithelial neoplasms contain a prominent proliferation of stromal smooth muscle.1, 2, 3, 4 However, the relationship of these tumors to other renal epithelial neoplasms, particularly clear cell renal cell carcinoma4 and clear cell papillary renal cell carcinoma,5, 6, 7, 8, 9, 10, 11 is incompletely understood.13 To shed light on these relationships, in this study we assessed clinicopathological, immunohistochemical, and molecular features of renal epithelial neoplasms with a prominent stromal smooth muscle component. We found a subset of tumors to comprise a homogeneous group that we regard as a distinct subcategory of renal neoplasia.
Previously, Michal et al1 reported an example of a renal tumor composed of cytologically bland epithelial elements dispersed in muscular and vascular stroma, which they termed ‘benign renal angiomyoadenomatous tumor.’ In a subsequent series of five similar tumors, Michal et al3 found these neoplasms to label for CK7 but not CD10 by immunohistochemistry and lack key molecular alterations of clear cell renal cell carcinoma, leading to the conclusion that they are distinct neoplasms. In another study, Kuhn et al2 reported five renal cell carcinomas that were noted to have a stroma with a prominent ‘angioleiomyoma-like’ appearance. The authors concluded that this angioleiomyoma-like component was unrelated to angiomyolipoma or tuberous sclerosis, and the epithelial component was interpreted as usual clear cell renal cell carcinoma. However, based on overlapping morphological and immunohistochemical features, others have hypothesized that these renal cell neoplasms with smooth muscle stroma are part of a morphological spectrum of clear cell papillary renal cell carcinoma.13, 28 In a contrasting study, Shannon et al4 found three renal cell carcinomas with smooth muscle stroma to be positive for both CK7 and CD10 by immunohistochemistry. By FISH, two demonstrated monosomy of chromosome 3 and one exhibited chromosome 3p deletion, leading the authors to suggest that these tumors are a variant of clear cell renal cell carcinoma.4 Based on the heterogeneous constellation of features yielded by these and other reports,29, 30, 31 it has been unclear whether these tumors encompass a single distinct entity or multiple subtypes of renal neoplasia.
In this study, we sought to reclassify renal epithelial neoplasms with a prominent smooth muscle component based on a panel of morphological, immunohistochemical, and molecular features. We identified a subgroup of tumors that demonstrated a remarkably homogeneous constellation of features, which we regarded as a distinct subcategory of renal neoplasm. As the morphological features of these neoplasms most closely resembles that described by Kuhn et al2 and as these tumors appear to be distinct from clear cell renal cell carcinoma, we chose to use the nomenclature ‘renal cell carcinoma with angioleiomyoma-like stroma’ for these tumors. These tumors were characterized by a prominent stromal smooth muscle proliferation that was uniformly positive for markers of smooth muscle differentiation by immunohistochemistry, best evidenced by diffuse and strong staining for caldesmon (2+ to 3+), often showing only minor labeling for desmin. Similar to the neoplasms described by Kuhn et al,2 the smooth muscle proliferation often extended into perinephric adipose tissue (75%), though the epithelial component uniformly did not, leading us to regard all tumors as stage pT1a. Within the kidney, this proliferation also commonly entrapped non-neoplastic renal elements.2 In some cases, entrapped non-neoplastic tubules became dilated, resembling cystic nephroma or mixed epithelial and stromal tumor. A prominent smooth muscle-like component was uniformly present as the basis for inclusion in this study; however, other areas of the stroma were edematous or hyalinized. Arguing against the possibility of a ‘collision’ between angiomyolipoma and renal cell carcinoma,2 the stroma lacked labeling for HMB45, melan-A, and cathepsin K.32
The epithelial component of the renal cell carcinomas with angioleiomyoma-like stroma was composed of glandular elements lined by cells with variably clear to eosinophilic or flocculent cytoplasm. Often the epithelium demonstrated a tufted growth into the lumen of the glands with an ill-defined apical cytoplasmic border, similar to the ‘blister-like’ appearance described by Michal et al.3 Surrounding the epithelial elements, a layer of capillaries was often prominent, imparting a dual-cell layer pattern resembling a basal cell or myoepithelial cell layer. The endothelial nature of these cells was supported by immunohistochemistry for CD34, with some areas showing an interrupted or discontinuous capillary network surrounding the glands.
The immunohistochemical profile of the epithelial component of these tumors was also uniform: epithelial cells were labeled with antibodies to carbonic anhydrase IX (with a membranous or ‘cup-shaped’8 pattern), CK7, CD10, vimentin, cytokeratin 34βE12, and PAX8. Immunohistochemical staining with antibody to AMACR typically yielded a negative reaction, with focal or weak labeling in a minority of tumors. Negative immunohistochemistry for HMB45, melan-A, cathepsin K, and TFE3 protein also argues against the possibility that these tumors are a manifestation of translocation-associated renal cell carcinoma.33, 34, 35
Comparison to Clear Cell Papillary Renal Cell Carcinoma
It has been hypothesized that these renal cell carcinomas with smooth muscle stroma are part of the morphological spectrum of clear cell papillary renal cell carcinoma.13 In keeping with such a possibility, we found the immunohistochemical profile of these two tumor types to overlap, including labeling for carbonic anhydrase IX (sometimes with a ‘cup-shaped’ staining pattern8), CK7,6, 8, 10 and cytokeratin 34βE128 and negative reactivity for AMACR.6, 8, 10 As a point of contrast, the tumors in this study showed more diffuse labeling for CD10 than expected of clear cell papillary renal cell carcinoma.10 In our experience, clear cell papillary renal cell carcinoma usually shows an entirely negative reaction for CD10 or apical labeling of only the cyst-lining cells; however, others have occasionally observed focal labeling for CD10 in non-cystic areas.6, 8, 10 Genetically, absence of chromosome 3p deletion is also a point of similarity between these tumor types that contrasts both to clear cell renal cell carcinoma.7, 36 However, features contrasting these tumors included a higher nuclear grade in renal cell carcinoma with angioleiomyoma-like stroma (grade 2-3) compared with clear cell papillary renal cell carcinoma (grade 1–2) and the absence of the ‘secretory cell’ pattern with nuclei aligned above the basement membrane in the former. In contradistinction to clear cell papillary renal cell carcinoma, none of the tumors in this study were associated with end-stage renal disease (including acquired cystic kidney disease),9 although one occurred in an allograft kidney. Renal cell carcinoma with angioleiomyoma-like stroma also appears quite rare compared with clear cell papillary renal cell carcinoma: in this study, none of the tumors that were ultimately classified as renal cell carcinoma with angioleiomyoma-like stroma were identified via a retrospective review of 341 consecutive cases of clear cell renal cell carcinoma, whereas 10 tumors from the same series were reclassified as clear cell papillary renal cell carcinoma, the latter accounting for approximately 3% of total renal cell carcinomas from a 3-year period.10 Therefore, if indeed these two neoplasms represent opposing ends of the spectrum of a single disease process, very few appear to occur at one end. If so, the presence of prominent smooth muscle that is ready recognizable morphologically appears to be associated with several other unique features, including increased labeling for CD10, a higher nuclear grade, infrequent cysts, and the absence of the ‘secretory cell’10 pattern.
Comparison to Clear Cell Renal Cell Carcinoma
The absence of chromosome 3p deletion by FISH and positive immunohistochemical labeling for CK7 and cytokeratin 34βE12 observed in this study support the idea that these tumors are not a manifestation of clear cell renal cell carcinoma. However, some patterns of clear cell renal cell carcinoma mimic the morphology of renal cell carcinoma with angioleiomyoma-like stroma.28, 37 In one such scenario, we found that areas of tangential sectioning of the tumor pseudocapsule may impart a similar appearance to the prominent smooth muscle stroma of these tumors. In such cases (n=2), the immunohistochemical staining pattern of clear cell renal cell carcinoma was observed (carbonic anhydrase IX+, CD10+, CK7−, cytokeratin 34βE12−), despite that the tumor pseudocapsule indeed resembled smooth muscle by light microscopy and immunohistochemistry. A helpful clue to this possibility is the presence of such a smooth muscle proliferation in only a single tissue block of a neoplasm that otherwise resembles clear cell renal cell carcinoma. Another four tumors had bundles of smooth muscle extending into the tumor in multiple sections, again coupled with the typical morphology and immunoprofile of clear cell renal cell carcinoma (carbonic anhydrase IX+, CD10+, CK7−, cytokeratin 34βE12−) and often deletion of chromosome 3p. Three of four of these tumors demonstrated invasion of the renal capsule or renal sinus, suggesting that this phenomenon may be a reactive change to invasion of adjacent structures. In both of these situations, the characteristic immunohistochemical profile of clear cell renal cell carcinoma may be helpful in pointing to the correct diagnosis.
Tumors that could not be Confidently Classified
Five other tumors were excluded from the category of renal cell carcinoma with angioleiomyoma-like stroma that could not be confidently classified: of these, one tumor exhibited a mixed morphology and immunoprofile of clear cell papillary renal cell carcinoma and renal cell carcinoma with angioleiomyoma-like stroma. As discussed above, the relationship between the tumors reported here and clear cell papillary renal cell carcinoma remains incompletely understood. The renal cell carcinomas with angioleiomyoma-like stroma in this study exhibited a higher nuclear grade (2–3) than typically seen in clear cell papillary renal cell carcinoma (usually 1–2); however, it seems highly unlikely that they represent a high-grade ‘transformation’ of clear cell papillary renal cell carcinoma, as all were small (⩽3.5 cm) and confined to the kidney. One possible explanation for the mixed features in this one tumor (a larger mass measuring 7.5 cm) is juxtaposition of two separate tumors that were not recognized grossly as distinct. In support of this possibility, the kidney in this case harbored multifocal tumors,10 and no single tissue section showed a transition between morphologies. Another tumor that could not be confidently classified (patient # 21) exhibited some of the features of renal cell carcinoma with angioleiomyoma-like stroma, including prominent stromal smooth muscle (caldesmon+) and a similar immunohistochemical and molecular profile in the epithelial component (carbonic anhydrase IX+, CK7+, cytokeratin 34βE12+, CD10+, AMACR−, 3p deletion absent); however, in contrast to the other renal cell carcinomas with angioleiomyoma-like stroma, this neoplasm was composed exclusively of papillary architecture. Tumors with these features may indeed be within the spectrum of renal cell carcinoma with angioleiomyoma-like stroma, though in the absence of a characteristic molecular alteration it is difficult to fully support such an assertion in light of the somewhat differing morphology.
As in the tumors reported by Shannon et al,4 two tumors in this study that were excluded from the category of renal cell carcinoma with angioleiomyoma-like stroma demonstrated chromosome 3p deletion and labeling for CK7 (patients 19 and 20). Labeling for carbonic anhydrase IX was also less than is typical of clear cell renal cell carcinoma (5–35%) in these two tumors. The significance of these findings is not entirely clear. It has been suggested that previously reported examples of CK7-positive clear cell renal cell carcinomas38 are in fact clear cell papillary renal cell carcinomas.12 However, as clear cell papillary renal cell carcinomas lack chromosome 3p deletion and these tumors did not exhibit such morphology, the possibility that another subtype of CK7-positive ‘clear cell’ renal neoplasm exists remains open to further study.
Based on these results, we suspect that a substantial fraction of the tumors previously categorized as ‘renal angiomyoadenomatous tumor’, ‘renal cell carcinoma associated with prominent angioleiomyoma-like proliferation’, and ‘clear cell renal cell carcinoma with smooth muscle stroma’2, 3, 4, 13, 28, 29, 30, 31, 37, 39 could be alternatively categorized as clear cell papillary renal cell carcinoma, clear cell renal cell carcinoma with prominent bundles of smooth muscle (reactive or related to the tumor pseudocapsule), or renal cell carcinoma, unclassified. In support of this hypothesis, after thorough morphological, immunohistochemical, and molecular study, almost 50% of the tumors originally considered for inclusion in this study were thought not to fit into the category of renal cell carcinoma with angioleiomyoma-like stroma, even some of which were originally diagnosed as such. Therefore discriminating between subtypes of renal tumors with prominent stroma can be a particular diagnostic challenge, requiring integration of the entire constellation of features.
Clinical Significance
Although follow-up information was available for very few patients in this study, none of the tumors reclassified as renal cell carcinoma with angioleiomyoma-like stroma pursued an aggressive course. Likewise, to our knowledge no tumor with these features reported to date has demonstrated evidence of malignant behavior. Our results also suggest that awareness of the mimics of renal cell carcinoma with angioleiomyoma-like stroma is important. One neoplasm in this study was originally thought to belong to this category, composed of cells with clear cytoplasm forming nests and tubular structures in a spindle cell and vascular stroma (patient # 18). After immunohistochemical staining (caldesmon−, CK7−, cytokeratin 34βE12−, PAX8+ spindle cell component, AMACR+) and evaluation in the context of the other tumors, it became apparent that this neoplasm was better regarded as unclassified renal cell carcinoma, possibly with an unusual manifestation of sarcomatoid change. Contrasting to the behavior of the renal cell carcinomas with angioleiomyoma-like stroma, this patient had metastases to a regional lymph node and developed distant metastasis to the soft tissue of the orbit 12 months after diagnosis.
Summary
In this study, we examined a series of 23 renal cell neoplasms with a prominent smooth muscle stromal component. Of these, 12 demonstrated a homogeneous constellation of morphological and immunohistochemical features, supporting classification into a distinct category that we regarded as renal cell carcinoma with angioleiomyoma-like stroma. These tumors demonstrated prominent stromal smooth muscle (caldesmon +) that often extended away from the epithelial component to entrap non-neoplastic renal tubules or interdigitate with perinephric fat. The epithelial component was characteristically comprised of glandular structures with variable clear to pale, flocculent, or eosinophilic cytoplasm, often with small tufts protruding into the lumina. The immunohistochemical and molecular profile of the epithelial component was also distinct (carbonic anhydrase IX+, CD10+, CK7+, cytokeratin 34βE12+, AMACR−, 3p deletion absent) and contrasted to clear cell renal cell carcinoma. Some features, particularly the labeling for carbonic anhydrase IX, CK7, and cytokeratin 34βE12 and absence of chromosome 3p deletion, raise the possibility of a relationship to clear cell papillary renal cell carcinoma; however, the increased labeling for CD10 and distinct morphological features, including well-formed bundles of stromal smooth muscle, are points of contrast. Malignant potential of these tumors is not yet established; however, awareness of morphological mimics, some of which are unequivocally malignant, is important to avoid misdiagnosis.
References
Michal M, Hes O, Havlicek F . Benign renal angiomyoadenomatous tumor: a previously unreported renal tumor. Ann Diagn Pathol 2000;4:311–315.
Kuhn E, De Anda J, Manoni S et al. Renal cell carcinoma associated with prominent angioleiomyoma-like proliferation: Report of 5 cases and review of the literature. Am J Surg Pathol 2006;30:1372–1381.
Michal M, Hes O, Nemcova J et al. Renal angiomyoadenomatous tumor: morphologic, immunohistochemical, and molecular genetic study of a distinct entity. Virchows Arch 2009;454:89–99.
Shannon BA, Cohen RJ, Segal A et al. Clear cell renal cell carcinoma with smooth muscle stroma. Hum Pathol 2009;40:425–429.
Adam J, Couturier J, Molinie V et al. Clear-cell papillary renal cell carcinoma: 24 cases of a distinct low-grade renal tumour and a comparative genomic hybridization array study of seven cases. Histopathology 2011;58:1064–1071.
Aydin H, Chen L, Cheng L et al. Clear cell tubulopapillary renal cell carcinoma: a study of 36 distinctive low-grade epithelial tumors of the kidney. Am J Surg Pathol 2010;34:1608–1621.
Gobbo S, Eble JN, Grignon DJ et al. Clear cell papillary renal cell carcinoma: a distinct histopathologic and molecular genetic entity. Am J Surg Pathol 2008;32:1239–1245.
Rohan SM, Xiao Y, Liang Y et al. Clear-cell papillary renal cell carcinoma: molecular and immunohistochemical analysis with emphasis on the von Hippel-Lindau gene and hypoxia-inducible factor pathway-related proteins. Mod Pathol 2011;24:1207–1220.
Tickoo SK, dePeralta-Venturina MN, Harik LR et al. Spectrum of epithelial neoplasms in end-stage renal disease: an experience from 66 tumor-bearing kidneys with emphasis on histologic patterns distinct from those in sporadic adult renal neoplasia. Am J Surg Pathol 2006;30:141–153.
Williamson SR, Eble JN, Cheng L et al. Clear cell papillary renal cell carcinoma: differential diagnosis and extended immunohistochemical profile. Mod Pathol 2013;26:697–708.
Bhatnagar R, Alexiev BA . Renal-cell carcinomas in end-stage kidneys: a clinicopathological study with emphasis on clear-cell papillary renal-cell carcinoma and acquired cystic kidney disease-associated carcinoma. Int J Surg Pathol 2012;20:19–28.
Srigley JR, Delahunt B, Eble JN et al. The International Society of Urological Pathology (ISUP) Vancouver Classification of Renal Neoplasia. Am J Surg Pathol 2013;37:1469–1489.
Verine J . Renal angiomyoadenomatous tumor: morphologic, immunohistochemical, and molecular genetic study of a distinct entity. Virchows Arch 2009;454:479–480.
Delahunt B, Cheville JC, Martignoni G et al. The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol 2013;37:1490–1504.
Chen YB, Tickoo SK . Spectrum of preneoplastic and neoplastic cystic lesions of the kidney. Arch Pathol Lab Med 2012;136:400–409.
Al-Ahmadie HA, Alden D, Fine SW et al. Role of immunohistochemistry in the evaluation of needle core biopsies in adult renal cortical tumors: an ex vivo study. Am J Surg Pathol 2011;35:949–961.
Williamson SR, Halat S, Eble JN et al. Multilocular cystic renal cell carcinoma: similarities and differences in immunoprofile compared with clear cell renal cell carcinoma. Am J Surg Pathol 2012;36:1425–1433.
Gobbo S, Eble JN, MacLennan GT et al. Renal cell carcinomas with papillary architecture and clear cell components: the utility of immunohistochemical and cytogenetical analyses in differential diagnosis. Am J Surg Pathol 2008;32:1780–1786.
Cheng L, MacLennan GT, Zhang S et al. Evidence for polyclonal origin of multifocal clear cell renal cell carcinoma. Clin Cancer Res 2008;14:8087–8093.
Halat S, Eble JN, Grignon DJ et al. Multilocular cystic renal cell carcinoma is a subtype of clear cell renal cell carcinoma. Mod Pathol 2010;23:931–936.
Williamson SR, Zhang S, Eble JN et al. Clear cell papillary renal cell carcinoma-like tumors in patients with von Hippel-Lindau disease are unrelated to sporadic clear cell papillary renal cell carcinoma. Am J Surg Pathol 2013;37:1131–1139.
Weller M, Berger H, Hartmann C et al. Combined 1p/19q loss in oligodendroglial tumors: predictive or prognostic biomarker? Clin Cancer Res 2007;13:6933–6937.
Wiens AL, Cheng L, Bertsch EC et al. Polysomy of chromosomes 1 and/or 19 is common and associated with less favorable clinical outcome in oligodendrogliomas: fluorescent in situ hybridization analysis of 84 consecutive cases. J Neuropathol Exp Neurol 2012;71:618–624.
Brunelli M, Fiorentino M, Gobbo S et al. Many facets of chromosome 3p cytogenetic findings in clear cell renal carcinoma: the need for agreement in assessment FISH analysis to avoid diagnostic errors. Histol Histopathol 2011;26:1207–1213.
Cossu-Rocca P, Eble JN, Delahunt B et al. Renal mucinous tubular and spindle carcinoma lacks the gains of chromosomes 7 and 17 and losses of chromosome Y that are prevalent in papillary renal cell carcinoma. Mod Pathol 2006;19:488–493.
Jones TD, Eble JN, Wang M et al. Molecular genetic evidence for the independent origin of multifocal papillary tumors in patients with papillary renal cell carcinomas. Clin Cancer Res 2005;11:7226–7233.
Hisaoka M, Quade B . Angioleiomyoma In: Fletcher CDM, Bridge JA, Hogendoorn PC, Mertens F, (eds). WHO Classification of Tumours of Soft Tissue and Bone 4th edn. IARC Press: Lyon, 2013, pp 120–121.
Petersson F, Grossmann P, Hora M et al. Renal cell carcinoma with areas mimicking renal angiomyoadenomatous tumor/clear cell papillary renal cell carcinoma. Hum Pathol 2013;44:1412–1420.
Iczkowski KA, Shanks JH, Burdge AH et al. Renal cell carcinoma with clear cells, smooth muscle stroma, and negative for 3p deletion: a variant of renal angiomyoadenomatous tumour? A case report. Histopathology 2013;62:522–524.
Singh C, Kendi AT, Manivel JC et al. Renal angiomyoadenomatous tumor. Ann Diagn Pathol 2012;16:470–476.
Petersson F, Yan B, Huang J et al. Low-grade renal carcinoma with histologic features overlapping with renal angiomyoadenomatous tumor and featuring polysomy 7 and 17 and a mutation in the von Hippel-Lindau gene: report of a hybrid tumor and a few comments on renal angiomyoadenomatous tumor and papillary renal tumors with clear cells. Ann Diagn Pathol 2011;15:213–216.
Martignoni G, Bonetti F, Chilosi M et al. Cathepsin K expression in the spectrum of perivascular epithelioid cell (PEC) lesions of the kidney. Mod Pathol 2012;25:100–111.
Argani P, Hicks J, De Marzo AM et al. Xp11 translocation renal cell carcinoma (RCC): extended immunohistochemical profile emphasizing novel RCC markers. Am J Surg Pathol 2010;34:1295–1303.
Argani P, Olgac S, Tickoo SK et al. Xp11 translocation renal cell carcinoma in adults: expanded clinical, pathologic, and genetic spectrum. Am J Surg Pathol 2007;31:1149–1160.
Camparo P, Vasiliu V, Molinie V et al. Renal translocation carcinomas: clinicopathologic, immunohistochemical, and gene expression profiling analysis of 31 cases with a review of the literature. Am J Surg Pathol 2008;32:656–670.
Cheng L, Williamson SR, Zhang S et al. Understanding the molecular genetics of renal cell neoplasia: implications for diagnosis, prognosis and therapy. Expert Rev Anticancer Ther 2010;10:843–864.
Kuroda N, Hosokawa T, Michal M et al. Clear cell renal cell carcinoma with focal renal angiomyoadenomatous tumor-like area. Ann Diagn Pathol 2011;15:202–206.
Mai KT, Kohler DM, Belanger EC et al. Sporadic clear cell renal cell carcinoma with diffuse cytokeratin 7 immunoreactivity. Pathology 2008;40:481–486.
Kuroda N, Michal M, Hes O et al. Renal angiomyoadenomatous tumor: fluorescence in situ hybridization. Pathol Int 2009;59:689–691.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Williamson, S., Cheng, L., Eble, J. et al. Renal cell carcinoma with angioleiomyoma-like stroma: clinicopathological, immunohistochemical, and molecular features supporting classification as a distinct entity. Mod Pathol 28, 279–294 (2015). https://doi.org/10.1038/modpathol.2014.105
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2014.105
This article is cited by
-
Renal cell carcinoma associated with tuberous sclerosis complex (TSC)/mammalian target of rapamycin (MTOR) genetic alterations
Modern Pathology (2022)
-
Renal cell carcinoma with leiomyomatous stroma in tuberous sclerosis complex: a distinct entity
Virchows Archiv (2021)
-
Novel, emerging and provisional renal entities: The Genitourinary Pathology Society (GUPS) update on renal neoplasia
Modern Pathology (2021)
-
Clinicopathologic analysis of renal cell carcinoma containing Intratumoral fat with and without osseous metaplasia
Diagnostic Pathology (2020)
-
Renal cell tumors with clear cell histology and intact VHL and chromosome 3p: a histological review of tumors from the Cancer Genome Atlas database
Modern Pathology (2017)