Abstract
The PD-1/B7-H1 pathway regulates immune responses and maintains homeostasis. Here, we identified a unique expression of B7 homolog 1 (B7-H1) on masticatory mucosae in the oral cavity. B7-H1 was physiologically expressed on the dorsal surface of the tongue, gingiva, and hard palate. Other squamous epithelia and other structures of the epithelia did not express B7-H1 in the steady state. Physiological B7-H1 expression on masticatory mucosae was limited on prickle cells, and its expression on basal keratinocytes (KCs) was strictly regulated. B7-H1 on prickle cells was upregulated by external topical stimuli, but B7-H1 on basal KCs was induced only by internal stimuli via infiltrating cells. The blocking of KC-associated B7-H1 or the lack of programmed cell death-1 (PD-1) on tissue effector CD4+ T cells in mice lacking B7-H1 on immune cells drastically exacerbated the tissue inflammation induced by topical OVA painting as an exogenous antigen, indicating direct interaction with KCs and CD4+ T cells. Trans-coinhibitory signals by KCs may modulate local T-cell/dendritic cell activation, resulting in inhibition of T-cell responses in both peripheral and secondary lymphoid tissues. Careful control of B7-H1 induction in KCs may play a crucial role in the protection from CD4+ T cell-mediated tissue inflammation by exogenous antigens delivered from the mucosal surface.
Similar content being viewed by others
INTRODUCTION
The oral mucosa is the type II mucosa lining the inside of the mouth and consists of stratified squamous epithelium. It shares many features with the skin and is remarkably different from type I mucosa such as the intestinal and respiratory mucosae.1 Oral mucosae are divided into the masticatory and lining mucosae by their function.2, 3 The gingiva, dorsal surface of the tongue (dorsal tongue), and hard palate are tough masticatory mucosa covered by keratinized stratified squamous epithelium. In contrast, the oral floor, buccal mucosa, soft palate, and ventral surface of the tongue (ventral tongue) are flexible lining mucosae covered by a nonkeratinized epithelium to accommodate chewing, speech, or swallowing. All oral epithelia function to protect the underlying tissues from environmental influences, such as mechanical, chemical, and microbial damage. Skin and keratinized oral epithelium contain four layers consisting of the stratum basale (basal layer), stratum spinosum (prickle layer), stratum granulosum (granular layer), and stratum corneum (keratinized layer), whereas nonkeratinized oral mucosae lack stratum granuloma and stratum corneum. In addition to the differential structured mucosae, masticatory mucosae may have unique mechanisms for protection against attacks on the immune system.
Immune checkpoints, such as co-inhibitory molecules and regulatory T cells, play crucial roles in maintaining self-tolerance and preventing tissue damage by excessive immune responses.4, 5, 6 Programmed cell death-1 (PD-1; CD279), in particular, plays an important role in regulating immune responses at sites of tissue inflammation and in the tumor microenvironment via the ligand binding expressed on parenchymal tissue cells.7, 8 B7 homolog 1 (B7-H1)/PD-1 ligand 1(PD-L1) (CD274) is one of the ligands for PD-1 and is often induced in nonlymphoid tissue cells, such as epithelial and endothelial cells during an inflammatory episode. B7-H1 binding to PD-1 on T cells negatively regulates T-cell activation, and blockade of the PD-1:B7-H1 pathway converts resting or exhausted T cells into functional effector T cells, resulting in augmentation of T-cell responses. Transgenic expression of B7-H1 on keratinocytes (KCs) impairs contact hypersensitivity reactions by the direct regulation of tissue-recruiting effector CD8+ T cells,9 and the knockdown of KC-associated B7-H1 enhanced activation of CD8+ T cells against self-antigen (Ag)-expressing KCs.10 These results indicate a contribution of onsite regulation by KC-associated B7-H1 to endogenous Ag-specific CD8+ T-cell responses. However, involvement of KC-associated B7-H1 in CD4+ T-cell responses against exogenous Ags has not been reported.
In this study, we examined the physiological expression of B7-H1 in mouse epithelia of multiple organs and identified unique B7-H1 expression on the masticatory mucosa. We further investigated the regulation of B7-H1 expression in each epithelial stratum and the function of KC-associated B7-H1 in CD4+ T-cell responses against exogenous Ags on the mucosal surface.
RESULTS
Prickle cells of the masticatory mucosa physiologically express B7-H1
Although B7-H1 expression has often been observed in inflammatory tissues, its expression in the steady state has not been fully analyzed. We performed histological analyses of B7-H1 expression in the epithelia of multiple organs, including the oral cavity and skin. Interestingly, B7-H1 was physiologically expressed in three keratinized masticatory mucosae, including the dorsal tongue, gingiva, and hard palate in the oral cavity (Figure 1aA–E). The epithelia of the dorsal tongue and gingiva in particular expressed B7-H1, and strong signals were seen in prickle cells, but not in basal KCs. Other oral nonkeratinized lining mucosae, including the ventral tongue, buccal mucosa, and soft palate, did not express B7-H1 (Figure 1aF–H). We also examined other organs with stratified squamous epithelium, including the esophagus, vagina, and three types of skin, the single squamous epithelium in the lung, and the single columnar epithelium of respiratory and digestive mucosae, including the nasal cavity, trachea, stomach, and small and large intestines. None of the epithelia expressed B7-H1 in the steady state (Supplementary Figure S1 online).
Prickle cells of masticatory mucosae physiologically express B7 homolog 1 (B7-H1). Sections of the indicated tissues from (a) 12-week-old, and (b) 3-, 15-, and 50-week-old BALB/c mice were stained with anti-B7-H1 monoclonal antibody (mAb). Representative images from three individuals are shown. Scale bars=50 μm. Images of higher magnification are shown in aD and aE and in b. The arrow in aB indicates the sulcular side.
As B7-H1 in the oral masticatory mucosa could be induced by stimuli from everyday life and other environmental stimuli, we examined the change of B7-H1 expression at three different ages (3, 15, and 50 weeks). Levels of B7-H1 expression in the dorsal tongue and gingiva at 3 weeks of age were notably lower and increased in an age-dependent manner (Figure 1b). In addition, the lack of B7-H1 expression in basal KCs was consistently observed, even in the older mice.
The existence of B7-H1+ epithelium may suggest the presence of its counter-receptor positive cells. Thus, we examined PD-1 expression in the dorsal tongue and gingiva in parallel with the detection of major histocompatibility complex (MHC) class II+ cells. Despite the clear presence of MHC class II+ cells, which were presumed to be Langerhans cells (LCs) and interstitial dendritic cells (DCs) in the epithelium and subepithelium of the dorsal tongue and sulcular epithelium of the gingiva, substantial expression of PD-1 was not observed (Supplementary Figure S2).
B7-H1 in nonmasticatory mucosa and skin is inducible by topical stimuli, whereas B7-H1 expression in basal KCs is impaired
We previously demonstrated that topical painting with 12-O-tetradecanoylphorbol-13-acetate (TPA) or 2,4-dinitro-1-fluorobenzene (DNFB) induced KC proliferation and skin inflammation in a PD-1:B7-H1 pathway-dependent manner.11, 12 Therefore, we examined the histological changes and expressions of Ki-67 (a marker of proliferation) and B7-H1 after topical painting on the abdominal skin and buccal mucosa that lack physiological B7-H1 expression, as we examined the dorsal tongue, because they all constitute stratified squamous epithelium. In the steady state, basal cells of abdominal skin did not express Ki-67 and B7-H1 (Figure 2a). In contrast, some basal cells in the buccal mucosa and dorsal tongue expressed low levels of Ki-67. TPA painting markedly increased the thickness of the epithelial layers as assessed by hematoxylin and eosin staining, and proliferation of basal cells, as assessed by Ki-67 expression, was seen in all three epithelia at 48 h (Figure 2b). B7-H1 was clearly induced in prickle cells and in the upper layers but not in basal cells of the skin and buccal mucosa. B7-H1 expression in the dorsal tongue was further upregulated by TPA painting, but B7-H1 expression was not observed in the basal cells. DNFB and ovalbumin (OVA) are commonly used as a hapten Ag and a protein Ag to induce contact hypersensitivity and to evaluate Ag-specific T-cell responses in murine models, respectively. OVA painting alone induced transient induction of B7-H1 after 6–10 h, but its expression declined to a similar level as observed in intact tissues at 48 h (data not shown). We evaluated the data at 24 h after DNFB alone (Supplementary Figure S3) or OVA/DNFB (Figure 2c) painting. Similar to TPA painting, DNFB paining alone strongly enhanced the epithelial thickness and proliferation of basal cells and induced mononuclear cell infiltration in the lamina propria. The staining profiles of DNFB alone were almost comparable to the OVA/DNFB-painted tissues. B7-H1 was also substantially induced in three epithelia. It should be noted that DNFB or OVA/DNFB painting induced B7-H1 expression in some basal cells, but expression on the basement membrane side was consistently impaired. Our results suggest that proliferating basal cells to the external layer induces B7-H1, but B7-H1 was not enriched at the boundary with the basement membrane.
Expression of B7 homolog 1 (B7-H1) and Ki-67 after topical painting with 12-O-tetradecanoylphorbol-13-acetate (TPA) or ovalbumin/2,4-dinitro-1-fluorobenzene (OVA/DNFB). Cryostat sections from the abdominal skin, buccal mucosa, and dorsal tongue of (a) intact and (b) TPA-painted mice at 48 h, or (c) OVA/DNFB-painted at 24 h mice were stained with hematoxylin and eosin (HE), anti-Ki-67, and anti-B7-H1 monoclonal antibody (mAb). Representative images from three individuals are shown. Scale bars=50 μm.
Blockade of B7-H1 on KCs exacerbates CD4+ T cell-mediated tissue inflammation
B7-H1 is broadly expressed in most immune cells, making it difficult to discriminate the contribution of B7-H1 to immune cells and KCs. We established B7-H1/PD-1 double-knockout (WKO) bone marrow (BM) chimera mice, in which all hematopoietic immune cells lacked B7-H1 and PD-1 and only nonlymphoid tissue cells could induce B7-H1 and PD-1. After hematopoietic reconstitution, in vitro activated DO11.10 CD4+ T cells were transferred; 1 day later, this was followed by topical OVA/DNFB painting onto the dorsal tongue to induce local T-cell inflammation. To examine the functional involvement of KC-associated B7-H1, a neutralizing anti-B7-H1 monoclonal antibody (mAb) was administrated 2 h before topical painting; 48 h after this, local tissues and the status of regional lymph node (LN) T cells were analyzed.
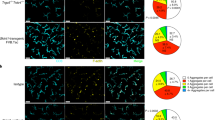
OVA/DNFB painting onto the dorsal tongue in the DO11.10 T cell-transferred BALB/c mice showed infiltration of some mononuclear cells (Figure 3aA) and CD4+ T cells (Figure 3aB) at 48 h. These cells were located only in the subepithelium. Interestingly, high levels of B7-H1 were present in all layers of the epithelium, including basal KCs (Figure 3aD), and few cells express low levels of PD-1 (Figure 3aC). In contrast, OVA/DNFB painting in the DO11.10 T cell-transferred B7-H1/PD-1WKO BM chimera mice induced abundant infiltration (Figure 3aE). Some lesions of basal cell layer were destroyed, and infiltrating cells located over the basement membrane. As compared with the results for BALB/c mice, more mononuclear cells (Figure 3aE), CD4+ T cells (Figure 3aF), and PD-1+ cells (Figure 3aG) were present. B7-H1 expression was consistently observed in all layers after DO11.10 T-cell transfer and OVA/DNFB painting (Figure 3aH), suggesting that T-cell infiltration induces B7-H1 in the basal KCs. B7-H1+ cells were undetectable in the lamina propria despite the abundant infiltration of MHC class II+ DCs and macrophages (Supplementary Figure S4), suggesting that interstitial DCs and macrophages have been replaced by donor-derived cells. Treatment with anti-B7-H1 mAb in the B7-H1/PD-1WKO BM chimera mice induced an abundant mononuclear cell infiltration and destruction of epithelial structures (Figure 3aI). Strong and abundant CD4 signals were seen in the whole field (Figure 3aJ), suggesting severe T-cell infiltration. PD-1+ cells were located in the same area as CD4+ T cells (Figure 3aK). These results indicate that KC-associated B7-H1 confers protection against epithelial tissue damage induced by CD4+ T-cell infiltration. Interactions with KC-associated B7-H1 and PD-1 on tissue-recruiting CD4+ T cells may inhibit T-cell activation at peripheral sites.
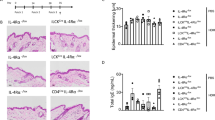
Topical ovalbumin/2,4-dinitro-1-fluorobenzene (OVA/DNFB) painting induces tissue inflammation in B7-H1 /PD-1 double-knockout (WKO) bone marrow (BM) chimera mice, and blockade of B7 homolog 1 (B7-H1) pathway further exacerbates this inflammation. B7-H1/PD-1WKO BM chimera mice were generated as described in the Methods. Violet tracker-labeled activated DO11.10 CD4+ T cells (2 × 106 cells/mouse) from RAG2−/− DO11.10 mice were adaptively transferred into intact BALB/c or B7-H1/PD-1WKO BM chimera mice at 1 day before topical OVA/DNFB painting onto the dorsal tongue. Recipient B7-H1/PD-1WKO BM chimera mice received either control rat IgG or anti-B7-H1 monoclonal antibody (mAb) 2 h before topical painting. After 48 h, tongue tissues were resected and histologically analyzed. (a) Representative images of mAb staining from three individuals are shown. Scale bars=50 μm. Thin dotted lines in (C) and (G) indicate the position of basement membrane. (b) Quantitative analysis for Foxp3 and CD4 was performed as described in the Methods. The number of CD4+ T cells and the proportion of Foxp3+ Tregs within KJ1-26+CD4+ T cells in three high-power field (HPF) images are shown. The values are the mean±s.d. from each group of three mice. HE, hematoxylin and eosin. *Significantly different (P<0.05).
Analysis of regional LN cells revealed that the total cell number and the percentage of KJ1-26+ DO11.10 CD4+ T cells were dramatically increased by the anti-B7-H1 mAb treatment (Figure 4a). Less than half of the violet-labeled DO11.10 CD4+ T cells did not divide in the control group, but the anti-B7-H1 mAb treatment markedly increased the proportion of divided cells and the division times (Figure 4b), suggesting increased proliferation of OVA-specific CD4+ T cells by the anti-B7-H1 mAb treatment. The proportion of interferon-γ (IFN-γ)-expressing cells in DO11.10 CD4+ T cells was markedly enhanced by the mAb treatment, and there was a reciprocal suppression of the proportion of IL-10+ or Foxp3+ cells (Figure 4a). The profiles of Foxp3 (forkhead box P3) expression and cell division showed that anti-B7-H1 mAb treatment inhibited the proliferation of regulatory T cells (Tregs) (Figure 4c). Simultaneous staining with interleukin-10 (IL-10) and Foxp3 revealed that the anti-B7-H1 mAb treatment selectively reduced the proportions of the IL-10+Foxp3+ double-positive Treg fraction but not of the Foxp3−IL-10+ Tr-1 fraction (Figure 4d). These results suggest that blockade of KC-associated B7-H1 at the local tissues inhibits activation of Foxp3+ Tregs in the secondary lymphoid tissues. To confirm whether the percentage of Tregs in the local tissues was also inhibited, we examined Foxp3 expression in the tissues. The number of Foxp3+ cells increased in anti-B7-H1 mAb-treated tissues, although the total number of CD4+ T cells also increased significantly (Figure 3aL–N). Quantitative analysis revealed that, despite the significant increase in infiltrating CD4+ T cells, the ratio of Foxp3+ cells in CD4+ T cells was significantly decreased by the anti-B7-H1 mAb treatment (Figure 3b). These results suggest that local blocking of KC-associated B7-H1 inhibits both the recruitment of Tregs in the tissues and activation of Tregs in the regional LNs.
Blockade of keratinocyte (KC)-associated B7 homolog 1 (B7-H1) enhances T-cell activation in the regional lymph nodes (RLNs). Generation of B7-H1/PD-1 double-knockout (WKO) bone marrow (BM) chimera mice, DO11.10 CD4+ T-cell transfer, and monoclonal antibody (mAb) treatment were performed as described in Figure 3. Regional LNs were obtained 48 h after topical painting. After counting, the cells were stimulated with phorbol 12-myristate 13-acetate (PMA) and ionomycin in the presence of breferdin A and then stained with FITC-anti-CD4, APC-anti-Foxp3, PE-anti-IL-10, and biotinylated-anti-KJ1-26, followed by APC-eFlour780-streptavidine, or the appropriate fluorochrome-conjugated control mAbs. (a) The total cell number and the proportions of DO11.10 (KJ1-26+) CD4+ T cells, IFN-γ+, IL-10+, or Foxp3+ cells within DO11.10 CD4+ T cells are shown. Values are the mean±s.d. from each group of three mice. Data shown are representative of two independent experiments. *Statistically different (P<0.05). (b) Violet-tracker dilutions of DO11.10 CD4+ T cells in the control Ig-treated (gray histogram) and anti-B7-H1 mAb-treated (open histogram) mice are displayed as histograms. (c) Dotted plot profiles of Foxp3 expression and violet dilution are shown. (d) Contour plots of interleukin-10 (IL-10) and Foxp3 (forkhead box P3) expression are shown. Data are representative from three individuals; the values in the profiles are the mean±s.d. from three mice. *Statistically different from the control group (P<0.05).
Tissue-recruiting PD-1+ T cells directly contribute to B7-H1-mediated protection
To examine the direct contribution of PD-1 expressed on DO11.10 CD4+ T cells to the regulation of local tissue inflammation, we compared the responses of PD-1−/− and PD-1+/+ DO11.10 CD4+ T-cell transfer in the B7-H1/PD-1WKO BM chimera mice. After 7 days, approximately half of the DO11.10 CD4+ T cells expressed PD-1 in the culture from the PD-1+/+ DO11.10 mice, but no expression was seen in the PD-1−/− DO11.10 CD4+ T cells (Figure 5a). PD-1−/− DO11.10 T cells proliferated much faster than PD-1+/+ DO11.10 T cells; however, the percentages of IFN-γ+ or Foxp3+ cells in both cultures were comparable to those before transfer. Consistent with the results of the anti-B7-H1 mAb treatment, PD-1−/− DO11.10 T-cell transfer elicited severe tissue inflammation with destruction of the basal cell layer and abundant CD4+ T-cell infiltration (Figure 5b). In the regional LNs, total cell numbers, the proportions of DO11.10 CD4+ T cells, and cell division assessed by violet tracker dilution were markedly increased by the PD-1−/− T-cell transfer (Figure 5c,d). The lack of PD-1 on DO11.10 T cells markedly increased the ratio of IFN-γ+ cells and decreased the proportions of Tregs, especially IL-10+ Tregs (Figure 5d). These results indicate the direct involvement of PD-1 on DO11.10 CD4+ T cells in the inhibition of local tissue inflammation. Taken together, our results suggest that KC-associated B7-H1 directly interacts with PD-1 expressed on tissue-recruiting effector CD4+ T cells, resulting in the inactivation of effector T cells and the generation of IL-10-expressing functional Tregs. KC-associated B7-H1 may play a crucial role in maintaining tissue homeostasis.
Deficiency of programmed cell death-1 (PD-1) on T cells accelerates tissue inflammation and regional lymph node (LN) T-cell activation. Cells from PD-1+/+ DO11.10 and PD-1−/− DO11.10 mice were cultured in the presence of ovalbumin (OVA) for 7 days. DO11.10 CD4+ T-cell transfer and OVA/2,4-dinitro-1-fluorobenzene (OVA/DNFB) painting onto the dorsal tongue was performed as described above. (a) Cultured cells were stimulated and stained with FITC-anti-CD4, PE-anti-IFN-γ, PE-Cy7-anti-PD-1, and biotinylated-anti-KJ1-26 monoclonal antibodies (mAbs), followed by APC-eFlour780-streptavidin, or the appropriate fluorochrome-conjugated control Ig. Expression profiles of PD-1, interferon-γ (IFN-γ), or Foxp3 (forkhead box P3) and KJ1-26 in the alive cell gate are shown as pseudocolor dotted plots. Representative data from three mice are shown. Values are the percentages of the indicated fraction. (b) Tongue tissues were obtained at 48 h after topical painting with OVA/DNFB, and sections were stained with hematoxylin and eosin (HE) and anti-CD4 mAb. Scale bars=50 μM. (c, d) Regional LN cells were stained and analyzed as described in Figure 4. Violet-tracker dilutions of PD-1+/+ (gray histogram) and PD-1−/− (open histogram) DO11.10 CD4+ T cells are displayed as histograms in c. Representative data from three mice are shown. Data shown in d are representative from two independent experiments; the values in the profiles are the mean±s.d. from three mice. *Statistically different (P<0.05).
KC-associated B7-H1 downregulates Ag-specific CD4+ T-cell responses in vitro
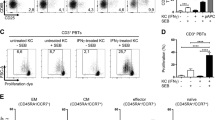
To demonstrate the functional involvement of KC-associated B7-H1 in CD4+ T-cell responses against OVA-Ag in vitro, we generated a KC-mediated coinhibition assay containing DO11.10 T cells, OVA-pulsed antigen-presenting cells (APCs), and KCs. Treatment of a murine KC cell line, Pam 212, with IFN-γ for 24 h markedly enhanced B7-H1 expression, but slightly induced MHC class II (Figure 6a). To negate involvement of B7-H1 and PD-1 on APCs, BM-derived dendritic cells (BMDCs) were generated from B7-H1/PD-1WKO mice. OVA-pulsed BMDCs from B7-H1/PD-1WKO mice induced high levels of MHC class II and CD86, but completely lacked B7-H1 and PD-1 (Figure 6b). Preactivated DO11.10 CD4+ T cells and OVA-pulsed B7-H1−PD-1− BMDCs were added to the IFN-γ-treated KC-seeded wells. DO11.10 CD4+ T cells stimulated with OVA-pulsed B7-H1−PD-1− BMDCs exhibited multiple cell dilution (maximum division 9) for 48 h, whereas similar cultures containing IFN-γ-treated KCs showed clearly impaired cell divisions (maximum division 6) and IFN-γ expression in a KC-cell number-dependent manner (Figure 6c,e). The addition of anti-B7-H1 mAb in the culture mostly rescued the impaired proliferative responses and IFN-γ expression induced by KCs to the comparable levels with DO11.10 T cells and OVA-pulsed B7-H1−PD-1− BMDCs alone (Figure 6d,e). The addition of anti-B7-H1 mAb in the culture with DO11.10 T and OVA-pulsed B7-H1−PD-1− BMDCs in the absence of KCs did not clearly affect cell division (Supplementary Figure S6), suggesting that B7-H1 pathway is not involved in the T–T interaction. These results demonstrated the actual interaction of B7-H1-expressing KCs with PD-1-expressing activated CD4+ T cells and their regulatory function in CD4+ T-cell activation in vitro.
B7 homolog 1 (B7-H1)-expressing keratinocytes (KCs) inhibit antigen (Ag)-specific CD4+ T-cell responses in vitro. (a) Pam 212 cells were stimulated in the presence or absence of interferon-γ (IFN-γ) for 24 h and cell surface expression of B7-H1 and major histocompatibility complex (MHC) class II was examined. Expression profiles are shown as histograms with control staining (shaded histograms). (b) Immature bone marrow-derived dendritic cells (BMDCs) from B7-H1/PD-1 double-knockout (WKO) mice were stimulated with tumor necrosis factor-α (TNF-α) and ovalbumin (OVA) for 24 h and cell surface expression of the indicated antigens was examined by flow cytometry. An electronic gate was placed on CD11c+ lymphocytes and then expression profiles are displayed as histograms with control staining (shaded histograms). (c, d) Activated DO11.10 CD4+ T cells were used as responder cells and OVA-pulsed mature B7-H1−PD-1− BMDCs were used as antigen-presenting cells (APCs). Responder T cells and APCs were added to the Pam 212-preseeded 24-well plate and cultured for 48 h. Cells were stained and analyzed as described in Figures 4 and 5. Violet-tracker dilutions of DO11.10 CD4+ T cells at the indicated conditions are shown as histograms; T and APC-alone culture (black line), T and APC culture with keratinocyte (KC) numbers at 1.25 × 103 (green line), 2.5 × 103 (blue line), 5 × 103 (shaded histograms with red line) cells, and T and APC culture with KCs (5 × 103 cells) in the presence of anti-B7-H1 monoclonal antibody (mAb; an open histogram with red line). (e) The percentages of proliferating cells assessed by over 3 times cell divisions and of IFN-γ+ cells within DO11.10 (KJ1-26+) CD4+ T cells at the indicated conditions are shown. The percentages of proliferating cells and IFN-γ expression in the DO11.10 CD4+ T-cell culture without APCs and KCs are 4.1% and 1.1%, respectively.
DISCUSSION
We identified the unique expression of B7-H1 on masticatory mucosae in the oral cavity. Other epithelial structure and squamous epithelium, including the skin, vaginal mucosa, and nonmasticatory oral mucosae, do not express B7-H1 in the steady state. More interestingly, physiological B7-H1 expression on the masticatory mucosae is limited to prickle cells, and its expression on basal cells is strictly regulated. This regulation is consistent in even older tissues. B7-H1 on prickle cells in the steady state appears to be induced by external dietary physical stimuli, accompanied by the functions of mastication and swallowing. In addition, microbial stimuli directly enhance B7-H1 expression in KCs. Microbial-induced Toll-like receptor-mediated signaling induces B7-H1 expression in gingival KCs and some cancer cells in vitro.13, 14 Both the dorsal tongue and gingiva possess complex anatomical structures that may receive various microbial stimuli through the mucosal surface, especially via the gingival sulcus. In fact, the sulcular epithelium expressed higher levels of B7-H1 (Figure 1) and initiated stronger MHC class II signals on the sulcular side (Supplementary Figure S2), suggesting activation of LCs and recruitment of interstitial DCs. This indicates that immune cell-related action, in addition to external stimuli, further augments the upregulation of B7-H1 on prickle cells. Induction of B7-H1 on basal KCs appears to be closely correlated with infiltrating mononuclear cells under the epithelium. Topical OVA/DNFB painting with DO11.10 T-cell transfer induced substantial CD4+ T-cell infiltration, whereas OVA/DNFB painting alone without T-cell transfer induced minimal infiltration. We previously demonstrated B7-H1-expressing KCs near the basement membrane in oral tissues from patients with oral lichen planus.15 Because IFN-γ is a potent cytokine for B7-H1 induction,8 IFN-γ secreted from recruiting effector CD4+ T cells augments B7-H1 expression in KCs from the internal side. Even in the physiological status, tissue-recruiting resident T cells increased age dependently and IFN-γ secreted by such T cells may contribute to upregulation of B7-H1. We previously demonstrated nonfunctional expression of B7-DC (another ligand for PD-1) on cultured human KCs in vitro.15 In this study, substantial B7-DC expression on KCs in vivo was not detected in both steady and inflammatory states. Although PD-1 expression has been reported on murine DCs under some conditions and in human immature LCs,16, 17 we could not detect clear PD-1 expression in LCs and interstitial DCs. In addition, no B7-H1 expression was seen on the cells with LC-like morphology in both steady and inflammatory status. Further studies are required to clarify the roles of prickle cell-associated B7-H1 in the masticatory mucosae under the steady state.
Here, we clearly demonstrated the substantial contribution of KC-associated B7-H1 in the regulation of CD4+ T cell-mediated local tissue inflammation. Recent reports demonstrated the involvement of KCs in CD4+ T-cell responses used an endogenous self-Ag system.18, 19 Here, we demonstrate for the first time the direct involvement of KCs in CD4+ T-cell responses in an exogenous Ag system. In our experimental setting, PD-1 was inducible only on transferred OVA-specific effector DO11.10 CD4+ T cells, and host-derived APCs were unable to express B7-H1. Thus, it is possible that interaction occurs between the PD-1 on tissue recruiting effector T cells and B7-H1 on KCs. KCs can induce MHC class II under inflammatory conditions or pathological change as nonprofessional APCs in both humans and mice.20, 21 Indeed, we found that some KCs expressed MHC class II in the OVA/DNFB-painted dorsal tongue, and its expression was further heightened in tissues from T cell-transferred PD-1/B7-H1WKO BM chimera mice and the anti-B7-H1 mAb treatment (Supplementary Figure S5). Nevertheless, the Ags in this study were exogenous protein Ags; therefore, KCs failed to efficiently present exogenous Ags on MHC class II, although KCs are able to present endogenous self-Ags.22 A more favorable explanation is that B7-H1-expressing KCs provide trans-coinhibitory signals to the PD-1-expressing recruiting T cells that receive the first signals via local professional APCs (LCs and interstitial DCs). It has been shown that “cis-costimulation” that delivers the first signal and the second costimulatory signal from one cell elicits much more efficient T-cell activation than “trans-costimulation” that delivers the first and second signals from separate cells.23 However, trans-costimulation functions under some conditions in vivo (e.g., under high B7 expression or the weak requirement of the first signal).24, 25, 26 Tissue-recruiting effector T cells are already primed and activated, and therefore may not require strong T cell receptor (TCR) signals from professional APCs. Indeed, we could demonstrate KC-mediated trans-coinhibition by co-culture experiments with CD4+ T cells, BMDCs, and KCs.
It is notable that blocking of the KC-associated B7-H1 at the local side enhanced Ag-specific T-cell responses in the regional LNs, suggesting modulation of Ag-captured DCs at the peripheral side. Blockade of KC-associated B7-H1 pathway augmented effector T cells and inhibited Foxp3+ Tregs at both peripheral and secondary lymphoid tissues. We previously demonstrated that blockade of the PD-1:B7-H1 pathway reduced T-cell motility, resulting in prolonged T cell-DC engagement.27 It is possible that blocking of KC-associated B7-H1 influences T-cell/DC interaction and that the enhanced T-cell activation modulates local cytokine profiles, resulting in further recruitment and activation of DCs at the local site. In addition, our results showed that blocking of KC-associated B7-H1 especially inhibited IL-10 expression by Tregs. This was consistent with previous reports that B7-H1-expressing KCs induce local immune tolerance by activating IL-10-secreting T cells.28, 29
In summary, we demonstrate the physiological expression of B7-H1 in the oral masticatory mucosae and the specific regulation of B7-H1 expression in basal KCs. B7-H1 that is expressed on KCs protects against excess tissue damage via the direct interaction with PD-1-expressing tissue-recruiting CD4+ T cells that respond to exogenous Ags delivered through the mucosal surface. Trans-coinhibitory signals by KCs modulate both local T-cell and DC activation and regulate T-cell immune responses at the peripheral and the secondary lymphoid tissues. Fine-tuning the control of B7-H1 induction in KCs may play a crucial role in mucosal homeostasis and protection from excess immune responses in disease.
METHODS
Mice. Female 5–7-week-old BALB/c mice were purchased from Japan SLC (Hamamatsu, Japan). B7-H1−/− mice in the C57BL/6 background (B7-H1KO/B6)30 and PD-1−/− mice in the BALB/c background (PD-1KO/c)31 were kindly provided by Lieping Chen from John Hopkins University (Baltimore, MD) and Tasuku Honjo from Kyoto University (Kyoto, Japan) through RIKEN RBC (Tsukuba, Ibaraki, Japan), respectively. B7-H1KO/B6 was backcrossed at least 10 generations to BALB/c, and B7-H1−/− mice on the BALB/c background (B7-H1KO/c) were obtained. B7-H1−/− PD-1−/− WKO mice were generated by breeding B7-H1KO/c to PD-1KO/c mutants. RAG2−/− DO11.10 TCR transgenic mice32, 33 were kindly supplied by Ko Okumura. RAG2−/− DO11.10 PD-1KO mice were generated by breeding RAG2−/− DO11.10 TCR to PD-1−/− mutants. Crosses were confirmed using flow cytometry with KJ1-26 (specific for the DO11.10 TCR), anti-B7-H1, or anti-PD-1 mAb and DNA genotyping. All mice were maintained under specific pathogen-free conditions and used according to institutional guidelines. All procedures were reviewed and approved by the animal care and use committee of Tokyo Medical and Dental University.
Topical application to oral mucosa and skin. Under anesthesia with a ketamine/xylazine mixture, 5 μl each of the following reagents were applied onto the dried surface of the dorsal tongue or the right side of the buccal mucosa. For skin painting, 20 μl of each reagent was applied to the shaved abdominal skin. TPA (400 μg ml−1 for the oral mucosa and 250 μg ml−1 for the skin, diluted in phosphate-buffered saline, Sigma-Aldrich, St Louis, MO), DNFB (0.2% dissolved in acetone/olive oil, 4:1, Sigma-Aldrich), and OVA (100 mg ml−1 in phosphate-buffered saline, Sigma-Aldrich) were used. For topical application with OVA/DNFB, DNFB was applied first, followed by OVA.
Immunohistochemistry. Skin and mucosal tissues were surgically dissected, embedded in Tissue-Tek (Sakura, Tokyo, Japan), frozen, and stored at −80 °C until use. Cryostat sections were fixed in cold absolute acetone and subjected to enzymatic immunohistochemistry as described previously.34 Briefly, after blocking with rabbit and rat serum, sections were incubated with primary mAbs against B7-H1 (MIH6, rat IgG2a), PD-1 (RMPI-14, rat IgG2a), CD4 (RM4-5, rat IgG2a), Foxp3 (FJK-16s, rat IgG2a), and Ki-67 (SolA15, rat IgG2a), or isotype control rat Igs; this was followed by biotinylated anti-rat IgG (Vector Laboratories, Burlingame, CA). For MHC class II staining, biotinylated anti-MHC class II (M5/114, rat IgG2b) was used. All incubation steps, except for anti-PD-1 mAb, were performed in a microwave processor (MI-7, Azumaya, Tokyo, Japan). Antibody binding was detected using avidin-biotin-peroxidase complex system (Vectastain; Vector Laboratories), visualized with the substrate diaminobenzidine, and counterstained with hematoxylin.
Quantitative analysis for Foxp3 and CD4 was performed to select three continuous high-power field images (× 200) that contained the basal layer in the center of each section. Positive cell numbers were counted on the print-out image and verified by two independent investigators.
Generation of dorsal tongue inflammation by DO11.10 T-cell transfer and OVA painting. A mixture of splenocytes and LN cells from RAG2−/− DO11.10 or RAG2−/− DO11.10 PD-1KO mice were stimulated with OVA protein (0.5 mg ml−1) and recombinant IL-2 (5 ng ml−1 at the final concentration; eBioscience, San Diego, CA) was added on day 3. Cultured cells were harvested on day 7 and dead cells were depleted by density gradient centrifugation. Over 98% of CD3+CD4+KJ1-26+ DO11.10 T cells were confirmed by flow cytometry. Cells were labeled with violet tracker (CellTrace Violet Cell proliferation Kit, Invitrogen, Carlsbad, CA) according to the manufacture’s protocol. Violet-labeled activated DO11.10 CD4+ T cells (2 × 106) were transferred and OVA/DNFB was then topically applied to the dorsal tongue after 24 h. For the mAb blocking experiments, each group of mice received an intraperitoneal injection of 500 μg of control rat IgG (Cappel) or anti-B7-H1 mAb (MIH5) 2 h before topical painting. At 48 h after the topical painting, regional (cervical and submandibular) LNs and tongue tissues were obtained for the flow cytometry and histological analyses, respectively.
Generation of BM chimera mice. BALB/c-recipient mice received a lethal dose of irradiation (7 Gy) using a 137Cs γ-irradiator (IBL437C, CIS Bio International, Bangnols sur Ceze, France), and then erythrocyte-depleted BM cells (2 × 107 cells) from 6-week-old B7-H1/PD-1WKO mice were intravenously transferred. In a prior experiment, the reconstitution of hematopoietic cells and the recovery of body weight were confirmed at 4 weeks. At 5 weeks after the irradiation, transfer of DO11.10 T cells and topical painting of OVA/DNFB were performed, as described above.
Multicolor immunofluorescence. Regional LN cells were isolated using collagenase I, as described previously.34 Cells were stimulated with phorbol 12-myristate 13-acetate and ionomycin in the presence of brefeldin A for 4 h, and multicolor staining for cell surface Ags and intracellular cytokines and transcription factors were performed, as described previously.35 For cell surface staining, PerCP-Cy5.5-conjugated anti-CD4 (RM4-5) and biotinylated-anti-KJ1-26 mAbs, followed by APC-eFlour780-streptavidin, were used. For intracellular staining, FITC-conjugated anti-IFN-γ (XMG1.2, rat IgG1), PE-anti-IL-10 (JES5-16E3, rat IgG2b), and APC-anti-Foxp3 (FJK-16s, rat IgG2a), or appropriate fluorochrome-conjugated isotype control Igs, were used. The protocol for intracellular staining was followed by the Foxp3 staining procedure. All mAbs were obtained from Affymetrix/eBioscience (San Diego, CA) or BD-Pharmingen (San Diego, CA). Stained cells were analyzed using a FACSVerse (BD Biosciences, San Jose, CA) with FACSuite software. Data were analyzed using a FlowJo software (Tree Star, Ashland, OR).
KC-mediated coinhibition assay. Immature BMDCs from B7-H1/PD-1WKO BM cells were generated in the presence of GM-CSF for 6 days as described previously.36 Collected immature BMDCs were pulsed with OVA (0.1 mg ml−1) in the presence of a maturation stimulus, tumor necrosis factor-α (10 ng ml−1, eBioscience), for an additional 24 h. Pam 212 cells (a murine KC cell line, BALB/c origin) were prestimulated with or without IFN-γ (10 ng ml−1, eBioscience) for 24 h in a 24-well plate. Violet-labeled activated DO11.10 CD4+ T cells (2.5 × 105 cells per well) and OVA-pulsed B7-H1/PD-1WKO BMDCs were added to the Pam 212-preseeded wells in the presence of either control rat IgG or anti-B7-H1 mAb (MIH5, 20 μg ml−1), and cells were cultured for 48 h. Cultured cells were stained and analyzed by flow cytometry as described above.
Statistical analysis. Statistical analyses were performed by Mann–Whitney U-test. Values of P<0.05 were considered significant.
References
Iwasaki, A. Mucosal dendritic cells. Annu. Rev. Immunol. 25, 381–418 (2007).
Presland, R.B. & Dale, B.A. Epithelial structural proteins of the skin and oral cavity: function in health and disease. Crit. Rev. Oral Biol. Med. 11, 383–408 (2000).
Squier, C.A. & Kremer, M.J. Biology of oral mucosa and esophagus. J. Natl. Cancer Inst. Monogr. 29, 7–15 (2001).
Waldmann, H. Tolerance: an overview and perspectives. Nat. Rev. Nephrol. 6, 569–576 (2010).
Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
McGrath, M.M. & Najafian, N. The role of coinhibitory signaling pathways in transplantation and tolerance. Front. Immunol. 3, 47 (2012).
Keir, M.E., Butte, M.J., Freeman, G.J. & Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 26, 677–704 (2008).
Ritprajak, P. & Azuma, M. Intrinsic and extrinsic control of expression of the immunoregulatory molecule PD-L1 in epithelial cells and squamous cell carcinoma. Oral Oncol. 51, 221–228 (2015).
Ritprajak, P., Hashiguchi, M., Tsushima, F., Chalermsarp, N. & Azuma, M. Keratinocyte-associated B7-H1 directly regulates cutaneous effector CD8+ T cell responses. J. Immunol. 184, 4918–4925 (2010).
Okiyama, N. & Katz, S.I. Programmed cell death 1 (PD-1) regulates the effector function of CD8 T cells via PD-L1 expressed on target keratinocytes. J. Autoimmun. 53, 1–9 (2014).
Tsushima, F. et al. Predominant expression of B7-H1 and its immunoregulatory roles in oral squamous cell carcinoma. Oral Oncol. 42, 268–274 (2006).
Cao, Y. et al. B7-H1 overexpression regulates epithelial-mesenchymal transition and accelerates carcinogenesis in skin. Cancer Res. 71, 1235–1243 (2011).
Qian, Y. et al. TLR4 signaling induces B7-H1 expression through MAPK pathways in bladder cancer cells. Cancer Invest. 26, 816–821 (2008).
Groeger, S., Domann, E., Gonzales, J.R., Chakraborty, T. & Meyle, J. B7-H1 and B7-DC receptors of oral squamous carcinoma cells are upregulated by Porphyromonas gingivalis. Immunobiology 216, 1302–1310 (2011).
Youngnak-Piboonratanakit, P. et al. The expression of B7-H1 on keratinocytes in chronic inflammatory mucocutaneous disease and its regulatory role. Immunol. Lett. 94, 215–222 (2004).
Yao, S. et al. PD-1 on dendritic cells impedes innate immunity against bacterial infection. Blood 113, 5811–5818 (2009).
Pena-Cruz, V., McDonough, S.M., Diaz-Griffero, F., Crum, C.P., Carrasco, R.D. & Freeman, G.J. PD-1 on immature and PD-1 ligands on migratory human Langerhans cells regulate antigen-presenting cell activity. J. Invest. Dermatol. 130, 2222–2230 (2010).
Okiyama, N. & Fujimoto, M. Clinical perspectives and murine models of lichenoid tissue reaction/interface dermatitis. J. Dermatol. Sci. 78, 167–172 (2015).
Meister, M. et al. Self-antigen presentation by keratinocytes in the inflamed adult skin modulates T-cell auto-reactivity. J. Invest. Dermatol. 135, 1996–2004 (2015).
Fan, L., Busser, B.W., Lifsted, T.Q., Oukka, M., Lo, D. & Laufer, T.M. Antigen presentation by keratinocytes directs autoimmune skin disease. Proc. Natl. Acad. Sci. USA 100, 3386–3391 (2003).
Nestle, F.O., Di, Meglio, P., Qin, J.Z. & Nickoloff, B.J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 9, 679–691 (2009).
Kim, B.S., Miyagawa, F., Cho, Y.H., Bennett, C.L., Clausen, B.E. & Katz, S.I. Keratinocytes function as accessory cells for presentation of endogenous antigen expressed in the epidermis. J. Invest. Dermatol. 129, 2805–2817 (2009).
Liu, Y. & Janeway, C.A. Jr. Cells that present both specific ligand and costimulatory activity are the most efficient inducers of clonal expansion of normal CD4 T cells. Proc. Natl. Acad. Sci. USA 89, 3845–3849 (1992).
Ding, L. & Shevach, E.M. Activation of CD4+ T cells by delivery of the B7 costimulatory signal on bystander antigen-presenting cells (trans-costimulation). Eur. J. Immunol. 24, 859–866 (1994).
Smythe, J.A., Fink, P.D., Logan, G.J., Lees, J., Rowe, P.B. & Alexander, I.E. Human fibroblasts transduced with CD80 or CD86 efficiently trans- costimulate CD4+ and CD8+ T lymphocytes in HLA-restricted reactions: implications for immune augmentation cancer therapy and autoimmunity. J. Immunol. 163, 3239–3249 (1999).
Mandelbrot, D.A., Kishimoto, K., Auchincloss, H. Jr, Sharpe, A.H. & Sayegh, M.H. Rejection of mouse cardiac allografts by costimulation in trans. J. Immunol. 167, 1174–1178 (2001).
Fife, B.T. et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat. Immunol. 10, 1185–1192 (2009).
Cao, Y. et al. Keratinocytes induce local tolerance to skin graft by activating interleukin-10-secreting T cells in the context of costimulation molecule B7-H1. Transplantation 75, 1390–1396 (2003).
Zhou, X. et al. High level expression of B7H1 molecules by keratinocytes suppresses xeno- and allo-reactions by inducing type I regulatory T cells. Transpl. Immunol. 21, 192–197 (2009).
Dong, H., Zhu, G., Tamada, K., Flies, D.B., van, Deursen, J.M. & Chen, L. B7-H1 determines accumulation and deletion of intrahepatic CD8+ T lymphocytes. Immunity 20, 327–336 (2004).
Nishimura, H. et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 291, 319–322 (2001).
Murphy, K.M., Heimberger, A.B. & Loh, D.Y. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science 250, 1720–1723 (1990).
Shinkai, Y. et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68, 855–867 (1992).
Chalermsarp, N. & Azuma, M. Identification of three distinct subsets of migrating dendritic cells from oral mucosa within the regional lymph nodes. Immunology 127, 558–566 (2009).
Sakurai, J. et al. Blockade of CTLA-4 signals inhibits Th2-mediated murine chronic graft-versus-host disease by an enhanced expansion of regulatory CD8+ T cells. J. Immunol. 164, 664–669 (2000).
Ritprajak, P, Hashiguchi, M. & Azuma, M. Topical application of cream-emulsified CD86 siRNA ameliorates allergic skin disease by targeting cutaneous dendritic cells. Mol. Ther. 16, 1323–1330 (2008).
Acknowledgements
We thank Drs L. Chen, T. Honjo, and K. Okumura for permission to use the PD-1−/− and B7-H1−/− mice and for providing RAG2−/− DO11.10 TCR transgenic mice. This work was supported by grants from Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (KAKENHI) (A26253086 and A23249082 to M.A.)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Supplementary information
Rights and permissions
About this article
Cite this article
Kang, S., Zhang, C., Ohno, T. et al. Unique B7-H1 expression on masticatory mucosae in the oral cavity and trans-coinhibition by B7-H1-expressing keratinocytes regulating CD4+ T cell-mediated mucosal tissue inflammation. Mucosal Immunol 10, 650–660 (2017). https://doi.org/10.1038/mi.2016.89
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2016.89