Abstract
Only a minority of patients who undergo surgical resection for pancreatic ductal adenocarcinoma are cured. Since patient outcome is not reliably predicted using pathological factors (tumor stage, differentiation, and resection margin status) alone, markers of tumor behavior are needed. One candidate predictor of pancreatic cancer outcome is E-cadherin status. CDH1 is a tumor suppressor gene encoding an important cell adhesion molecule (E-cadherin). The aim of this study was to determine if, among patients undergoing pancreaticoduodenectomy for pancreatic adenocarcinoma, loss of E-cadherin expression was an independent predictor of poor outcome. We examined patterns of loss of E-cadherin by immunohistochemistry in tissue microarrays of 329 surgically resected pancreatic ductal adenocarcinomas. E-cadherin expression was then correlated with outcome. Kaplan–Meier analysis and Cox proportional hazards regression modeling were used to assess the mortality risk. One hundred forty-one pancreatic adenocarcinomas (43%) had partial or complete loss of E-cadherin expression within the analyzed tissue cores. In most instances (134 cases, 41%), this loss was partial. Patients whose pancreatic adenocarcinomas had either complete loss (n=7; median survival, 5.5 months) or partial loss (n=134; 12.7 months) of E-cadherin expression had significantly worse median survival than those with uniformly intact E-cadherin expression (n=188; 18.5 months) by univariate (P=0.002) and multivariate (P=0.006) analyses. In subgroup analysis, patients with poorly differentiated cancers had a worse prognosis if their cancers had partial loss of E-cadherin expression (P=0.02). Among patients undergoing pancreaticoduodenectomy for pancreatic ductal adenocarcinoma, partial loss of tumoral E-cadherin expression is an independent predictor of poor outcome.
Similar content being viewed by others
Main
Pancreatic ductal adenocarcinoma is the 4th leading cause of cancer death in the United States. In 2010, it is estimated that 43 140 Americans will be diagnosed with pancreatic cancer1 and only about 4% will live 5 years after diagnosis. One important reason for this poor survival is that ∼85% of patients present with advanced unresectable disease.2 Furthermore, pancreatic cancer remains unresponsive to most chemotherapeutic agents. Hence, there is a great need to understand the biological mechanisms that contribute to pancreatic cancer development and progression. Survival is better for patients with pancreatic cancers localized to the pancreas, as surgical resection at present offers the only chance of cure. For patients with resectable ductal adenocarcinoma of the pancreatic head, the average 5-year overall survival is ∼20–25%.3 Clinicopathological parameters provide important prognostic information. The presence of positive resection margins, poor tumor differentiation, large tumor, and positive lymph nodes all portend a worse prognosis.3, 4
Several molecular markers are also independently associated with outcome among patients who undergo pancreaticoduodenectomy for pancreatic cancer. Among the common somatic genetic alterations, including activating point mutations in the KRAS (KRAS2) oncogene and inactivating mutations of CDKN2A, TP53, and DPC4/SMAD4 tumor suppressor genes, only the presence of SMAD4 mutations and loss of Smad4 protein (an accurate marker of SMAD4 mutation) has been shown to be associated with an adverse outcome.5, 6
Gene silencing by DNA methylation (including CDKN2A, SPARC, RELN, TFPI2, and numerous others) also contributes to the development and progression of pancreatic cancer,7, 8, 9, 10, 11, 12, 13, 14, 15, 16 although these methylated genes have not been shown to be independent predictors of outcome. In contrast, Sparc expression in pancreatic cancer-associated fibroblasts does portend an adverse outcome, and Sparc expression is being evaluated to determine if it predicts response to albumin-bound Paclitael (Abraxane) therapy.17 Similarly, loss of BNIP3 expression, a gene commonly methylated in pancreatic cancers has been shown to predict responsiveness to gemcitabine therapy.18, 19
One gene that undergoes genetic and epigenetic inactivation in pancreatic and other cancers and is associated with poor outcome in multiple cancer types is CDH1. Among pancreatic adenocarcinomas, those with an undifferentiated phenotype have a poor outcome and typically lack E-cadherin expression20, 21 and pancreatic adenocarcinomas lacking E-cadherin expression are more likely to be poorly differentiated.15, 22, 23, 24, 25
Mutational inactivation (≤5%)26 or complete silencing of CDH1 by DNA methylation20, 27 has been identified only occasionally (∼5%) in xenografts of primary pancreatic cancers and in pancreatic cancer cell lines. CDH1 expression, however, is also controlled by other epigenetic mechanisms besides DNA methylation, including transcriptional repression by ZEB1, SIP1 (ZEB2), Snail,28 and Slug29, 30, 31, 32 by certain microRNAs (miR-200 and miR-205) that influence levels of the transcriptional repressors of CDH1. For example, we recently reported that loss of miR-200 expression and associated SIP1-mediated suppression of CDH1 occurs in some pancreatic cancer cell lines, but in most pancreatic cancers, SIP1 is silenced by promoter methylation, and miR-200a/miR-200b is hypomethylated and overexpressed, and CDH1 expression is retained.15
E-cadherin is important for cell-to-cell cohesion, cell-to-cell recognition, and epithelial polarity.33 The extracellular domain of E-cadherin binds to other cadherins from neighboring cells, while the intracellular cytoplasmic tail of E-cadherin interacts with several proteins, such as β-catenin, p120 catenin, and Hakai protein.33, 34 E-cadherin regulates β-catenin signaling in the canonical Wnt pathway. Free cytosolic β-catenin is regulated by binding of the cytoplasmic domain of E-cadherin or by catenin destruction complexes that includes APC, Axin, GSK3β, and cytokeratin-1.33 Interestingly, while nuclear β-catenin (and transcriptional activation) is characteristic of the pancreatic variant neoplasm known as solid-pseudopapillary neoplasmas, and can be seen in pancreaticoblastomas, it is not a feature of most pancreatic ductal adenocarcinomas,35 and undifferentiated pancreatic adenocarcinomas lacking E-cadherin expression typically also lack nuclear β-catenin expression.20
The adhesive phenotype of a cell can be lost when CDH1 is down-regulated allowing neoplastic cells to become more mobile.36 Although E-cadherin loss in cancers is often attributed to the induction of an epithelial mesenchymal transition program,28, 37, 38 there is little evidence that primary pancreatic cancers undergo phenotypic evidence of true epithelial mesenchymal transition.39
In our prior investigation of undifferentiated pancreatic adenocarcinomas, those with E-cadherin loss had a poorer prognosis.20 In the current study, we sought to determine the role of E-cadherin loss as a predictor of outcome in an unselected group of pancreatic ductal adenocarcinomas with usual histology and to determine if such loss is independent of tumor grade and other prognostic factors. Since many of the pancreatic cancers we analyzed had focal areas of tumoral E-cadherin loss involving only a subset of the neoplastic cells, we examined if partial loss of E-cadherin was also an independent predictor of a poor outcome.
Materials and methods
Patients and Tissues
We retrospectively analyzed 329 patients with resectable infiltrating ductal adenocarcinoma of the pancreas who underwent surgical pancreatic resection at the Johns Hopkins Medical Institutions from January 1998 to June 2006. Histologic variants of pancreatic neoplasms, such as adenosquamous carcinomas, colloid carcinomas, medullary carcinomas, undifferentiated adenocarcinomas, intraductal papillary mucinous neoplasms, and mucinous cystic neoplasms, were excluded because of their different natural history to conventional pancreatic ductal adenocarcinomas. Otherwise all patients with available cancer tissues were included.
All clinical and pathologic patient information is maintained in a regularly updated clinical database. Overall tumor differentiation was obtained from the pathology report and defined according to WHO criteria.40
The primary outcome of the study was overall postoperative survival as determined from date of surgical resection to time of death or last follow-up. Date of death was obtained from the Johns Hopkins pathology database. This study was conducted as part of a Johns Hopkins Hospital institutional review board-approved protocol. The manuscript followed REMARK guidelines for reporting on prognostic markers.41
Tissue Microarray Construction
Tissue microarrays were constructed to obtain uniform immunohistochemical labeling of pancreatic tissues and limit intra-assay variation. Tissue microarrays were constructed from the archival formalin-fixed paraffin-embedded tissue blocks of 360 surgically resected primary pancreatic ductal adenocarcinomas as described previously17 using a manual tissue microarrayer (Beecher Instruments, Silver Spring, MD, USA). Four cores (1.5 mm size) were punched from each patient's tumor and non-neoplastic pancreatic tissue into harvested into recipient blocks.
Immunohistochemistry
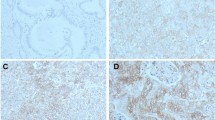
Immunohistochemistry was performed at the Johns Hopkins Hospital Department of Pathology Immunohistochemical laboratory. Briefly, tissue sections were deparaffinized and hydrated in xylene and serial alcohol solutions, respectively. Endogenous peroxidase was blocked by incubation in 3% H2O2 for 10 min. The antigen retrieval step was carried out in a steam pressure cooker containing preheated high pH antigen retrieval buffer (DakoCytomation, Glostrup, Denmark) at 95C, 20 min. Primary anti-E-cadherin mouse monoclonal antibody (clone ECH-6, Cell Marque, Rocklin, CA, USA) was incubated for 32 min at room temperature and sections were labeled with an automated immunostaining system with a I-View detection kit (Benchmark XT, Ventana medical systems). Immunostained sections were lightly counterstained in with hematoxylin, dehydrated in ethanol, and cleared in xylene. Immunohistochemical labeling was successfully performed on 329 of the 360 pancreatic cancers on the tissue microarrays. The reason some tissues did not immunolabel successfully was because of loss of tissue cores during the sectioning or labeling process. To be included for analysis each cancer had to have sufficient numbers of E-cadherin labeled cells to permit quantification of the percent of cells with E-cadherin labeling (>100 cancer cells). E-cadherin expression was evaluated primarily according to the percentage of cells that labeled, although we also evaluated if labeling intensity was an important variable. E-cadherin labeling predominates on the cell membranes, although there is some cytoplasmic labeling in normal ductal and acinar cells and in most neoplastic cells (Figure 1a). E-cadherin immunolabeling was categorized as absent when the labeling was absent or present in <5% of cancer cells. Immunolabeling was considered positive for expressing if the intensity was strong (2+) or weak (1+), and if the extent was ≥5% of cancer cells. The intensity of E-cadherin labeling of tumor cells was compared relative to that of normal ductal and acinar cells (Figure 1a): strong (2+) intensity was assigned if the labeling was similar to that of normal ductal and acinar cells and (1+) intensity if the labeling was weaker. We also counted the proportion of E-cadherin labeled cancer cells of ∼100 cancer cells in each tissue microarray core. We classified E-cadherin expression as previously described as ‘intact’ when 100% of cancer cells on the tissue cores labeled (Figure 1b), and arbitrarily into ‘focal loss’ when ≥51% and <99% of cancer cells labeled, ‘diffuse loss’ when ‘≥6% and ≤50%’ of cancer cells labeled and ‘total loss (Figure 1d)’ when <5% of cancer cells labeled.16, 42 We did not evaluate other cutoffs of expression percentages. We also combined cases with ‘focal loss’ and ‘diffuse loss’ and categorized them as cases with ‘partial loss of E-cadherin’ (Figure 1c). The immunohistochemical scoring was performed by SMH and MG and scoring was done blinded to any other patient data, including outcome.
Representative images of E-cadherin labeling of normal pancreas and pancreatic ductal adenocarcinoma. (a) Normal acinar and ductal cells label E-cadherin. Pancreatic ductal adenocarcinomas with (b) intact E-cadherin labeling and (c) partial E-cadherin loss. Approximately 30% of cancer cells show intact membranous E-cadherin labeling and (d) complete loss of E-cadherin.
Statistical Methods
The sample size was chosen based on our prior experience of the patient sample sizes required for markers to independently predict outcome after pancreaticoduodenectomy for pancreatic cancer.17 Means/s.d. were compared using the unpaired Student t-test. The χ2 and Fisher's exact tests were performed to examine associations between E-cadherin expression and each clinicopathologic factor. Overall patient survival was defined as the time from surgical resection of each patient's pancreatic ductal adenocarcinoma to their death or the date of last follow-up. Survival rates were calculated using the Kaplan–Meier method and statistical significance was evaluated using the log-rank test. The Cox proportional hazards regression models were used to investigate the significance of prognostic factors. A P-value of <0.05 was considered statistically significant. Statistical analyses were performed using SPSS (v17, Chicago, IL, USA).
Results
Patient and Tumor Characteristics
The patient population included 54% men, mean age (±s.d.) 66.5±10.5 years, with 86% of patients having cancers with lymph node metastasis, 58% of cancers were >3 cm, 94% T3 TNM stage, 44% poor differentiated, 69% margin positive, 51% with microvascular invasion and 93% with perineural invasion. Fifty-three percent of patients underwent adjuvant chemotherapy and/or radiation therapy, most of whom receiving 5-fluorouracil and radiation.43 The follow-up period ranged from 3 to 134 months (median, 21.8 months). The number of events (patient death) was observed in 206 cases (63%). The overall median survival was 16.3 months and 9% of patients were alive after 5 years of follow-up.
E-cadherin Expression Patterns
Of the 329 pancreatic cancers, 141 (43%) showed partial or complete loss of E-cadherin expression. Of these 141 pancreatic cancers, 134 (41%) had partial loss of E-cadherin and 7 (2%) had complete loss of expression. When loss of expression was evident in cancer cells, both membranous and cytoplasmic expression were lost. We did not observe any cancer cells with more cytoplasmic expression than membranous expression, or cells with only membranous loss of expression, a pattern would indicate mislocalization of E-cadherin.
E-cadherin Expression and Clinicopathologic Variables
The association of E-cadherin expression with clinicopathologic characteristics is summarized in Table 1. Poorly differentiated pancreatic cancers were more likely to have loss of E-cadherin expression than were well and moderately differentiated cancer (P=0.007, χ2 test). When we stratified loss of expression and differentiation separately into partial and total loss of E-cadherin, there was a significant association between tumor differentiation and the extent of E-cadherin loss (P=0.02) (Table 1). Considering only cancers with intact E-cadherin vs those with partial loss, we found that cancers with partial loss of E-cadherin were more likely to be poorly differentiated (P=0.01).
There was no association between E-cadherin expression and other clinicopathologic factors, including gender, ethnicity, age (<60 vs ≥60 years), pT classification, lymph node metastasis, microscopic vascular invasion, perineural invasion, or adjuvant therapy.
Histology of Pancreatic Cancer Cells Lacking E-cadherin Expression
Since cells with loss of E-cadherin expression are often non-cohesive, we classified pancreatic ductal adenocarcinoma cells lacking E-cadherin expression as ‘gland forming’ or ‘non-gland forming’. Note, this classification is distinct from our previous study in which we classified the overall differentiation of a pancreatic cancer as undifferentiated (non-cohesive or non-gland forming) or differentiated (cohesive or gland forming).20 None of the pancreatic cancers in the current series were undifferentiated; instead, our 329 pancreatic ductal adenocarcinomas all had varying degrees of gland-forming foci. However, many well and moderately differentiated pancreatic cancers had foci of isolated non-gland-forming cancer cells. We classified pancreatic cancer cells with loss of E-cadherin as ‘gland forming’ if they formed glands or ‘non-gland forming’ if they existed as single or scattered clusters of cancer cells that did not form cancer glands. Representative images of gland-forming and non-gland-forming foci are depicted in Figure 2.
Representative images of E-cadherin labeling in gland-forming and non-gland-forming pancreatic ductal adenocarcinomas. (a, b) Gland-forming cells, (a) intact E-cadherin labeling in gland-forming cells and (b) E-cadherin labeling loss in gland-forming cells. (c, d) Non-gland-forming cells, (c) intact E-cadherin labeling in non-gland-forming cells and (d) E-cadherin loss in non-gland-forming cells.
Of 134 cases with partial loss of E-cadherin, 128 contained some non-gland-forming cells and all 134 cases had at least some gland-forming elements. The association of tumor differentiation and the morphology of cells with E-cadherin loss of expression (gland-forming or non-gland-forming cells) are summarized in Supplemental Table 1. Among the 128 cases with non-gland-forming/single cancer cells, loss of E-cadherin was more frequently observed in poorly differentiated cancers (85%, 58/68 cases) compared with well or moderately differentiated cancers (48%, 29/60 cases, P<0.0001). Within gland-forming regions, loss of E-cadherin was more common in well to moderately differentiated adenocarcinomas (89%, 58/65 cases) than in poorly differentiated tumors (73%, 51/69 cases, P=0.03). These data indicate that although E-cadherin loss is more common in poorly differentiated cancers, loss of E-cadherin expression is also often evident in cells that retain glandular features.
Patient’ Survival and E-cadherin Expression
We compared patient survival to E-cadherin expression classified as either intact vs any loss of expression. The median survival for patients with intact E-cadherin cancer labeling was 18.5 months (5-year survival rate, 18%), whereas for those with any loss of tumoral E-cadherin labeling, it was only 12.1 months (5-year survival rate, 10%, P=0.005, log-rank test; Figure 3a).
Kaplan–Meier survival curves in pancreatic ductal adenocarcinoma patients with E-cadherin expression according to (a) E-cadherin expression classified as on intact vs any loss of expression. The median survival for patients with intact E-cadherin labeling was 18.5 months, and the median survival for those with loss of E-cadherin labeling was 12.1 months (P=0.005, log-rank test). (b) E-cadherin expression classified as intact vs partial loss, vs total loss of expression. The median survival for patients with intact E-cadherin labeling was 18.5 months. The median survival in patients with partial E-cadherin loss was 12.7 months, while that of total E-cadherin loss was 5.5 months. There was a significant survival difference among three groups (P=0.002, log-rank test, overall comparison). When compared in a pairwise manner, there was a significance survival difference between patients with intact E-cadherin labeling and those with partial E-cadherin loss (P=0.009) and between patients with intact E-cadherin labeling and those with total E-cadherin loss (P=0.005). There was a marginal significance (P=0.07), between patients with partial and total E-cadherin loss. (c, d) Differentiation and E-cadherin expression status. (c) In poorly differentiated carcinomas, there is a significant survival difference between patients with intact (median survival, 15.1 months) and those with partial loss of E-cadherin cancer labeling (median survival, 10.8 months, P=0.02). (d) In well to moderately differentiated carcinomas, there was no statistic difference between patients with intact (median survival, 21.9 months) and those with partial loss of E-cadherin cancer labeling (median survival, 19.0 months, P=0.29).
We next compared pancreatic cancers classified as intact E-cadherin expression, partial E-cadherin loss and total E-cadherin loss and found that there was a significant difference in survival among these three groups (P=0.002, log-rank test, overall comparison). The median survival for patients with intact E-cadherin labeling (5-year survival rate, 18%; n=188) was 18.5 months, for patients with partial E-cadherin loss (5-year survival rate, 10%; n=134), 12.7 months, and for those with total E-cadherin loss (5-year survival rate, 0%; n=7), 5.5 months. When compared in a pairwise manner, there was a statistically significant survival difference between patients whose cancers that had intact E-cadherin labeling vs those with partial E-cadherin loss (P=0.009), and between those with intact E-cadherin labeling vs those with total E-cadherin loss (P=0.005).
Although the numbers of pancreatic cancers with total E-cadherin loss in our series was small (n=7), there was a trend toward statistical significance between patients whose cancers had partial vs those with total E-cadherin loss (P=0.07; Figure 3b). In order to further determine if the extent of E-cadherin loss portends a worse patient survival, we subdivided pancreatic cancers with partial E-cadherin loss (6–99% of cells expressing E-cadherin) into those with focal (6–50% of cells expressing E-cadherin) and diffuse loss (51–99% of cells expressing E-cadherin). The median survival among patients with cancers with focal E-cadherin loss (n=99) was 14.3 months, while those that had diffuse E-cadherin loss (n=35) was 10.6 months (P=0.13). Because loss of E-cadherin is associated with differentiation of pancreatic cancer, we performed subgroup analysis based on E-cadherin expression (intact vs partial loss of E-cadherin) and differentiation (well or moderately differentiated cancer) of pancreatic cancer. In poorly differentiated carcinomas, there is a significant survival difference between patients with intact (median survival, 15.1 months) and those with partial loss of E-cadherin cancer labeling (median survival, 10.8 months, P=0.02; Figure 3c). However, in well to moderately differentiated carcinomas, no statistical difference was present between patients with intact (median survival, 21.9 months) and those with partial loss of E-cadherin cancer labeling (median survival, 19.0 months, P=0.29; Figure 3d).
We also determined if there was any relationship between the intensity of E-cadherin labeling and survival. The median survival for patients with pancreatic cancers with strong labeling for E-cadherin (n=136) and those with weak labeling of E-cadherin (n=187) was 18.4 and 15.1 months, respectively. No significant survival difference was observed among patients whose cancers had strong vs weak intensity of E-cadherin labeling (P=0.13).
Based on these results, our multivariate model classified E-cadherin expression of pancreatic cancer cells into three groups (intact expression, partial loss, and total loss).
Univariate Analysis for Other Clinicopathologic Factors
By univariate survival analysis, the following clinicopathologic factors were associated with shorter patient survival (Table 2): poor tumor differentiation, the presence of lymph node metastasis, the presence of microscopic vascular invasion, positive resection margins, and the presence of adjuvant therapy.
Multivariate Analysis
Multivariate analyses were performed to assess which factors remained independent predictors of survival after adjusting for factors that were significant by univariate analyses. Loss of E-cadherin expression (P<0.001), poor tumor differentiation (P<0.001), positive resection margin status (P<0.001), and having received adjuvant therapy (P<0.001) were all independently prognostic in our model (Table 3). The hazard ratio for cancers with partial and total E-cadherin loss was 1.57 (95% confidence interval, 1.18–2.09) and 6.34 (95% confidence interval, 2.50–16.10), respectively, compared with those with intact E-cadherin labeling.
Discussion
In this single-institution study, we find that among patients undergoing pancreaticoduodenectomy, partial or total loss of E-cadherin expression is an independent predictor of an adverse outcome. When E-cadherin expression loss occurs in primary pancreatic cancer cells, it is usually lost in only a small percentage of cells. Interestingly, the prevalence of CDH1 inactivation by intragenic mutation or DNA methylation in pancreatic adenocarcinomas is much less common26, 27, 44, 45 than the prevalence of focal loss of E-cadherin protein expression observed in this study. Inactivating mutations and DNA methylation-induced silencing of CDH1 are expected to typically cause diffuse loss of E-cadherin expression throughout the cancer. The number of pancreatic cancers with diffuse loss of E-cadherin in our study was small (∼2%, 7 of 329 cases). We found a trend indicating that pancreatic cancers with complete loss of E-cadherin had a poorer prognosis (P=0.07), consistent with previous investigators who have found that pancreatic cancers with complete loss of E-cadherin is associated with a poor prognosis.20, 21 Previous studies that have evaluated the prognostic significance of E-cadherin loss in pancreatic and other cancers22, 46, 47, 48, 49 (and reviewed in Gould Rothberg and Bracken50) have generally dichotomized cancers into those with intact/lost E-cadherin expression, rather than evaluating the significance of focal E-cadherin loss. For example, our group has previously found that undifferentiated pancreatic cancer cells often show complete loss of E-cadherin.20, 21 Our finding that focal loss of E-cadherin is common and has prognostic significance indicates that the mechanisms responsible for focal loss within a subset of infiltrating pancreatic adenocarcinoma cells are likely to be biologically significant. Epigenetic inactivation of CDH1 in cultured cells is unstable51 and so epigenetic mechanisms could be responsible for this focal loss within a primary infiltrating adenocarcinoma. Given the focal nature of the loss of E-cadherin expression in many pancreatic cancers, it is plausible that local environmental factors could suppress E-cadherin expression by transcriptional repression or by other mechanisms.52, 53, 54 For example, hypoxia and local inflammatory changes mediated by tumor–stromal interactions have been shown to induce signaling changes that can affect the expression of transcriptional repressors of E-cadherin.39
Consistent with previous studies, we did find that loss of E-cadherin was more common in poorly differentiated pancreatic adenocarcinomas.25, 55, 56, 57 However, we also found partial E-cadherin loss in pancreatic cancers that were not classified as poorly differentiated and often found loss of expression in both gland-forming and non-gland-forming cells of the invasive pancreatic adenocarcinomas. Subgroup analysis indicated that it was in the group of patients with poorly differentiated cancers that E-cadherin expression status had additional prognostic value. These observations indicate that E-cadherin has independent prognostic value beyond classification by tumor grade. In addition, the finding of loss of E-cadherin in gland-forming cancer cells suggests that up-regulation of other adhesion molecules may occur to compensate for loss of E-cadherin in these cells.
Although the patients all underwent a similar pancreaticoduodenectomy and postoperative care at one institution for their pancreatic cancer, they did not all undergo the same treatment postoperatively. However, our patients were selected and analyzed for E-cadherin status without regard to their postoperative treatment and the effect of E-cadherin status remained prognostically significant even after adjustment for adjuvant chemotherapy.
Currently, prognostic markers have limited utility for most patients with pancreatic ductal adenocarcinoma given the overall poor outcome for patients with this disease. However, there are a number of therapeutic agents that have shown some promise in clinical trials58 and prognostic markers should still be evaluated, so they are available for further evaluation when the clinical setting is appropriate. Our results indicate that including E-cadherin expression status in the evaluation of pancreatic adenocarcinomas may better predict patient survival following surgical resection.
In summary, we find that partial loss of E-cadherin in primary pancreatic ductal adenocarcinomas is an independent predictor of an adverse outcome among patients undergoing pancreaticoduodenectomy.
References
Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277–300.
Niederhuber JE, Brennan MF, Menck HR . The National Cancer Data Base report on pancreatic cancer. Cancer 1995;76:1671–1677.
Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg 2000;4:567–579.
Geer RJ, Brennan MF . Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg 1993;165:68–72;discussion 72–63.
Tascilar M, Skinner HG, Rosty C, et al. The SMAD4 protein and prognosis of pancreatic ductal adenocarcinoma. Clin Cancer Res 2001;7:4115–4121.
Blackford A, Serrano OK, Wolfgang CL, et al. SMAD4 gene mutations are associated with poor prognosis in pancreatic cancer. Clin Cancer Res 2009;15:4674–4679.
Sato N, Maitra A, Fukushima N, et al. Frequent hypomethylation of multiple genes overexpressed in pancreatic ductal adenocarcinoma. Cancer Res 2003;63:4158–4166.
Sato N, Fukushima N, Maitra A, et al. Discovery of novel targets for aberrant methylation in pancreatic carcinoma using high-throughput microarrays. Cancer Res 2003;63:3735–3742.
Sato N, Goggins M . Epigenetic alterations in intraductal papillary mucinous neoplasms of the pancreas. J Hepatobiliary Pancreat Surg 2006;13:280–285.
Sato N, Goggins M . The role of epigenetic alterations in pancreatic cancer. J Hepatobiliary Pancreat Surg 2006;13:286–295.
Sato N, Matsubayashi H, Abe T, et al. Epigenetic down-regulation of CDKN1C/p57KIP2 in pancreatic ductal neoplasms identified by gene expression profiling. Clin Cancer Res 2005;11:4681–4688.
Fukushima N, Sato N, Ueki T, et al. Preproenkephalin and p16 gene CpG island hypermethylation in pancreatic intraepithelial neoplasia (PanIN) and pancreatic ductal adenocarcinoma. Am J Pathol 2002;160:1573–1581.
Sato N, Ueki T, Fukushima N, et al. Aberrant Methylation of CpG Islands in intraductal papillary mucinous neoplasms of the pancreas increases with histological grade. Gastroenterology 2002;123:1365–1372.
Sato N, Fukushima N, Hruban RH, et al. CpG island methylation profile of pancreatic intraepithelial neoplasia. Mod Pathol 2008;21:238–244.
Li A, Omura N, Hong SM, et al. Pancreatic cancers epigenetically silence SIP1 and hypomethylate and overexpress miR-200a/200b in association with elevated circulating miR-200a and miR-200b levels. Cancer Res 2010;70:5226–5237.
Sato N, Fukushima N, Chang R, et al. Differential and epigenetic gene expression profiling identifies frequent disruption of the RELN pathway in pancreatic cancers. Gastroenterology 2006;130:548–565.
Infante JR, Matsubayashi H, Sato N, et al. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J Clin Oncol 2007;25:319–325.
Akada M, Crnogorac-Jurcevic T, Lattimore S, et al. Intrinsic chemoresistance to gemcitabine is associated with decreased expression of BNIP3 in pancreatic cancer. Clin Cancer Res 2005;11:3094–3101.
Erkan M, Kleeff J, Esposito I, et al. Loss of BNIP3 expression is a late event in pancreatic cancer contributing to chemoresistance and worsened prognosis. Oncogene 2005;24:4421–4432.
Winter JM, Ting AH, Vilardell F, et al. Absence of E-cadherin expression distinguishes noncohesive from cohesive pancreatic cancer. Clin Cancer Res 2008;14:412–418.
Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol 2009;27:1806–1813.
Karayiannakis AJ, Syrigos KN, Polychronidis A, et al. Expression patterns of alpha-, beta- and gamma-catenin in pancreatic cancer: correlation with E-cadherin expression, pathological features and prognosis. Anticancer Res 2001;21:4127–4134.
Li YJ, Ji XR . Relationship between expression of E-cadherin-catenin complex and clinicopathologic characteristics of pancreatic cancer. World J Gastroenterol 2003;9:368–372.
Masugi Y, Yamazaki K, Hibi T, et al. Solitary cell infiltration is a novel indicator of poor prognosis and epithelial-mesenchymal transition in pancreatic cancer. Hum Pathol 2010;41:1061–1068.
Joo YE, Rew JS, Park CS, et al. Expression of E-cadherin, alpha- and beta-catenins in patients with pancreatic adenocarcinoma. Pancreatology 2002;2:129–137.
Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008;321:1801–1806.
Ueki T, Toyota M, Sohn T, et al. Hypermethylation of multiple genes in pancreatic adenocarcinoma. Cancer Res 2000;60:1835–1839.
von Burstin J, Eser S, Paul MC, et al. E-cadherin regulates metastasis of pancreatic cancer in vivo and is suppressed by a SNAIL/HDAC1/HDAC2 repressor complex. Gastroenterology 2009;137:361–371, 371 e1–5.
Cano A, Perez-Moreno MA, Rodrigo I, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2000;2:76–83.
Park SM, Gaur AB, Lengyel E, et al. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev 2008;22:894–907.
Gregory PA, Bert AG, Paterson EL, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB and SIP. Nat Cell Biol 2008;10:593–601.
Korpal M, Lee ES, Hu G, et al. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem 2008;283:14910–14914.
Jeanes A, Gottardi CJ, Yap AS . Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene 2008;27:6920–6929.
Halbleib JM, Nelson WJ . Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev 2006;20:3199–3214.
Cao D, Maitra A, Saavedra JA, et al. Expression of novel markers of pancreatic ductal adenocarcinoma in pancreatic nonductal neoplasms: additional evidence of different genetic pathways. Mod Pathol 2005;18:752–761.
Kalluri R, Weinberg RA . The basics of epithelial-mesenchymal transition. J Clin Invest 2009;119:1420–1428.
Wang Z, Li Y, Kong D, et al. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res 2009;69:2400–2407.
Arumugam T, Ramachandran V, Fournier KF, et al. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res 2009;69:5820–5828.
Klymkowsky MW, Savagner P . Epithelial-mesenchymal transition: a cancer researcher′s conceptual friend and foe. Am J Pathol 2009;174:1588–1593.
Kloppel G, Hruban RH, Longnecker DS, et al. Ductal adenocarcinoma of the pancreas. In: Hamilton SR, Aaltonen LA (eds). World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System Vol 2 IARCPress: Lyon, 2000;211–230.
McShane LM AD, Sauerbrei W, Taube SE, et al. Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics. Reporting recommendations for tumor marker prognostic studies. J Clin Oncol 2005;23:9067–9072.
Cao D, Zhang Q, Wu LS, et al. Prognostic significance of maspin in pancreatic ductal adenocarcinoma: tissue microarray analysis of 223 surgically resected cases. Mod Pathol 2007;20:570–578.
Asiyanbola B GA, Herman JM, Choti MA, et al. Determining pattern of recurrence following pancreaticoduodenectomy and adjuvant 5-flurouracil-based chemoradiation therapy: effect of number of metastatic lymph nodes and lymph node ratio. J Gastrointest Surg 2009;13:752–759.
House MG, Guo M, Iacobuzio-Donahue C, et al. Molecular progression of promoter methylation in intraductal papillary mucinous neoplasms (IPMN) of the pancreas. Carcinogenesis 2003;24:193–198.
Dansranjavin T, Mobius C, Tannapfel A, et al. E-cadherin and DAP kinase in pancreatic adenocarcinoma and corresponding lymph node metastases. Oncol Rep 2006;15:1125–1131.
Lim SC, Lee MS . Significance of E-cadherin/beta-catenin complex and cyclin D1 in breast cancer. Oncol Rep 2002;9:915–928.
Rakha EA, Abd El Rehim D, Pinder SE, et al. E-cadherin expression in invasive non-lobular carcinoma of the breast and its prognostic significance. Histopathology 2005;46:685–693.
Shin SJ, Kim KO, Kim MK, et al. Expression of E-cadherin and uPA and their association with the prognosis of pancreatic cancer. Jpn J Clin Oncol 2005;35:342–348.
Torer N, Kayaselcuk F, Nursal TZ, et al. Adhesion molecules as prognostic markers in pancreatic adenocarcinoma. J Surg Oncol 2007;96:419–423.
Gould Rothberg BE, Bracken MB . E-cadherin immunohistochemical expression as a prognostic factor in infiltrating ductal carcinoma of the breast: a systematic review and meta-analysis. Breast Cancer Res Treat 2006;100:139–148.
Graff JR, Gabrielson E, Fujii H, et al. Methylation patterns of the E-cadherin 5′ CpG island are unstable and reflect the dynamic, heterogeneous loss of E-cadherin expression during metastatic progression. J Biol Chem 2000;275:2727–2732.
Fujita Y, Krause G, Scheffner M, et al. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol 2002;4:222–231.
Yang J, Weinberg RA . Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell 2008;14:818–829.
Marambaud P, Shioi J, Serban G, et al. A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. EMBO J 2002;21:1948–1956.
Karayiannakis AJ, Syrigos KN, Chatzigianni E, et al. Aberrant E-cadherin expression associated with loss of differentiation and advanced stage in human pancreatic cancer. Anticancer Res 1998;18:4177–4180.
Li YJ, Meng YX, Ji XR . Relationship between expressions of E-cadherin and alpha-catenin and biological behaviors of human pancreatic cancer. Hepatobiliary Pancreat Dis Int 2003;2:471–477.
Pignatelli M, Ansari TW, Gunter P, et al. Loss of membranous E-cadherin expression in pancreatic cancer: correlation with lymph node metastasis, high grade, and advanced stage. J Pathol 1994;174:243–248.
Vincent A, Herman JM, Schulick R, et al. Pancreatic cancer. Lancet 2011 (in press).
Acknowledgements
This work was supported by NIH grants (P50-CA62924, R01-CA120432) and the Michael Rolfe Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Modern Pathology website .
Supplementary information
Rights and permissions
About this article
Cite this article
Hong, SM., Li, A., Olino, K. et al. Loss of E-cadherin expression and outcome among patients with resectable pancreatic adenocarcinomas. Mod Pathol 24, 1237–1247 (2011). https://doi.org/10.1038/modpathol.2011.74
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2011.74
Keywords
This article is cited by
-
Epithelial-mesenchymal transition in undifferentiated carcinoma of the pancreas with and without osteoclast-like giant cells
Virchows Archiv (2021)
-
Efficacy of the highly selective focal adhesion kinase inhibitor BI 853520 in adenocarcinoma xenograft models is linked to a mesenchymal tumor phenotype
Oncogenesis (2018)
-
Identification of potential biomarkers to differentially diagnose solid pseudopapillary tumors and pancreatic malignancies via a gene regulatory network
Journal of Translational Medicine (2015)
-
Nectin expression in pancreatic adenocarcinoma: nectin-3 is associated with a poor prognosis
Surgery Today (2015)
-
Reduced expression of Raf kinase inhibitor protein correlates with poor prognosis in pancreatic cancer
Clinical and Translational Oncology (2012)