Abstract
A distinct renal tumor has recently been described as “high-grade oncocytic renal tumor” and “sporadic renal cell carcinoma with eosinophilic and vacuolated cytoplasm”. The Genitourinary Pathology Society (GUPS) consensus proposed a unifying name “eosinophilic vacuolated tumor” (EVT) for this emerging entity. In this multi-institutional study, we evaluated 19 EVTs, particularly their molecular features and mutation profile, using next-generation sequencing. All cases were sporadic and none of the patients had a tuberous sclerosis complex. There were 8 men and 11 women, with a mean age of 47 years (median 50; range 15–72 years). Average tumor size was 4.3 cm (median 3.8 cm; range 1.5–11.5 cm). All patients with available follow-up data (18/19) were alive and without evidence of disease recurrence or progression during the follow-up, ranging from 12 to 198 months (mean 56.3, median 41.5 months). The tumors were well circumscribed, but lacked a well-formed capsule, had nested to solid growth, focal tubular architecture, and showed ubiquitous, large intracytoplasmic vacuoles, round to oval nuclei, and prominent nucleoli. Immunohistochemically, cathepsin K, CD117, CD10, and antimitochondrial antigen were expressed in all cases. Other positive stains included: PAX8, AE1/AE3 and CK18. CK7 was typically restricted only to rare scattered cells. Vimentin, HMB45, melan-A, and TFE3 were negative in all cases. All tumors showed retained SDHB. All cases (19/19) showed non-overlapping mutations of the mTOR pathway genes: TSC1 (4), TSC2 (7), and MTOR (8); one case with MTOR mutation showed a coexistent RICTOR missense mutation. Low mutational rates were found in all samples (ranged from 0 to 6 mutations/Mbp). Microsatellite instability and copy number variations were not found in any of the 17 analyzable cases. EVT represents an emerging renal entity that shows a characteristic and readily identifiable morphology, consistent immunohistochemical profile, indolent behavior, and mutations in either TSC1, TSC2, or MTOR genes.
Similar content being viewed by others
Introduction
Recently, two studies have described a distinct renal tumor, previously unrecognized and not listed in the 2016 WHO classification, that emerged from the group of difficult to classify eosinophilic (oncocytic) renal tumors1,2. This novel type of renal tumor had a readily recognizable morphology and was composed of eosinophilic (oncocytic) cells with large intracytoplasmic vacuoles, atypical nuclear features with prominent nucleoli, and exhibited a relatively consistent immunohistochemical profile. The initial names proposed for this tumor were “high-grade oncocytic renal tumor (HOT)” by He et al.1 and “sporadic renal cell carcinoma with eosinophilic and vacuolated cytoplasm” by Chen et al.2. All patients included in these two studies presented with non-syndromic, solitary tumors, and both studies described virtually the same tumor morphology and immunoprofile. All reported tumors had indolent behavior, although with relatively limited follow-up data. One additional case was subsequently documented in a patient with tuberous sclerosis complex (TSC)3, in contrast to the previously reported sporadic cases. The prevalence of this type of tumor in a sporadic and syndromic setting is currently unknown.
The molecular insights into this entity have so far been limited. Chen et al. found somatic inactivating mutations of TSC2 in 3/5 tumors tested, activating mutations of MTOR in 2/5 tumors tested, additionally loss of chromosome 1 in both cases showing an MTOR mutation, consistent with a hyperactive MTOR complex. He et al. also found loss of chromosome 1 (3/9), but also of chromosome 19 (4/9 cases), and loss of heterozygosity at 16p11 (3/3 cases) and 7q31 (2/3 cases) were observed. No other chromosomal gains or losses were found in both studies.
Most recently, a unifying consensus name for this entity, “eosinophilic vacuolated tumor” (EVT) has been proposed by the Genitourinary Pathology Society (GUPS)4, to reflect the most salient morphologic features of this entity. GUPS proposed that EVT should be considered an “emerging renal entity”, requiring additional work and validation.
Therefore, the aim of this study was to further characterize EVT, to evaluate and validate its molecular features and mutation profile, focusing specifically on investigating the alterations of the mTOR pathway.
Material and methods
Case selection
A total of 25 cases were initially considered as possible EVT, primarily based on the morphology. Only 3 of these were previously included in the He et al. study (cases #1, #3, and #9 in the current study)1. However, these cases were initially not analyzed more comprehensively for molecular-genetic changes, and in the current study we provide an updated follow-up for these cases. The 22 new cases were identified and collected from the files of the University Hospital Plzen, University of Calgary, Cleveland Clinic, Institute Nacional de Cancerologia Mexico City, University of Washington, Seattle, University of Sydney, Hôpital Tenon Paris, University of Alabama at Birmingham, Stradin´s University, Riga, University of Toronto, McGill University, University of Szeged, University of Erlangen, Universitätsklinikum Hamburg-Eppendorf, Alfa Medical, Bratislava, University Hospital Ostrava, University Hospital Nitra, and Cruces University Hospital, Barakaldo. All available clinical and other data were obtained from the files of the participating institutions.
Two pathologists performed a final review of all cases (OH and KT) with a critical evaluation of the morphology, immunohistochemical profile, and molecular-genetic features. The final cohort included in the study consisted of 19 cases, based on the morphologic features, immunohistochemical profile, and molecular-genetic features. Six cases were excluded from the final cohort. One case was excluded because the DNA quality was insufficient for a complete molecular-genetic analysis, although this case fulfilled the morphologic criteria and the immunohistochemical profile for an EVT. Two patients had FLCN mutations, but not TSC1, TSC2, and MTOR mutations, despite some morphologic and immunohistochemical similarities to EVT. On further investigation, one patient had a single renal tumor (1.7 cm), skin lesions, family history of renal tumors, and a pneumothorax in the past, but was never tested genetically for Birt-Hogg-Dubé (BHD); the second patient had a single renal tumor (1.5 cm), but no history of BHD. Finally, 3 additional cases demonstrated no mutations of TSC1, TSC2, and MTOR when analyzed by the next-generation sequencing (NGS) panel used in this study, although they showed a morphologic similarity with EVT on the initial evaluation. No other significant molecular changes were identified in these 3 cases. Detailed information about the 6 excluded cases is provided in the Supplementary Tables 1 and 2.
Immunohistochemistry
All immunohistochemical (IHC) stains were performed at a single laboratory (University Hospital Plzen), using a Ventana Benchmark XT automated stainer (Ventana Medical System, Inc., Tucson, AZ, USA). The following primary antibodies were used: cytokeratin (polyclonal: AE1-AE3 and PCK26, Ventana, RTU), CK18 (DC 10, monoclonal, DakoCytomation, Carpinteria CA, 1: 100), CK7 (OV-TL12/30, monoclonal, DakoCytomation, 1:200), cytokeratin 20 (M7019, monoclonal; Dako; 1:100), racemase/AMACR (P504S, monoclonal; Zeta, Sierra Madre, CA; 1:50), vimentin (V9, monoclonal; Cell Marque, Rocklin, CA; RTU), Ki-67 (monoclonal, MIB-1, 1:400, Dako), TFE3 (monoclonal, MRQ-37, RTU, Ventana Medical System, Inc.), c-kit (CD117, polyclonal, DakoCytomation, 1:300), CD10 (Sp67, monoclonal, Ventana, RTU), anti-melanosome (HMB45, monoclonal, DakoCytomation, 1:200), Melan A (A103, monoclonal, Ventana, RTU), PAX 8 (MRQ-50, monoclonal, CellMarque, Rocklin, CA, RTU), antimitochondrial antibody (113-1, monoclonal, Biogenex, San Ramon, CA, 1:500), SDHB (polyclonal, Sigma Aldrich, St. Luis, MO, 1:50), cathepsin K (3F9, monoclonal, Abcam, Cambridge, UK, 1:400). Primary antibodies were visualized using a supersensitive streptavidin-biotin-peroxidase complex (BioGenex). Internal biotin was blocked using the standard protocol for the Ventana Benchmark XT automated stainer (hydrogen peroxide based). Appropriate positive and negative controls were used. IHC result were interpreted as follows: (−) if 0% of neoplastic cells were positive; (+/−) <10% of cells positive); (+) 10–25% of cells positive; (++) >25–50% of cells positive; (+++); >50–75% of cells positive; and (++++) >75–100% of cells positive.
Next-generation sequencing
The tumor DNA samples were profiled using massively parallel sequencing of exons from 592 genes (SureSelect XT, Agilent, Santa Clara, CA and the NextSeq instrument, Illumina, San Diego, CA), as previously described5. The tumor mutational burden (TMB) was assessed by calculating the number of nonsynonymous missense mutations, excluding common germline variants, in one megabase of DNA. TMB was considered high if ≥11 mutations/megabase (muts/Mb) were detected6 Microsatellite instability (MSI) was calculated from the NGS data by direct analysis of short tandem repeat tracts in the target regions of sequenced genes. The count only included alterations that resulted in increases or decreases in the number of repeats; high microsatellite instability (MSI-H) was defined as ≥46 altered microsatellite loci. This threshold was established by comparing NGS results with PCR-based microsatellite fragment analysis results in ~2100 samples7.
Results
Clinicopathologic and immunohistochemistry results
Clinicopathologic data and immunohistochemistry results are summarized in Table 1. There were 8 men and 11 women (M:F = 1:1.4), with a mean patient age of 47 years (median 50; range 15–72 years). Average tumor size was 4.3 cm (median 3.8; range 1.5–11.5 cm). Pathologic stage pT1a was found in 12/18 cases, 5/18 were pT1b, and 1/18 was pT2b (tumor size and stage information was not available for case #19). All patients (18/19) with available follow-up data were alive and without evidence of disease (recurrence or progression) during the follow-up, ranging from 12 to 198 months (mean 56.3, median 41.5 months).
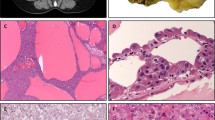
Grossly, all tumors were circumscribed and solid, lacked a well-formed capsule, macrocysts, and necrosis. The cut surface was gray, tan-mahogany or dark brown (Fig. 1). On microscopy, the common architectural patterns included solid, compact acinar, nested, or broad trabecular, as well as focal tubular or tubulocystic architecture. Prominent thick-walled vessels were typically present at the periphery and small non-neoplastic tubules were often entrapped at the periphery. The neoplastic cells had prominent membranes and voluminous eosinophilic cytoplasm, usually non-homogeneous, with variable granularity, typically condensed toward the cell periphery, reminiscent of chromophobe RCC (ChrRCC). Importantly, the cells had ubiquitous and large intracytoplasmic vacuoles and round to oval nuclei, with more granular to coarse chromatin, and prominent nucleoli (corresponding to WHO/ISUP grade 3), although often even larger inclusion-like nucleoli were found in virtually all cases. Mitoses were an exceptionally rare finding.
A EVT is a grossly a circumscribed and solid tumor, and lacks a well-formed capsule, macrocysts, and necrosis. The cut surface can be gray (as shown), tan-mahogany or dark brown. B On microscopy, they often show solid growth, and large thick-walled vessels are typically found at the periphery, with entrapped non-neoplastic tubules. C The neoplastic cells have large intracytoplasmic vacuoles. D, E Other growth patterns include tubular and nested, often set in stromal areas in the background. F The neoplastic cells, in addition to large vacuoles, have voluminous eosinophilic cytoplasm, round to oval nuclei, and often very prominent nucleoli, that focally may appear as inclusions.
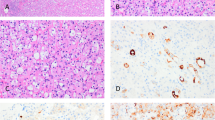
On immunohistochemistry, cathepsin K was expressed in all cases, as well as CD117 (in some cases, both were focal) (Fig. 2). All tumors were also positive for CD10 and antimitochondrial antigen (MIA). Other positive stains (not listed in Table 1) included PAX8, AE1/AE3, and cytokeratin (CK) 18; SDHB were retained in all cases, exhibiting strong and diffuse reactivity. AMACR was also variably positive in the majority of cases (16/19). CK7 was typically very focal and limited, restricted to scattered positive cells, usually not exceeding 10% of neoplastic cells overall. Focal CK20 reactivity was also noted in 6/18 evaluated tumors, typically limited and very focal. All tumors were completely negative for vimentin. Other negative stains (not listed in Table 1) included HMB45, Melan-A, and TFE3. Ki67 reactivity was very low in most of the cases (<1%), typically with 0–3 positive cells/high-power field.
A On immunohistochemistry, cathepsin K was expressed in all cases. Of note, in some cases we have also noted a focal membranous (or submembranous) pattern, in addition to the cytoplasmic one, that was more common. B Reactivity for CD117 was also found in all cases (in some cases, both cathepsin K and CD117 were focal). C All tumors were also positive for CD10. D CK7 was typically very focal and found in only scattered cells; in some cases, only very rare CK7 positive cells were present (inset).
Molecular results
Molecular-genetic findings are summarized in Table 2 and Fig. 3. All 19 analyzed cases showed non-overlapping mTOR pathway mutations in either TSC1, TSC2, or MTOR genes. Four cases showed exclusive pathogenic TSC1 gene mutations. TSC2 gene mutations were identified in 7 cases. Single pathogenic (or likely pathogenic) MTOR gene mutations were identified in 8 cases; one of these showed a coexistent RICTOR missense mutation (case #7). Low mutational rates were found in all samples, ranging from 0 to 6 mutations/Mbp. Microsatellite instability and copy number variations were not found in any of the 17 analyzable cases. Оf note, two cases (#3 and #9) that lacked copy number variations in the current study, were also analyzed in the previous study by He et al. and showed −19p and −1, +19p, respectively (shown as cases #2 and #12)1. The differences in the results for these two cases likely stem from the methodological differences in the techniques used. While He et al. used CGH microarray analysis, which is comprehensive genome-wide screen for copy gains and losses, in the current study we only screened for isolated gene amplifications (at least six copies) involving a specific gene panel, which allows for a less detailed chromosomal analysis.
All 19 analyzed cases showed non-overlapping mTOR pathway substitution mutations in either TSC1, TSC2, or MTOR genes. In some cases, combinations of splice-missense, splice-frameshift, frameshift-missense, or dual frameshift mutations were found. The mutation details are shown in Table 2.
Discussion
This study represents the largest molecular evaluation series of EVT reported to-date, assembled through a multi-institutional collaboration. Importantly, we confirmed non-overlapping mTOR pathway genes mutations in all studied cases, which further confirms and validates the molecular profile of EVT2. All EVT cases included in this series showed mutually exclusive alterations of TSC1 (4/19), TSC2 (7/19), or MTOR (8/19) genes. Although only single inactivating TSC1 and TSC2 gene mutations were detected in some samples, two different mutations were found in other cases (e.g., case #1, #3, #4, #14, #15, and #19); it is presumed that the samples with only one detected mutation had biallelic inactivation either due to synonymous mutations or LOH. This discrepancy can be explained by the limitations of the methodology used, which does not capture the intronic or promoter regions of the gene, including the LOH or epigenetic changes. We can only speculate on the significance of the concomitant MTOR/RICTOR alterations observed in one tumor (case #7) and their role as possible co-oncogenic drivers, as postulated recently in non-small cell lung cancer8. Nevertheless, the consistent molecular profile observed in this study, in addition to the characteristic morphology and immunoprofile, further support the notion that EVT truly represents a distinct renal entity. Of note, none of the 19 patients showed clinical features or other findings (for example renal angiomyolipomas) typically associated with TSC, indicating the sporadic nature of the studied tumors. Lastly, all patients with renal EVTs were alive and without evidence of disease recurrence or progression during the follow-up, which further supports the initial observations that this likely represents an indolent tumor type.
In 2018, He et al. presented a study that included a multi-institutional series of 14 cases, and proposed that this renal tumor potentially represents a distinct entity; some of the authors of the current study also participated in that study1. He et al. initially designated this type of tumor as “high-grade oncocytic tumor” (HOT)1. 9/14 cases of the initial study were analyzed by aCGH, although a more extensive molecular-genetic analysis was not performed. Subsequently, in 2019, Chen et al. reported a single-institution series of 7 cases, designated “sporadic renal cell carcinomas with eosinophilic and vacuolated cytoplasm”, demonstrating virtually identical clinicopathologic and histomorphologic features2. In 5/7 cases, in which they performed molecular analysis, 3 had a TSC2 inactivating mutations (2 with independent TSC2 mutations). The other 2 analyzed cases harbored a MTOR c.7280T>G, p.(L2427R) mutation. In the current study, we found that MTOR mutations were slightly more frequent than the TSC2 ones (8 and 7 cases, respectively); in addition, we also found 4 cases with TSC1 mutations. Both cases with MTOR activating mutations in the study by Chen et al.2 also had a loss of chromosome 1, as shown initially by He et al.1. The presence of hyperactive mTORC1 signaling was supported by the diffuse immunohistochemical staining for p-S6 and p-4EBP12.
Similar to the current series, all patients in the two initial studies presented with solitary, tan-yellow to brown tumors, and had no prior history of syndromic associations, including TSC1,2. All initially reported tumors were stage pT1a or pT1b, with identical mean size of 3.4 cm, ranging from 1.5 to 7 cm1,2. Regarding the immunoprofile, the evaluated cases in these two series were Cathepsin K positive (negative in only 1 case); 9/14 cases in the series by He et al. also had CD117 expression, while CK7 was either negative or only focally positive in both studies1,2. CD10 expression was documented in 12/13 cases in the He et al. study (not performed by Chen et al.)1. The remaining immunoprofile documented in the He et al. study essentially mirrored the findings of the current study. Examples of EVT (HOT) were also studied by electron microscopy and demonstrated numerous intracytoplasmic mitochondria resembling the findings in renal oncocytoma9. In the study by He et al., 10 patients with available follow-up were without disease progression after a mean follow-up of 28 months (range 1–112 months)1. Similarly, Chen et al. also reported an indolent behavior in all 5 patients with available median follow-up of 12 months (range 10–128 months)2.
One female patient with EVT (designated as “HOT”) was subsequently reported in a TSC patient3. However, this patient had a known history of TSC and also had multiple small angiomyolipomas and one small renal cell carcinoma with fibromyomatous (angioleiomyomatous) stroma, adjacent to the EVT3, as typically seen in TSC patients10. In our opinion, similar or virtually identical tumors to EVT, according to our assessment of the available illustrations and provided data, have also been included in some recent studies, albeit designated with different names. For example, such tumors with similar morphology harboring TSC mutations have been included in a series of “eosinophilic renal tumors“11. Further, some examples illustrated in the study by Palsgrove et al. (listed as cases #6 and #7) may also represent EVT, rather than eosinophilic solid and cystic renal cell carcinoma (ESC RCC), as proposed by the authors12. Taking into account that several studies used different terminology to describe essentially the same renal tumor type, the Genitourinary Pathology Society (GUPS) consensus group has recently proposed a new unifying name “eosinophilic vacuolated tumor“ (EVT) for this entity4. The proposed name reflects the typical morphologic features (“eosinophilic and vacuolated”), and avoids the “high-grade” descriptive characterization used initially; the term “tumor” instead of “carcinoma” was preferred, given that all reported cases so far had indolent behavior4.
In our view, the most important differential diagnostic category for EVT are those tumors that are difficult to classify and exhibit ‘borderline’ or overlapping features between oncocytoma and ChrRCC, which can be found either in syndromic or sporadic setting10,13,14,15,16,17. Such eosinophilic (oncocytic) tumors with overlapping morphologies pose a common diagnostic dilemma and do not fit into any of the currently recognized renal tumor categories in the WHO classification13,18. In brief, the typical EVT morphology is beyond the morphologic spectrum of renal oncocytoma, despite the IHC similarities that include reactivity for CD117, accompanied by focal CK7 expression, typically restricted to scattered cells or cell clusters. Although the “plant cell-like” pattern of classic ChrRCC can superficially resemble EVT, ChrRCC lacks marked cytoplasmic vacuoles, “atypical” nuclear features with very prominent nucleoli, and exhibits irregular (“raisinoid”) nuclei, not seen in EVT. Although both oncocytoma and ChrRCC demonstrate CD117 reactivity, they are negative for cathepsin K, as seen in EVT. Of note, ChrRCC also typically shows diffuse CK7 reactivity, unlike EVT.
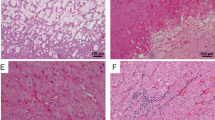
The recent GUPS update on the novel developments in existing renal tumors proposed the term “oncocytic renal neoplasm of low malignant potential, not further classified”, when referring to solitary and sporadic, difficult to classify ‘borderline’ tumors with overlapping features between oncocytoma and ChrRCC.19. Hereditary cases, that are typically multifocal and bilateral, should exclusively be named “hybrid oncocytic tumors”. Such cases with “hybrid” morphology in the setting of BHD syndrome are often characterized by scattered clusters and individual cells with clear cytoplasm, exhibiting a “checkerboard” mosaic pattern. Indeed, we found a significant morphologic and immunoprofile overlap between EVT and two similar tumors that had FLCN mutation. These tumors were initially considered as possible EVTs, but were excluded from the final cohort because both lacked TSC/MTOR mutations and showed instead FLCN mutations (Fig. 4A, B). One of these patients likely had an inherited BHD syndrome, and one likely had a new somatic mutation, without the BHD stigmata. These two cases demonstrated similar morphology to EVT, despite some subtle differences, such as a mosaic-type pattern and absence of prominent nucleoli (i.e., high-grade nuclear features) and they also had immunohistochemical similarities with EVT (see Supplementary Tables 1 and 2). From a practical standpoint, we would consider those cases with documented FLCN mutations (and absent TSC/MTOR mutations) as “hybrid oncocytic tumors”. For the sake of the study clarity, we also excluded 4 additional cases: one case because it had low DNA quality that did not allow to perform all genetic studies, and 3 cases that had similar morphology and immunohistochemical profile (CD117, CD10, cathepsin K, and MIA positive) to EVT, but had no identifiable mutations of TSC1, TSC2, and MTOR (Fig. 4C, D). It is possible that these cases may have had undetected TSC/MTOR mutations when analyzed by the NGS panel applied in this study; however, they also lacked other identifiable mutations. Such cases are currently best considered “renal oncocytic neoplasms, not otherwise specified”. If signing out such cases in practice, one may add“with EVT-like features”.
Two cases with FLCN mutations are illustrated in A and B. These two cases had similar morphology to EVT, despite some subtle differences, including a more mosaic-type growth and absence of prominent nucleoli. One of these patients (A) likely had a true BHD syndrome, and one (B) had a new mutation, without the BHD stigmata. Both cases lacked TSC/MTOR mutations. They also showed immunohistochemical similarities with EVT, including cathepsin K reactivity, either more diffuse (A – inset), or focal (B – inset). Both cases were also positive for CD117 and for CK7 (both > 30%) (not shown). Two additional cases that had no identifiable mutations of TSC1, TSC2, and MTOR, but with very similar morphology to EVT are illustrated in C and D. It is possible that these cases may have had undetected TSC/MTOR mutations, but they also lacked other identifiable mutations.
Other, less common renal tumors that can potentially mimic EVT include MiTF RCC (TFEB and TFE3), SDH-deficient RCC, and ESC RCC, another novel renal entity and their distinguishing features have been covered in several recent reviews4,9,18,20. Regarding the similarities with ESC RCC, EVT indeed shares molecular similarities that include the presence of TSC2 and TSC1 mutations and activation of the mTOR pathway; additionally, rare examples of both entities have been found in TSC patients. EVT can be distinguished from ESC RCC primarily on morphology, as it typically lacks a macrocystic gross component (typically found in ESC RCC), has large cytoplasmic vacuoles (not usually seen in ESC RCC), and generally lacks the coarse cytoplasmic granularity seen in ESC RCC (such granularity can however rarely be found in some EVTs). There are also IHC differences between EVT and ESC RCC. EVT typically exhibits CD117+/vimentin−/CK20− profile (though rare cases may show focal CK20+ cells), which is different from the typical ESC RCC immunoprofile CD117−/vimentin+/CK20+4. Cathepsin K is also positive in the majority of ESC RCC and in EVT. In EVT, the positivity is either diffuse (most of the cases) or focal; in some cases, we have also noticed a focal membranous pattern (possibly a submembranous condensation), in addition to the more common cytoplasmic one. In ESC RCC, the positivity for cathepsin K is often patchy, cytoplasmic, with rare cases showing either diffuse reactivity or, very rarely, complete absence of staining4,12,21.
TSC/MTOR mutations appear to be more commonly found in some other novel renal entities, for example RCC with fibromyomatous stroma9,12,18,22,23,24,25. However, such mTOR pathway mutations have also been found in AML (or PEComas), and in some common renal tumors, such as metastatic clear cell RCC or papillary RCC26,27,28,29, acquired cystic disease associated (ACD) RCC25, and in rare examples of “unclassified aggressive RCCs”30. It appears that the tumors with TSC/MTOR mutations represent a diverse group of renal neoplasms showing variable morphologies and immunoprofiles, and different biologic behaviors. Thus, the introduction of a potential concept of “TSC-associated renal tumor family”, although appealing, is currently unjustifiable, based on the available evidence, and requires further study4.
In summary, EVT is an emerging low-grade renal entity that can be either diagnosed or suspected on morphology, and shows a relatively uniform immunohistochemical profile. We confirmed in this study that EVT is consistently associated with mTOR pathway abnormalities, including non-overlapping mutations in MTOR, TSC2, and TSC1. All reported cases so far, including the ones from this study, exhibited an indolent behavior. The findings from our study strongly support the conclusion that EVT should be recognized as a distinct and novel renal entity.
Data availability
All data are shown in the article.
Change history
05 October 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41379-021-00941-4
02 November 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41379-021-00967-8
References
He, H. et al. “High-grade oncocytic renal tumor”: morphologic, immunohistochemical, and molecular genetic study of 14 cases. Virchows Arch 473, 725–738 (2018).
Chen, Y. B. et al. Somatic mutations of TSC2 or MTOR characterize a morphologically distinct subset of sporadic renal cell carcinoma with eosinophilic and vacuolated cytoplasm. Am. J. Surg. Pathol. 43, 121–131 (2019).
Trpkov, K. et al. High-grade oncocytic tumour (HOT) of kidney in a patient with tuberous sclerosis complex. Histopathology 75, 440–442 (2019).
Trpkov, K. et al. Novel, emerging and provisional renal entities: The Genitourinary Pathology Society (GUPS) update on renal neoplasia. Mod. Pathol. 34, 1167–1184 (2021).
Gatalica, Z., Xiu, J., Swensen, J. & Vranic, S. Comprehensive analysis of cancers of unknown primary for the biomarkers of response to immune checkpoint blockade therapy. Eur. J. Cancer 94, 179–186 (2018).
Samstein, R. M. et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet 51, 202–206 (2019).
Vanderwalde, A., Spetzler, D., Xiao, N., Gatalica, Z. & Marshall, J. Microsatellite instability status determined by next-generation sequencing and compared with PD-L1 and tumor mutational burden in 11,348 patients. Cancer Med. 7, 746–756 (2018).
Ruder, D. et al. Concomitant targeting of the mTOR/MAPK pathways: novel therapeutic strategy in subsets of RICTOR/KRAS-altered non-small cell lung cancer. Oncotarget 9, 33995–34008 (2018).
Siadat, F. & Trpkov, K. ESC, ALK, HOT and LOT: Three letter acronyms of emerging renal entities knocking on the door of the WHO Classification. Cancers 12, 1 (2020).
Guo, J. et al. Tuberous sclerosis-associated renal cell carcinoma: a clinicopathologic study of 57 separate carcinomas in 18 patients. Am. J. Surg. Pathol. 38, 1457–1467 (2014).
Tjota, M. et al. Eosinophilic renal cell tumors with a TSC and MTOR gene mutations are morphologically and immunohistochemically heterogenous: clinicopathologic and molecular study. Am. J. Surg. Pathol. 44, 943–954 (2020).
Palsgrove, D. N. et al. Eosinophilic solid and cystic (ESC) renal cell carcinomas harbor TSC mutations: Molecular analysis supports an expanding clinicopathologic spectrum. Am. J. Surg. Pathol. 42, 1166–1181 (2018).
Williamson, S. R. et al. Diagnostic criteria for oncocytic renal neoplasms: a survey of urologic pathologists. Hum. Pathol. 63, 149–156 (2017).
Petersson, F. et al. Sporadic hybrid oncocytic/chromophobe tumor of the kidney: a clinicopathologic, histomorphologic, immunohistochemical, ultrastructural, and molecular cytogenetic study of 14 cases. Virchows Arch 456, 355–365 (2010).
Delongchamps, N. B. et al. Hybrid tumour ‘oncocytoma-chromophobe renal cell carcinoma’ of the kidney: a report of seven sporadic cases. BJU Int. 103, 1381–1384 (2009).
Mai, K. T., Dhamanaskar, P., Belanger, E. & Stinson, W. A. Hybrid chromophobe renal cell neoplasm. Pathol. Res. Pr. 201, 385–389 (2005).
Hes, O., Petersson, F., Kuroda, N., Hora, M. & Michal, M. Renal hybrid oncocytic/chromophobe tumors - a review. Histol. Histopathol. 28, 1257–1264 (2013).
Trpkov, K. & Hes, O. New and emerging renal entities: a perspective post-WHO 2016 classification. Histopathology 74, 31–59 (2019).
Trpkov, K. et al. New developments in existing WHO entities and evolving molecular concepts: the Genitourinary Pathology Society (GUPS) update on renal neoplasia. Mod. Pathol. 34, 1392–1424 (2021).
Alaghehbandan, R., Perez Montiel, D., Luis, A. S. & Hes, O. Molecular genetics of renal cell tumors: a practical diagnostic approach. Cancers 12, 1 (2019).
Tretiakova, M. S. Eosinophilic solid and cystic renal cell carcinoma mimicking epithelioid angiomyolipoma: series of 4 primary tumors and 2 metastases. Hum. Pathol. 80, 65–75 (2018).
Trpkov, K. et al. Eosinophilic, solid, and cystic renal cell carcinoma: Clinicopathologic study of 16 unique, sporadic neoplasms occurring in women. Am. J. Surg. Pathol. 40, 60–71 (2016).
Trpkov, K. et al. Eosinophilic solid and cystic renal cell carcinoma (ESC RCC): Further morphologic and molecular characterization of ESC RCC as a distinct entity. Am. J. Surg. Pathol. 41, 1299–1308 (2017).
Parilla, M. et al. Are sporadic eosinophilic solid and cystic renal cell carcinomas characterized by somatic tuberous sclerosis gene mutations? Am. J. Surg. Pathol. 42, 911–917 (2018).
Shah, R. B. et al. “Renal cell carcinoma with leiomyomatous stroma” harbor somatic mutations of TSC1, TSC2, MTOR, and/or ELOC (TCEB1): Clinicopathologic and molecular characterization of 18 sporadic tumors supports a distinct entity. Am. J. Surg. Pathol. 44, 571–581 (2020).
Schultz, L. et al. Immunoexpression status and prognostic value of mTOR and hypoxia-induced pathway members in primary and metastatic clear cell renal cell carcinomas. Am. J. Surg. Pathol. 35, 1549–1556 (2011).
Kwiatkowski, D. J. et al. Mutations in TSC1, TSC2, and MTOR are associated with response to rapalogs in patients with metastatic renal cell carcinoma. Clin. Cancer Res. 22, 2445–2452 (2016).
Roldan-Romero, J. M. et al. Molecular characterization of chromophobe renal cell carcinoma reveals mTOR pathway alterations in patients with poor outcome. Mod. Pathol. 33, 2580–2590 (2020).
Chaux, A. et al. Dysregulation of the mammalian target of rapamycin pathway in chromophobe renal cell carcinomas. Hum. Pathol. 44, 2323–2330 (2013).
Chen, Y. B. et al. Molecular analysis of aggressive renal cell carcinoma with unclassified histology reveals distinct subsets. Nat. Commun. 7, 13131 (2016).
Funding
This study was supported by the Charles University Research Fund (project number Q39) and by the grant of Ministry of Health of the Czech republic-Conceptual Development of Research Organization (Faculty Hospital in Plzen- FNPl 00669806).
Author information
Authors and Affiliations
Contributions
M.F.: drafted paper, evaluated results; G.Z., J.S., P.N.: performed genetic analyses; Z.M., A.R., W.S.R., M.-G.C., G.A., T.M., M.M.: helped with design and interpretation of results; L.J.I., P.-M.D., S.M., C.E., B.F., Y.A., S.F., S.A., G.Y., K.L., P.K., R.J., A.A., H.A., F.C., R.B., H.M., D.D., B.M.: provided cases with full details, G.G., M.H.: evaluated clinical information, T.K. and O.H.: coordinated whole study, edited draft and constructed discussion.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study was performed in accordance with the Declaration of Helsinki. Ethic committee approval was not required by Charles University and University Hospital Plzen.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Due to errors on author names.
Supplementary information
Rights and permissions
About this article
Cite this article
Farcaş, M., Gatalica, Z., Trpkov, K. et al. Eosinophilic vacuolated tumor (EVT) of kidney demonstrates sporadic TSC/MTOR mutations: next-generation sequencing multi-institutional study of 19 cases. Mod Pathol 35, 344–351 (2022). https://doi.org/10.1038/s41379-021-00923-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-021-00923-6
This article is cited by
-
Genomic alterations and diagnosis of renal cancer
Virchows Archiv (2024)
-
Clinicopathological correlation of Cathepsin K expression in salivary gland carcinomas; relation to patients` outcome
Diagnostic Pathology (2023)
-
mTOR eosinophilic renal cell carcinoma: a distinctive tumor characterized by mTOR mutation, loss of chromosome 1, cathepsin-K expression, and response to target therapy
Virchows Archiv (2023)
-
Do we need an updated classification of oncocytic renal tumors?
Modern Pathology (2022)
-
Low grade oncocytic tumors of the kidney: a clinically relevant approach for the workup and accurate diagnosis
Modern Pathology (2022)