Abstract
Airway diseases, including cigarette smoke-induced chronic bronchitis, cystic fibrosis, and primary ciliary dyskinesia are associated with decreased mucociliary clearance (MCC). However, it is not known whether a simple reduction in MCC or concentration-dependent mucus adhesion to airway surfaces dominates disease pathogenesis or whether decreasing the concentration of secreted mucins may be therapeutic. To address these questions, Scnn1b-Tg mice, which exhibit airway mucus dehydration/adhesion, were compared and crossed with Muc5b- and Muc5ac-deficient mice. Absence of Muc5b caused a 90% reduction in MCC, whereas Scnn1b-Tg mice exhibited an ∼50% reduction. However, the degree of MCC reduction did not correlate with bronchitic airway pathology, which was observed only in Scnn1b-Tg mice. Ablation of Muc5b significantly reduced the extent of mucus plugging in Scnn1b-Tg mice. However, complete absence of Muc5b in Scnn1b-Tg mice was associated with increased airway inflammation, suggesting that Muc5b is required to maintain immune homeostasis. Loss of Muc5ac had few phenotypic consequences in Scnn1b-Tg mice. These data suggest that: (i) mucus hyperconcentration dominates over MCC reduction alone to produce bronchitic airway pathology; (ii) Muc5b is the dominant contributor to the Scnn1b-Tg phenotype; and (iii) therapies that limit mucin secretion may reduce plugging, but complete Muc5b removal from airway surfaces may be detrimental.
Similar content being viewed by others
Introduction
Secretion and clearance of mucus constitute a conserved mechanism for host protection across species. Airway mucus, a heterogeneous mixture of water, mucins, proteins, salts, and lipids, is traditionally listed among lung innate defenses owing to its ability to trap and mediate swift removal of inhaled particles/pathogens by mechanical clearance.1 Effective mucus clearance is essential for respiratory health, as illustrated by the poor prognosis of lung diseases characterized by airway mucus stasis, e.g., cystic fibrosis, primary ciliary dyskinesia, the bronchitis associated with chronic obstructive pulmonary disease, and bronchiectasis. Regardless of etiology, all of these bronchitic diseases share the common hallmarks of abnormal sputum production and an airway pathology reflecting epithelial remodeling, mucus accumulation, and inflammation. Increasing efforts have been focused on understanding airway mucus biology and, in particular, how the abnormal structure and function of mucus may produce disease. A complex picture is emerging, where the particular features of diseased mucus might include abnormal concentration (“hydration”),2, 3, 4 biogenesis and pH,5, 6, 7, 8 macromolecular organization,9, 10 and/or functional relationships with other components of the host defense, including inflammatory cells.11, 12, 13
The gel-forming mucins MUC5AC and MUC5B, as well as their murine homologs Muc5ac and Muc5b, are the principal macromolecular components of airway mucus and dominate its biophysical properties.14, 15, 16 Once secreted into the extracellular space, gel-forming mucins are found as mucin/protein complexes with seemingly organized structures.10 MUC5AC and MUC5B are different in terms of domain structure, glycosylation, assembly, and secretion sites,17 and are thus predicted to have different functions. In humans, cigarette smoke-induced chronic bronchitis, cystic fibrosis, and primary ciliary dyskinesia are characterized predominately by increases in MUC5B, whereas MUC5AC may be the predominant mucin expressed in asthma.3, 15, 18 Recent reports have identified Muc5b as essential for mucociliary clearance (MCC)19 and Muc5ac hypersecretion as a major determinant of hyperreactivity to inhaled methacholine in allergen-challenged mouse airways.20 However, the functional significance of these two mucins in the pathogenesis of bronchitic lung diseases remains to be fully elucidated.
Defective mucus clearance has been experimentally generated in Scnn1b-transgenic (Scnn1b-Tg) mice by airway-targeted overexpression of the epithelial Na+ channel β subunit (βENaC, encoded by the Scnn1b gene).21 Expression of the Scnn1b transgene causes airway surface dehydration with a consequent increase in mucus concentration, which leads to osmotic compression of cilia, mucus adhesion, and airway obstruction. As noted above, airway clearance is also reduced in Muc5b knock-out (Muc5b−/−) mice,19 but because these two mouse models have been studied independently, the relative contributions of “low” vs. “high” mucin concentration mechanisms that degrade mucus clearance to the pathogenesis of bronchitic lung diseases are unknown.
The overall goal of the present study was to test which specific defect(s) in the mucus clearance system, i.e., mucus hyperconcentration/adhesion as observed in the Scnn1b-Tg model, or selective reduction in mucus flow, as observed in the Muc5b−/− model, produce the pathologic correlate of bronchitis, e.g., airway remodeling, inflammation, and mucus accumulation. Further, we investigated which of the major secreted mucins (Muc5ac or Muc5b) dominates the muco-obstructive phenotype of Scnn1b-Tg mice and whether genetic reductions in mucin concentration might be advantageous. To address these questions, we crossed Muc5b−/− mice or Muc5ac−/− mice with Scnn1b-Tg mice and characterized the phenotype of the progeny.
Results
Survival and body weights of the Muc5b−/− × Scnn1b-Tg cross progeny
The Muc5b−/− × Scnn1b-Tg cross generated mice of six different genotypes: Muc5b+/+, Muc5b+/−, and Muc5b−/− either positive (Scnn1b-Tg) or negative for Scnn1b overexpression (see Methods for the mouse nomenclature adopted in the main text and legends). Scnn1b-Tg mice exhibited only mild lethality compared with wild-type (WT) littermates (Supplementary Figure S1a online), regardless of their Muc5b genotype, as expected for mice in a mixed C57BL/6N22:129/SV23 genetic background. At postnatal day (PND) 35, Muc5b−/−Scnn1b-Tg mice exhibited a slight reduction in body weight as compared with Muc5b+/+ littermates (Supplementary Figure S1b and c), but this finding was not apparent in the progeny of congenic C57 Muc5b−/− × Scnn1b-Tg mice (Supplementary Figure S1d–f).
Mucus hyperconcentration/adhesion is necessary to produce bronchitic lung pathology
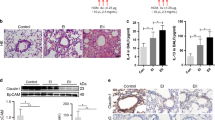
To determine whether mucus hyperconcentration/adhesion or reduced mucus flow per se can produce bronchitic lung pathology, we first compared Muc5b−/− and Muc5b+/+Scnn1b-Tg mice vs. Muc5b+/+ littermates. Histologically, Muc5b−/− mice were indistinguishable from Muc5b+/+ littermates ( Figure1a–c ). In contrast, Muc5b+/+Scnn1b-Tg mice exhibited a severe bronchitic pathology characterized by inflammatory infiltrates, luminal secretions, and emphysematous lesions ( Figure 1a–c ). Airway mucus content was evaluated histologically with Alcian Blue-Periodic Acid Schiff staining ( Figure 1d ) and quantified morphometrically as mucus volume density (mucus VS24) in the airways (epithelial+lumen, Figure 1e ) and in the epithelia alone ( Figure 1f ). The mucus content in Muc5b+/+ and Muc5b−/− mice was minimal, and mostly confined to the intraepithelial compartment ( Figure1e ). In contrast, Muc5b+/+Scnn1b-Tg mice exhibited substantial intraluminal mucus accumulation, consistent with muco-obstructive lung disease. Secreted mucins were also evaluated by western blots of bronchoalveolar lavage (BAL) ( Figure 1f, g and Supplementary Figure S2, upper panels). Muc5b was absent in Muc5b−/− mice, and the Muc5b BAL content was greatly increased in Muc5b+/+Scnn1b-Tg mice, consistent with previous reports.25 Parallel quantification of Muc5ac content revealed a modest increase in Muc5b−/− mice as compared with Muc5b+/+ mice (∼3-fold, P<0.05. Figure 1h ), in agreement with previous mRNA and immunohistological data.19 Muc5b+/+Scnn1b-Tg mice exhibited a significant increase in Muc5ac (∼18-fold vs. Muc5b+/+ mice, Figure 1h ), in agreement with previous mRNA data24 and suggesting accumulation of Muc5ac due to poor clearance.
Mucus hyperconcentration/adhesion is necessary to produce bronchitic lung pathology, but Muc5b deletion ameliorates airway mucus obstruction in Scnn1b-Tg mice. (a) Representative photomicrographs of airway lumens cut in cross section proximal to the hilum from PND35 mice of the indicated genotypes, stained with H&E illustrating airway mucus obstruction and inflammatory infiltrates characteristic of bronchitic lung pathology in Scnn1b-Tg mice. (b and c) Semiquantitative histology scores for airway inflammation (b) and air space enlargement (c) in PND35 mice from the Muc5b−/− × Scnn1b-Tg cross in the C57:129 genetic background. n=6–9 mice/genotype. ANOVA *P<0.05 vs. Muc5b+/+ mice. (d) Equivalent sections as in (a) stained with AB-PAS for mucopolysaccharides, illustrating significant amelioration of mucus obstruction in Muc5b−/−Scnn1b-Tg vs. Muc5b+/+Scnn1b-Tg mice. Scale bar 0.1 mm. (e and f) Morphometric analysis of total (epithelial+luminal, e) and epithelial (f) airway mucus volume density (Vs) in PND35 mice (C57:129 genetic background). n=6–9 mice/genotype. ANOVA *P<0.05 vs. Muc5b+/+ mice, #P<0.05 vs. Muc5b+/+Scnn1b-Tg mice. (g and h) Densitometric analysis of mucin agarose western blots of BAL from the progeny of the Muc5b−/− × Scnn1b-Tg cross in the C57 congenic background, at PND35. Blots were probed with anti-Muc5b (g) or anti-Muc5ac (h) antibodies. n=6 mice/genotype. ANOVA *P<0.05 vs. Muc5b+/+ mice, #P<0.05 vs. Muc5b+/+Scnn1b-Tg mice. AB-PAS, Alcian Blue-Periodic Acid Schiff staining; ANOVA, analysis of variance; BAL, bronchoalveolar lavage; H&E, hematoxylin and eosin; PND, postnatal day.
Muc5b deletion ameliorates airway mucus obstruction in Scnn1b-Tg mice
The effect of Muc5b genetic deletion on the phenotype of Scnn1b-Tg mice was evaluated by comparing Muc5b−/−Scnn1b-Tg and Muc5b+/−Scnn1b-Tg mice vs. Muc5b+/+Scnn1b-Tg littermates. Histologically, all three genotypes exhibited prominent airway inflammation ( Figure 1a, b ) and parenchymal remodeling ( Figure 1a, c ).
Notably, Muc5b−/−Scnn1b-Tg mice exhibited a significant reduction in intraluminal mucus as compared with Muc5b+/+Scnn1b-Tg mice, but secretions adherent to airway surfaces were still detected ( Figure 1d, e ). A genotype-dependent reduction in BAL Muc5b content was noted in Muc5b+/−Scnn1b-Tg and Muc5b−/−Scnn1b-Tg mice ( Figure 1g ), reflecting allelic insufficiency and complete deletion of Muc5b, respectively. Also, there was a genotype-dependent increase in BAL Muc5ac content in Muc5b−/−Scnn1b-Tg mice (∼46-fold vs. Muc5b+/+ mice and∼2.5-fold vs. Muc5b+/+Scnn1b-Tg mice, Figure 1h ), likely contributing to the residual mucus observed in the airways ( Figure 1d ).
Muc5ac deletion does not ameliorate airway mucus obstruction in Scnn1b-Tg mice
Initial crosses between Muc5ac−/− and Muc5ac+/−Scnn1b-Tg mice in the C57:129 background generated mice of four different genotypes (Muc5ac+/−; Muc5ac−/−; Muc5ac+/−Scnn1b-Tg; and Muc5ac−/−Scnn1b-Tg) in the expected Mendelian proportions, and all exhibited high survival (Supplementary Figure S3a). Analyses performed on the progeny of congenic C57BL/6N mice bred to generate all six possible genotypes (Muc5ac+/− × Muc5ac+/−Scnn1b-Tg) confirmed that Muc5ac deletion did not significantly affect survival (Supplementary Figure S3b) or body mass (Supplementary Figure S3c and d).
Similar to the cross with Muc5b−/− mice, only Scnn1b-Tg mice exhibited bronchitic lung pathology when evaluated on hematoxylin and eosin-stained sections, regardless of Muc5ac genotype (not shown). However, in contrast to what was observed for the Muc5b−/− cross, there were no differences in Alcian Blue-Periodic Acid Schiff staining-positive mucus content, either luminal or epithelial, between Muc5ac-deficient or -sufficient Scnn1b-Tg mice ( Figure 2a–c ). These data suggest that Muc5ac did not significantly contribute to the intraluminal mucus plugging observed in Scnn1b-Tg mice. Quantitation of BAL mucin content ( Figure 2d, e and Supplementary Figure S2, lower panels) revealed that, contrary to Muc5b−/− mice, Muc5ac−/− mice did not exhibit a compensatory increase in Muc5b compared with Muc5ac+/+ mice. Muc5b signal was increased in Scnn1b-Tg mice, regardless of Muc5ac genotype. As for Muc5ac BAL content, the signal for Muc5ac+/+ and Muc5ac+/− mice was just above background, but an increased level of Muc5ac was observed in Muc5ac+/+Scnn1b-Tg mice. Of note, there was a genotype-dependent reduction of harvested Muc5ac in Muc5ac+/−Scnn1b-Tg and Muc5ac−/−Scnn1b-Tg mice, reflecting the Muc5ac allelic make-up of the mice.
Muc5ac deletion does not ameliorate airway mucus obstruction in Scnn1b-Tg mice. (a) Representative photomicrographs of proximal left lobe main stem bronchus from PND35 mice of the indicated genotypes, stained with AB-PAS for mucopolysaccharides, illustrating no changes in mucus obstruction in Muc5ac−/−Scnn1b-Tg vs. Muc5ac+/−Scnn1b-Tg mice. Scale bar 0.1 mm. (b and c) Morphometric analysis of total (epithelial+luminal, b) and epithelial (c) airway mucus volume density (Vs) in PND35 mice (C57:129 genetic background). n=6–11 mice/genotype. ANOVA *P<0.05 vs. Muc5ac+/+ mice. (d and e) Densitometric analysis of mucin agarose western blots of BAL from the progeny of the Muc5ac−/− × Scnn1b-Tg cross in the C57 congenic background, at PND35. Blots were probed with anti-Muc5b (d) or anti-Muc5ac (e) antibodies. n=6 mice/genotype. ANOVA *P<0.05 vs. Muc5ac+/+ mice, #P<0.05 vs. Muc5ac+/+Scnn1b-Tg mice. AB-PAS, Alcian Blue-Periodic Acid Schiff staining; ANOVA, analysis of variance; BAL, bronchoalveolar lavage; PND, postnatal day.
Relative contribution of Muc5ac vs. Muc5b to neonatal survival in a model of lethal mucus obstruction, the F1 C57:FVB Scnn1b-Tg mouse
As previously reported, the severity of the Scnn1b-Tg muco-obstructive phenotype is proportional to airway mucin secretory capacity, which is age- and mouse strain-dependent.22 Specifically, we and others have reported that neonatal mice experience a transient increase in Muc5ac and Muc5b secretion during the early postnatal period (PND5–10).25, 26 The developmentally regulated increase in mucin concentration on airway surfaces, coupled to the small caliber of neonatal airways, makes Scnn1b-Tg pups more sensitive to the impact of salt and water depletion produced by Scnn1b-Tg overexpression and causes fatal tracheal mucus obstruction. We have previously described a variant of Scnn1b-Tg mice, F1 C57:FVB Scnn1b-Tg mice,22 characterized by extremely high postnatal mortality (90%) and higher BAL mucus content as compared with congenic C57BL/6N Scnn1b-Tg mice.
Accordingly, we crossed Muc5ac−/−Scnn1b-Tg mice or Muc5b−/−Scnn1b-Tg mice with inbred FVB/NJ mice to obtain mice of four different genotypes (Muc+/+, Muc+/−, Muc+/+Scnn1b-Tg, Muc+/−Scnn1b-Tg) in a homogeneous F1 C57:FVB genetic background. The effect of decreased Muc5ac or Muc5b concentration on survival was evaluated. Muc5b+/+ and Muc5b+/− mice had the expected normal, high survival, whereas Muc5b+/+Scnn1b-Tg mice were all dead within 7 days from birth ( Figure 3a ). Importantly, heterozygosity for Muc5b significantly improved the survival of Muc5b+/−Scnn1b-Tg mice (∼70% at PND10) as compared with Muc5b+/+Scnn1b-Tg mice (∼0% at PND10). When similar studies were performed with Muc5ac−/− mice, we observed no protection from lethality ( Figure 3b ) but a modest though significant delay in mortality of the Muc5ac+/−Scnn1b-Tg mice as compared with Muc5ac+/+Scnn1b-Tg mice. Collectively, these data suggest that Muc5b is the dominant mucin that leads to lethality of Scnn1b-Tg mice in the “high mucus producer” FVB background.
Relative contribution of Muc5ac vs. Muc5b to neonatal survival in a model of lethal mucus obstruction, the F1 C57:FVB/NJ Scnn1b-Tg mice. Survival curves for the progeny of the Muc5b−/−Scnn1b-Tg mice (a) or Muc5ac−/−Scnn1b-Tg mice (b) crossed with inbred FVB/NJ mice. Muc5b heterozygosity improved overall survival of F1 C57:FVB Scnn1b-Tg mice, whereas Muc5ac heterozygosity only resulted in delayed time of death (median survival PND9 vs. PND5 for Muc5ac+/−Scnn1b-Tg mice vs. Muc5ac+/+Scnn1b-Tg mice, respectively). *P<0.05 vs. Muc+/+ littermates, #P<0.05 vs. Muc+/+Scnn1b-Tg littermates. PND, postnatal day.
Mucociliary transport only partially correlates with the severity of muco-obstructive lung disease
A key measurement that has been used to relate airway mucus function to disease has been the rate of MCC.2 Accordingly, we compared the relative rates of mucus transport in the two models of defective airway clearance, i.e., Scnn1b-Tg and Muc5b−/− mice. Furthermore, to test whether a reduction in mucin concentration in the lungs of Scnn1b-Tg mice produced by absence of Muc5b might rescue mucus transport, we also measured MCC in Muc5b−/−Scnn1b-Tg.
As shown in Figure 4 , Muc5b+/+ mouse lungs cleared ∼75% of the tracer particles within 15 min after instillation. In contrast, there was almost a complete loss of MCC in Muc5b−/− mice, as previously reported.19 Also, similar to previous reports,21 a ∼50% reduction in MCC was observed in Muc5b+/+Scnn1b-Tg mice. Notably, deletion of Muc5b in Scnn1b-Tg mice did not rescue mucus transport, but rather caused a reduction in MCC towards the levels exhibited by Muc5b−/− mice.
Mucociliary transport only partially correlates with the severity of muco-obstructive lung disease. Mucociliary clearance measurements in selected genotypes from the progeny of the Muc5b−/− × Scnn1b-Tg cross in the C57 congenic background, at PND35. n=6–14 mice/genotype. ANOVA *P<0.05 vs. Muc5b+/+ mice, #P<0.05 vs. Muc5b+/+Scnn1b-Tg mice. ANOVA, analysis of variance; PND, postnatal day.
Deletion of Muc5b or Muc5ac does not affect bacterial burden or ameliorate airway inflammation in Scnn1b-Tg mice
Because both Scnn1b-Tg and Muc5b−/− mice have increased susceptibility to spontaneous and experimentally induced airway bacterial infection,19, 21, 27 we tested whether the combination of the two genotypes would produce an additive phenotype. As effects of Muc5ac deletion on intestinal pathogen clearance have also been reported,28 microbiology experiments were also performed for the Muc5ac−/− × Scnn1b-Tg cross.
At PND5–7, Muc5b−/− mice exhibited an incidence of infection of ∼50% compared with 0% in Muc5b+/+ mice, with a bacterial burden of ∼1.5 Log CFU per mouse ( Figure 5a ). In contrast, virtually all neonatal Scnn1b-Tg mice were infected with a bacterial burden of ∼3 Log CFU per mouse, and this infection was not affected by the Muc5b genotype. At PND35, none of the Muc5b−/− mice were infected, and sporadic infections were detected in Scnn1b-Tg mice ( Figure 5b ), in agreement with previous reports.27 Similar to neonatal Scnn1b-Tg mice, there was no appreciable difference in bacterial burden due to Muc5b genotype in adult WT or Scnn1b-Tg mice. A similar picture emerged from the microbiological analysis of BAL samples harvested from mice derived from Muc5ac−/− × Scnn1b-Tg crosses at PND5–7 (Supplementary Figure S4), with the notable exception that Muc5ac−/− neonatal mice did not exhibit the incidence of spontaneous bacterial infection observed in neonatal Muc5b−/− mice.
Deletion of Muc5b does not affect bacterial burden or ameliorate airway inflammation in Scnn1b-Tg mice. (a and b) Quantification of CFU in BAL samples from the progeny of the Muc5b−/− × Scnn1b-Tg cross in the C57:129 genetic background, at PND5–7 (a) or PND35 (b). (Log10+1)-transformed data. n=8–24 mice/genotype (a) and n=5–8 mice/genotype (b). ANOVA *P<0.05 vs. Muc5b+/+ mice. (c and d) BAL neutrophil counts for the progeny of the Muc5b−/− × Scnn1b-Tg cross in the C57:129 genetic background, at PND5–7 (c) or PND35 (d) n=7–24 mice/genotype (c) and n=11–15 mice/genotype (d). ANOVA *P<0.05 vs. Muc5b+/+ mice. ANOVA, analysis of variance; BAL, bronchoalveolar lavage; CFU, colony-forming units; PND, postnatal day.
To test whether genetic ablation of Muc5ac or Muc5b affected the airway inflammatory profile of Scnn1b-Tg mice, we characterized BAL cells in neonatal (PND5–7) and adult (PND35) mice. Neutrophil counts were elevated in both Muc5b−/− and Muc5b+/+Scnn1b-Tg neonatal mice as compared with Muc5b+/+ littermates ( Figure 5c ), in agreement with the presence of bacteria. Rather than rescuing this phenotype, Muc5b deletion caused a trend towards worsening of the neutrophil infiltration in Scnn1b-Tg mice. In adult mice, there was a modest but significant increase in neutrophil count in Muc5b−/− mice, whereas the neutrophil infiltrate was much greater in Muc5b+/+Scnn1b-Tg mice ( Figure 5d ). However, the airway neutrophilia typical of adult Scnn1b-Tg mice was not ameliorated by either partial (Muc5b+/−Scnn1b-Tg mice) or total (Muc5b−/−Scnn1b-Tg mice) ablation of Muc5b. Rather, a gene-dosage-dependent trend towards worsening of airway neutrophilia was observed (Muc5b+/+>Muc5b+/−>Muc5b−/−). Muc5b deletion alone was not associated with significant changes in macrophage numbers either in neonatal (Supplementary Figure S5a) or adult WT mice (Supplementary Figure S5b). Neonatal Scnn1b-Tg mice exhibited a modest increase in BAL macrophages as compared with WT littermates, regardless of Muc5b genotype, but this increase was normalized in adult mice with only Muc5b−/−Scnn1b-Tg mice exhibiting a significant difference vs. WT littermates.
As a parallel readout of airway inflammation, we evaluated the BAL chemokine and cytokine profiles in adult mice. The neutrophil chemoattractant chemokines (C-X-C motif) ligand 1 (CXCL1 or KC) and lipopolysaccharide-induced CXC chemokine (LIX) were significantly elevated in Scnn1b-Tg mice as compared with both WT and Muc5b−/− mice ( Figure 6a,b ). Genetic deletion of Muc5b did not alter the levels of these inflammatory markers in Scnn1b-Tg mice. Although it did not reach significance, macrophage inflammatory protein (MIP)-2 was elevated in all Scnn1b-Tg samples, regardless of Muc5b genotype (Supplementary Figure S5c), whereas IL-6 and TNFα were below the lower limit of detection in all samples (data not shown).
Deletion of Muc5b does not alter the BAL chemokine and cytokine profile in Scnn1b-Tg mice. KC (a) and LIX (b) levels in cell-free BAL from the progeny of the Muc5b−/− × Scnn1b-Tg cross in the C57:129 genetic background at PND35. The dotted line represents the assay LOD. n=5–8 mice/genotype. ANOVA *P<0.05 vs. Muc5b+/+ mice. ANOVA, analysis of variance; BAL, bronchoalveolar lavage; LOD, lower detection limit.
As for the progeny of the Muc5ac−/− × Scnn1b-Tg cross, partial or total Muc5ac deletion did not cause neutrophilia or altered BAL macrophage counts in either neonatal (Supplementary Figure S6a and c) or adult (Supplementary Figure S6b and d) WT mice. Of note, Muc5ac deletion did not rescue the BAL neutrophilia in either neonatal or adult Scnn1b-Tg mice (Supplementary Figure S6a and b), and only a slight increase in BAL macrophage numbers was detected in neonatal Muc5ac−/−Scnn1b-Tg mice as compared with WT littermates (Supplementary Figure S6d).
Deletion of Muc5b, but not Muc5ac, worsens the incidence of bronchus-associated lymphoid tissue (BALT) in Scnn1b-Tg mice
Histopathological analysis of lung sections stained with hematoxylin and eosin ( Figure 7a ) using a semiquantitative score25 (Supplementary Figure S7a and b) and quantitative morphometry ( Figure 7b ) indicated that BALT was absent in PND35 WT mice, regardless of Muc5ac or Muc5b genotype, whereas low level accumulation of this ectopic lymphoid tissue could be detected in mucin-sufficient Scnn1b-Tg mice at this time point. Of note, Muc5b deletion significantly increased the incidence of BALT in Scnn1b-Tg mice, whereas Muc5ac deletion did not modify this phenotype. This increase in BALT was not reflected in the total number of BAL lymphocytes, which was elevated in Scnn1b-Tg mice regardless of Muc5b genotype ( Figure 7c ). Both B and T cells were present in these lymphoid nodules ( Figure 7d ), and their rather loose organization suggested recently formed, inducible BALT.29
Deletion of Muc5b, but not Muc5ac, worsens the incidence of BALT in Scnn1b-Tg mice. (a) Representative micrographs of proximal left lobe main stem bronchus from PND35 mice, stained with H&E, illustrating typical histopathology for the indicated genotypes. Scale bar 0.2 mm. Airway mucus obstruction was evident in Muc5b+/+Scnn1b-Tg mice and Muc5ac−/−Scnn1b-Tg mice (asterisks), but it was less severe in Muc5b−/−Scnn1b-Tg mice. However, Muc5b−/−Scnn1b-Tg mice presented with a higher incidence of BALT (arrow and high magnification inset, scale bar 20 μm). (b) Morphometric analysis of BALT in PND35 mice (C57:129 genetic background). n=6–9 mice/genotype. ANOVA *P<0.05 vs. Muc5b+/+ mice. (c) BAL lymphocyte counts for the progeny of the Muc5b−/− × Scnn1b-Tg cross in the C57:129 genetic background, at PND35. n=11–15 mice/genotype. ANOVA *P<0.05 vs. Muc5b+/+ mice. (d) Representative confocal images of BALT immunostained with B and T cells specific markers (B220 in red and CD3 in green, respectively), and relevant isotype negative controls (rat IgG2a,k and goat IgG, respectively). Nuclei are stained in blue (DAPI). DIC image is provided to illustrate the typical localization of BALT in the airway submucosal compartment. ANOVA, analysis of variance; BAL, bronchoalveolar lavage; BALT, bronchus-associated lymphoid tissue; DIC, differential interference contrast; H&E, hematoxylin and eosin; PND, postnatal day.
We sought to determine whether there was a correlation between incidence of BALT and immunoglobulin concentrations in BAL harvested from the progeny of the Muc5b−/− × Scnn1b-Tg cross. A striking increase in IgA was observed in all Scnn1b-Tg samples, regardless of Muc5b genotype ( Figure 8a ). Except for an increase in IgG1 in Muc5b−/−Scnn1b-Tg mice ( Figure 8b ), no systematic differences were observed for other IgG subtypes or IgM as a function of Muc5b genotype (Supplementary Figure S8a–d). Longitudinal studies comparing Scnn1b-Tg and WT littermates showed that IgA levels are increased in the BAL of Scnn1b-Tg mice beginning at PND10 (Supplementary Figure S8e). As it has been proposed that IgA binding to mucus through the polymeric Ig receptor secretory component (SC) is essential for IgA function in the lung,30 we tested whether selective deletion of Muc5b or Muc5ac would modify the localization of SC in WT or Scnn1b-Tg mice. As shown in Figure 8c , SC was exclusively localized to the surface epithelium in both Muc5b+/+ and Muc5b−/− mice (as well as Muc5ac−/− mice, not shown), whereas it was abundant in the luminal mucous secretions of Scnn1b-Tg mice, and its localization was not altered in the absence of either Muc5ac or Muc5b.
Airway mucus hyperconcentration/adhesion stimulates IgA secretion. (a and b) IgA (a) and IgG1 (b) levels in cell-free BAL from the progeny of the Muc5b−/− × Scnn1b-Tg cross in the C57:129 genetic background at PND35. The dotted line represents the assay LOD. n=5–8 mice/genotype. ANOVA *P<0.05 vs. Muc5b+/+ mice. (c) Immunohistochemical localization of the polymeric Ig receptor SC in the main stem bronchus of mice for the indicated genotypes. A serial section to the one used for Muc5b+/+Scnn1b-Tg SC stain is shown as negative IgG control (IgG control). ANOVA, analysis of variance; BAL, bronchoalveolar lavage; LOD, lower detection limit; SC, secretory component.
Discussion
Many aspects of lung mucus biology have become better defined in recent years. For example, it appears that Muc5b, not Muc5ac, is the dominant mucin that confers to the mucus layer the properties required for transport in the healthy lung.19 Moreover, the dominant role of mucins in producing the biophysical properties governing mucus flow in health vs. reduced/no flow in disease has also become better appreciated. Notably, a novel paradigm posits that healthy airway surfaces are populated by two mucus hydrogel layers, one comprised of the mobile layer (where Muc5b is dominant) and the other comprised of the periciliary layer, enriched in tethered mucins including Muc1, Muc4, and Muc16.4 The identification of this two-gel topology for the airway surface9 is key to quantify biophysical variables related to the distribution of water between the two hydrogels, which ultimately determines the efficiency of mucus transport.4 Of particular relevance for this study is the notion that mucus clearance is inversely proportional to its concentration, a concept recently translated to the clinic.2
It is clear that mucins have an important role in the pathogenesis of bronchitic lung diseases, which are clinically characterized by increased sputum production over defined periods of time, and pathologically associated with airway epithelial remodeling, including goblet cell metaplasia, inflammation, and mucus plugging. Despite the likely importance of mucus in the pathogenesis of these diseases, it is not clear whether abnormal qualitative or quantitative properties of mucus produce disease. Specifically, it is not clear whether the simple absence of mucus clearance, abnormal ratios of MUC5AC to MUC5B, or abnormal mucus concentration are required features to initiate pathology. Heretofore, it has been difficult to experimentally separate the two different pathologic mechanisms, e.g., absence of transport vs. presence of hyperconcentration, which is key to address this question.
Recently, the opportunity to study the role of mucins subtypes, mucus concentration, and mucus transport in the pathogenesis of bronchitic lung disease has been afforded by the generation of mouse models that produced different perturbations of airway mucus biology. Specifically, the availability of Muc5b−/− mice, which exhibit very slow airway mucus transport,19 and Scnn1b-Tg mice, which exhibit airway mucus hyperconcentration and adhesion to airway surfaces,21 has provided the opportunity to assess the relative roles and possible interactions of these dysfunctions in a common cohort.
In the studies reported here, the progenies from the mucin-deficient × Scnn1b-Tg mice crosses were compared utilizing metrics of bronchitic disease severity. Mucin-sufficient Scnn1b-Tg mice exhibited a relatively severe bronchitic phenotype, including: (i) increased airway mucus burden ( Figures1 and 2 ); (ii) highly penetrant spontaneous bacterial infection ( Figure 5 ); (iii) significant inflammatory infiltrates ( Figure 5 ); (iv) epithelial/parenchymal remodeling ( Figures 1 and7 ); and (v) increase in pro-inflammatory cytokines ( Figure 6 ) consistent with previous reports.24, 25, 27 Novel to this study, we observed another index of increased immune responses, i.e., high concentrations of IgA in Scnn1b-Tg BALF ( Figure 7e ). In contrast, Muc5b−/− mice exhibited a milder disease, with a lesser incidence of bacterial infection, fewer BAL neutrophils, lower levels of pro-inflammatory cytokines and IgA, and importantly no histological evidence of airway epithelial remodeling or emphysema. In parallel, no evidence of spontaneous lung disease was observed in Muc5ac−/− mice, consistent with previous reports.20
Interestingly, the severity of the obstructive and inflammatory airway phenotype did not simply reflect the rates of airway mucus clearance. As shown in Figure 4 , airway MCC in Muc5b−/− mice was indeed lower than in Scnn1b-Tg mice, despite the milder phenotype of the former. Accordingly, we speculate that it is not a reduction in the absolute rate of mucus clearance that dominates the pathogenesis of bronchitis, but it is the presence of hyperconcentrated mucus adherent to the airway surfaces, as evident in Scnn1b-Tg mice ( Figures 1, 2, and 7 ). This interpretation is consistent with findings in other mouse models that exhibit defective mucus transport but lack a mucus-adhesive component, e.g., models of primary ciliary dyskinesia, which also exhibit a mild lower airway phenotype.31, 32, 33
Crossing the Muc5b−/− and Muc5ac−/− mice with Scnn1b-Tg mice also offered the opportunity to query the role of the two major secreted mucins in the development of the Scnn1b-Tg phenotype. Muc5ac-deficient Scnn1b-Tg mice exhibited little/no change as compared with Muc5ac-sufficient Scnn1b-Tg mice ( Figure 2 ). In contrast, the magnitude of mucus obstruction in Scnn1b-Tg mice was significantly reduced in the absence of Muc5b, as evaluated both morphometrically and by BAL western blot ( Figure 1e–h ), consistent with the notion that Muc5b is the major secreted mucin in mouse airways.19, 26, 34 Despite the reduction in mucus burden, areas of mucus adhesion persisted histologically ( Figure 1d, e ). These plugs likely contained Muc5ac, consistent with the significant accumulation of Muc5ac in the BAL fluid of Muc5b−/−Scnn1b-Tg mice ( Figure 1h ).
The availability of a mouse strain that exhibits a lethal muco-obstructive phenotype, i.e., F1 C57:FVB Scnn1b-Tg mice, allowed us to investigate whether decreased levels of Muc5b or Muc5ac could rescue survival. Heterozygosity for Muc5b, but not Muc5ac, was sufficient to significantly rescue the survival of F1 C57:FVB Scnn1b-Tg mice ( Figure 3 ). On the basis of the data that Muc5b+/−Scnn1b-Tg mice exhibit ∼50% the levels of BAL Muc5b as compared with Muc5b+/+Scnn1b-Tg mice ( Figure 1g ), it is likely that the reduction of Muc5b was responsible for the large increase in survival. In contrast, Muc5ac+/−Scnn1b-Tg mice exhibited a modest increase in time to death as compared with Muc5ac+/+Scnn1b-Tg mice, suggesting that a Th2- or developmentally driven increase in Muc5ac might be detrimental to survival early in life.
Importantly, inflammation in Muc5b−/−Scnn1b-Tg mice was not reduced proportionately to mucus obstruction. Instead, there was an overall increase in the severity of pulmonary inflammation as indexed by increased number of BAL neutrophils and BALT ( Figures 5 and 7 ), These findings suggest that other factors in addition to mucus obstruction can lead to pulmonary inflammation. Perhaps the simplest explanation for the increased inflammatory phenotype in Muc5b−/−Scnn1b-Tg mice is that reduction of secreted Muc5b did not rescue the defective airway clearance of Scnn1b-Tg mice but, rather, further reduced mucus clearance to the levels of Muc5b−/− mice ( Figure 4 ). This reduction could promote further adhesion of other mucus components, possibly Muc5ac, to airway surfaces as well as prolong the residence time of pro-inflammatory particles or debris initiating a predictable inflammatory response. Alternatively, it has been reported that mucins may “communicate” with inflammatory cells, including macrophages and dendritic cells,12, 13, 35, 36, 37 and perhaps the absence of Muc5b contributed per se to an exaggerated inflammatory response in Muc5b−/−Scnn1b-Tg mice. Finally, gel-forming mucins, and particularly Muc5b, are associated with a network of proteins forming macromolecular complexes, known as “mucin interactomes,” which are thought to be involved in maintaining airway immune homeostasis through their anti-microbial/anti-inflammatory/anti-oxidant functions.38, 39 Derangement of this network may have adverse effects in controlling airway inflammation.
Another unexpected finding of this study was that Muc5b deletion did not affect bacterial burden in Scnn1b-Tg mice ( Figure 5 a, b ) despite a reduction in mucus plugs. A possible interpretation is that while Muc5b deletion decreased mucus plugging, it also reduced clearance of inhaled bacteria (as indicated by the presence of bacteria in 50% of the neonatal Muc5b−/− mice), offsetting the beneficial effect of reduced plugging. We can also speculate that the composition of neonatal mucus, enriched in Muc5ac as compared with adult mice (26 and A. Livraghi-Butrico, unpublished data), might also contribute to increased, Muc5b-independent trapping of bacteria.
As noted above, we found that IgA BAL levels were elevated in Scnn1b-Tg mice, suggesting that mucus hyperconcentration/adhesion stimulates local IgA production and secretion. IgA levels were elevated independently of Muc5b genotype, suggesting that the residual degree of mucus obstruction was sufficient to stimulate IgA synthesis and secretion. Importantly, previous reports have shown that SC-mediated binding of IgA to mucus is required for its function.30 Our immunohistological data ( Figure 8c ) suggest that SC binding to the mucus is not Muc5ac- or Muc5b-dependent. However, we cannot rule out the possibility that the overall decrease in intraluminal Muc5b in Muc5b−/−Scnn1b-Tg mice removed an essential “scaffold” for secreted IgA, impairing immune exclusion40 and promoting inflammation.
In conclusion, mucus biology in the normal and diseased lung is complex and likely involves not only the biophysical contributions of mucins to mucus transport but also interactions with host immune cells and defense proteins. Our data suggest that mucus hyperconcentration/adhesion rather than the loss of mucus flow dominates the pathophysiology of bronchitic lung diseases, and, consequently, these diseases may be characterized as “muco-obstructive” lung diseases. So, what is pathogenic in static/adherent mucus? Mucus adhesion certainly blocks airflow, and it is in part responsible for the reduced airflow observed in muco-obstructive lung diseases. Adherent mucus is also the site of most bacterial airways infections, including those causing exacerbations in cystic fibrosis and chronic obstructive pulmonary disease, and, even when “sterile,” it appears to be a pro-inflammatory DAMP.27 Moreover, mucus plugging induces local, epithelia hypoxia,24 which is an increasingly recognized feature of the CB syndrome,41 important for both inflammatory responses and anaerobic infection. Notably, therapies designed to clear mucus from airway surfaces are predicted to be therapeutic for these conditions. The simplest therapies involve the “rehydration” of mucus so that its concentration is restored to levels compatible with transport. It is also likely that therapies designed to reduce mucin secretion, and thus reduce concentrations, may also be useful. However, our data suggest that a complete loss of secreted mucins, especially MUC5B, may worsen pathology, so a more moderate titration might be needed therapeutically.
Methods
Animals Animals were maintained and studied under protocols approved by the University of North Carolina Institutional Animal Care and Use Committee, according to the principles outlined by the Animal Welfare and the National Institutes of Health guidelines. Mice were housed in individually ventilated micro-isolator cages in a specific pathogen-free facility at the University of North Carolina at Chapel Hill, on a 12-h day/night cycle. Mice were fed a regular chow diet and given water ad libitum.
Muc5ac−/−20 and Muc5b−/−19 mice were obtained from the Laboratory of Dr Christopher Evans as C57BL/6J:129/Sv line. Congenic C57BL/6N Scnn1b-Tg mice and WT littermates were maintained as a hemizygous as described22 and referred to as WT (i.e., Scnn1b-Tg− or Scnn1b-Tg negative) or Scnn1b-Tg (i.e., Scnn1b-Tg+ or Scnn1b-Tg positive). Mucin-deficient Scnn1b-Tg mice and appropriate littermate controls were generated by sequential breeding of congenic C57BL/6N hemizygous Scnn1b-Tg mice with Muc5ac−/−or Muc5b−/− mice, using a breeding strategy previously described.25 For clarity, genotypes of the progenies are indicated in the main text and figure legends as follows: Muc5ac+/+/Scnn1b-Tg−=Muc5ac+/+; Muc5ac+/−/Scnn1b-Tg−=Muc5ac+/−; Muc5a−/−/Scnn1b-Tg−=Muc5ac−/−; Muc5ac+/+/Scnn1b-Tg+=Muc5ac+/+Scnn1b-Tg; Muc5ac+/−/Scnn1b-Tg+=Muc5ac+/−Scnn1b-Tg; Muc5a−/−/Scnn1b-Tg+=Muc5ac−/−Scnn1b-Tg; and similarly for the Muc5b−/− × Scnn1b-Tg cross. Congenic C57BL/6N Muc5ac−/− and Muc5b−/− mice were generated by crossing heterozygous mice to inbred C57BL/6N mice (Taconic, Hudson, NY) for more than 12 generations. Mixed-strain or congenic mice were used as described in the text. At PND1 or 2, pups were toe clipped for identification and genotyping, as previously described.21 Mice studied were littermates, age-matched, and of both sexes. Congenic FVB/NJ mice were purchased from The Jackson Laboratory (Bar Harbor, ME).
Bronchoalveolar lavage (BAL), cell counts, analyses of soluble contents, and bacteriology BAL was performed in neonatal and adult mice as previously described.25 For microbiology studies, BAL was performed aseptically and colony-forming units (CFUs) were enumerated in serially plated dilutions (plated onto Columbia anaerobe sheep blood agar (Becton Dickinson, NJ) and incubated in a candle jar to facilitate the growth of microaerophilic bacteria at 37 °C for 24 h), as previously described.27 Mouse TNFα, KC, MIP-2, LIX, IL-6, IgG1, IgG2a, IgG2b, IgG3, IgA, and IgM were measured in cell-free BAL using a Luminex-based assay (EMD Millipore, Billerica, MA), according to the manufacturer’s instructions.
Lung histology Lungs were immersion-fixed in 10% neutral-buffered formalin to prevent dislodging of airway luminal contents. Paraffin-embedded sections were stained with hematoxylin and eosin and Alcian Blue-Periodic Acid Schiff staining, and lung pathology graded as previously described using a semiquantitative histology score25 or morphometry.24 Tissue blocks received a numerical code at time of embedding and scoring was performed by an investigator blinded to specimen genotype.
Agarose gel mucin western blot Secreted mucin quantification was carried out using a slight modification of the protocol described in Livraghi et al.25 BAL samples were solubilized by addition of urea to reach a 6 M concentration. Samples were reduced with 10 mM dithiothreitol for 90 min at 37 °C and alkylated with 25 mM iodoacetamide for 30 min at room temperature (RT) in the dark. Equal volumes of reduced samples (40 μl) were run on 1% agarose gel at 80 V for 90 min. Gels were vacuum-blotted onto nitrocellulose membranes with 4 × sodium citrate buffer for 2 h, blocked with Odyssey blocking buffer (OBB, Li-COR Biosciences, Lincoln, NE), and probed with rabbit polyclonal antibodies against Muc5b (UNC223, 1:2000 in OBB34) or Muc5ac (UNC294, 1:1000 in OBB+0.1% Tween-20,42). The secondary antibody was IRDye 680LT donkey anti-rabbit IgG (Li-COR Biosciences), diluted 1:15,000 in OBB. Detection and densitometry were performed using the Odyssey Infrared Imaging System (LI-COR Biosciences).
Mucociliary clearance (MCC) assay PND35 mice were anesthetized with 2–3% isoflurane and a small incision was made through the tracheal ventral wall. Using a fine-bore cannula, 200 nl of phosphate-buffered saline (PBS) containing a known number of fluorescent microspheres (3 μm Molecular Probes FluoSpheres, Nile Red, Invitrogen, Thermo Fisher Scientific, Rochester, NY) was deposited near the tracheal bifurcation. After the cannula was removed and the tracheostomy closed, the anesthetized mouse was allowed to breath spontaneously for 15 min. After this period, the mouse was killed, the lungs and trachea (up to the larynx) were removed and solubilized in KOH, and the beads left in the tissue were counted. MCC was determined as % of delivered beads that were cleared.
Immunofluorescence analysis of BALT Lungs were inflated with a 1:1 mixture of OCT:PBS, embedded in 100% OCT and sectioned. Slides were air dried, fixed in ice cold 100% acetone for 5 min, and washed in PBS. Blocking was performed in 5% normal goat serum (Jackson ImmunoResearch Laboratories, West Grove, PA), 1:50 Fc Block (BD Biosciences, San Jose, CA, rat anti-mouse CD16/CD22 clone 2.4G2), 0.1% Tween-20, and 0.1% Triton-X in PBS, for 30 min at RT. Primary antibodies and isotype controls (goat anti-mouse CD3-ɛ (M-20) Santa Cruz Biotechnology (Dallas, TX) and goat IgG Jackson ImmunoResearch 0.2 mg ml−1, dil. 1:100; rat anti-mouse CD45R/B220 and rat IgG2a, κ both from BD Biosciences, dil. 1:50) were diluted in PBS+0.1% Tween-20+0.1% Triton-X (PBS-TT) and incubated over night at 4 °C. Sections were washed in PBS+0.1% Tween-20 (PBST) and secondary antibodies (donkey anti-goat AlexaFluor 633 and donkey anti-rat AlexaFluor 594, Jackson ImmunoResearch, both at 1:200 dilution in PBSTT) were applied for 60 min at RT in the dark. After washing in PBST, slides were mounted with Vectashield Soft Mount media (Vector Laboratories, Burlingame, CA) containing DAPI for nuclear staining, and imaged by confocal microscopy, using a Leica SP2 microscope with an Apochromat × 40/1.25 NA oil immersion lens (Leica Microsystems, Buffalo Grove, IL).
Immunohistochemical localization of SC Lungs were immersion fixed in 10% neutral-buffered formalin for 24 h. Paraffin-embedded sections were incubated at 65 °C for 2–4 h, and deparaffinized with xylene (2 changes × 5’) and graded ethanol (100% 2 × 5’, 95% 1 × 5’, 70% 1 × 5’). After rehydration, antigen retrieval was performed by boiling the slides in 0.1 M sodium citrate pH 6.00 (3 cycles with microwave settings: 100% power for 6.5 min, 60% for 6 min, and 60% for 6 min, refilling the Coplin jars with deionized water after each cycle). After cooling and rinsing with dH2O, quenching of endogenous peroxidase was performed with 0.5% H2O2 in methanol for 15 min, slides were washed in PBS, and blocked with 5% normal donkey serum, 1:50 Fc block in PBS-T, for 1 h at RT. Primary antibodies and isotype control (goat anti-mouse pIgR R&D Systems (Minneapolis, MN) AF2800 and goat IgG Jackson ImmunoResearch 0.2 mg ml−1) were diluted in 5% normal donkey serum in PBST and incubated over night at 4 °C. Sections were washed in PBS and secondary antibody (biotinylated donkey anti-goat IgG, Jackson ImmunoResearch, at 1:200 dilution in 5% normal donkey serum in PBST) was applied for 30 min at RT. After washing in PBST, slides were incubated with avidin-peroxidase complex according to the manufacturer instruction (Vectastain kit, Vector laboratories), washed, incubated with the chromogenic substrate (Immpact Novared, Vector laboratories) and counterstained with Mayer hematoxylin. Coverslipped slides were imaged by transmitted light microscopy, using an Olympus BX60 microscope with an UPlanFLN 40x/0.75 NA lens.
Statistics Data are shown as means±s.e.m., with the number of mice (n). Survival curves were compared using Kaplan–Meier followed by Log-rank analysis (Mantel-Cox) with Bonferroni correction for multiple comparisons. Analysis of variance followed by Tukey’s post hoc test for multiple comparisons was used to determine significant differences among groups for body weight, BAL cell and CFUs counts, MCC, BAL mucins content, BALF cytokines and immunoglobulin content, histology scores, and morphometric analyses. P<0.05 was considered statistically significant.
References
Linden, S.K., Sutton, P., Karlsson, N.G., Korolik, V. & McGuckin, M.A. Mucins in the mucosal barrier to infection. Mucosal Immunol. 1, 183–197 (2008).
Anderson, W.H. et al. The relationship of mucus concentration (hydration) to mucus osmotic pressure and transport in chronic bronchitis. Am. J. Respir. Crit. Care Med. 192, 182–190 (2015).
Henderson, A.G. et al. Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J. Clin. Invest. 124, 3047–3060 (2014).
Button, B. et al. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science 337, 937–941 (2012).
Garcia, M.A., Yang, N. & Quinton, P.M. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J. Clin. Invest. 119, 2613–2622 (2009).
Gustafsson, J.K. et al. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J. Exp. Med. 209, 1263–1272 (2012).
Tang, X.X. et al. Acidic pH increases airway surface liquid viscosity in cystic fibrosis. J. Clin. Invest. 126, 879–891 (2016).
Shah, V.S. et al. Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science 351, 503–507 (2016).
Kesimer, M. et al. Molecular organization of the mucins and glycocalyx underlying mucus transport over mucosal surfaces of the airways. Mucosal Immunol. 6, 379–392 (2013).
Kesimer, M., Makhov, A.M., Griffith, J.D., Verdugo, P. & Sheehan, J.K. Unpacking a gel-forming mucin: a view of MUC5B organization after granular release. Am. J. Physiol. Lung Cell. Mol. Physiol. 298, L15–L22 (2010).
Kiwamoto, T. et al. Endogenous airway mucins carry glycans that bind Siglec-F and induce eosinophil apoptosis. J. Allergy Clin. Immunol. 135, 1329–1340, e1329 (2015).
McDole, J.R. et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 483, 345–349 (2012).
Shan, M. et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 342, 447–453 (2013).
Thornton, D.J. & Sheehan, J.K. From mucins to mucus: toward a more coherent understanding of this essential barrier. Proc. Am. Thorac. Soc. 1, 54–61 (2004).
Kirkham, S., Sheehan, J.K., Knight, D., Richardson, P.S. & Thornton, D.J. Heterogeneity of airways mucus: variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B. Biochem. J. 361, 537–546 (2002).
Thornton, D.J., Rousseau, K. & McGuckin, M.A. Structure and function of the polymeric mucins in airways mucus. Annu. Rev. Physiol. 70, 459–486 (2008).
Rose, M.C. & Voynow, J.A. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol. Rev. 86, 245–278 (2006).
Kirkham, S. et al. MUC5B is the major mucin in the gel phase of sputum in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 178, 1033–1039 (2008).
Roy, M.G. et al. Muc5b is required for airway defence. Nature 505, 412–416 (2014).
Evans, C.M. et al. The polymeric mucin Muc5ac is required for allergic airway hyperreactivity. Nat. Commun. 6, 6281 (2015).
Mall, M., Grubb, B.R., Harkema, J.R., O'Neal, W.K. & Boucher, R.C. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat. Med. 10, 487–493 (2004).
Livraghi-Butrico, A. et al. Genetically determined heterogeneity of lung disease in a mouse model of airway mucus obstruction. Physiol. Genomics 44, 470–484 (2012).
O’Neal, W.K. et al. Assessment of genetic modifiers for phenotypic severity of Scnn1b-transgenic mice. Pediatr Pulmunol 42 (Supplement 30), 266 (abstract #184) (2007).
Mall, M.A. et al. Development of chronic bronchitis and emphysema in beta-epithelial Na+ channel-overexpressing mice. Am. J. Respir. Crit. Care Med. 177, 730–742 (2008).
Livraghi, A. et al. Airway and lung pathology due to mucosal surface dehydration in {beta}-epithelial Na+ channel-overexpressing mice: role of TNF-{alpha} and IL-4R{alpha} signaling, influence of neonatal development, and limited efficacy of glucocorticoid treatment. J. Immunol. 182, 4357–4367 (2009).
Roy, M.G. et al. Mucin production during prenatal and postnatal murine lung development. Am. J. Respir. Cell. Mol. Biol. 44, 755–760 (2011).
Livraghi-Butrico, A. et al. Mucus clearance, MyD88-dependent and MyD88-independent immunity modulate lung susceptibility to spontaneous bacterial infection and inflammation. Mucosal Immunol. 5, 397–408 (2012).
Hasnain, S.Z. et al. Muc5ac: a critical component mediating the rejection of enteric nematodes. J. Exp. Med. 208, 893–900 (2011).
Foo, S.Y. & Phipps, S. Regulation of inducible BALT formation and contribution to immunity and pathology. Mucosal Immunol. 3, 537–544 (2010).
Phalipon, A. et al. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity 17, 107–115 (2002).
Gilley, S.K. et al. Deletion of airway cilia results in noninflammatory bronchiectasis and hyperreactive airways. Am. J. Physiol. Lung Cell. Mol. Physiol. 306, L162–L169 (2014).
Livraghi, A. & Randell, S.H. Cystic fibrosis and other respiratory diseases of impaired mucus clearance. Toxicol. Pathol. 35, 116–129 (2007).
Ostrowski, L.E. et al. Conditional deletion of dnaic1 in a murine model of primary ciliary dyskinesia causes chronic rhinosinusitis. Am. J. Respir. Cell. Mol. Biol. 43, 55–63 (2010).
Zhu, Y. et al. Munc13-2−/− baseline secretion defect reveals source of oligomeric mucins in mouse airways. J. Physiol. 586, 1977–1992 (2008).
Rajavelu, P. et al. Airway epithelial SPDEF integrates goblet cell differentiation and pulmonary Th2 inflammation. J. Clin. Invest. 125, 2021–2031 (2015).
Contreras-Ruiz, L. & Masli, S. Immunomodulatory cross-talk between conjunctival goblet cells and dendritic cells. PLoS One 10, e0120284 (2015).
Knoop, K.A., McDonald, K.G., McCrate, S., McDole, J.R. & Newberry, R.D. Microbial sensing by goblet cells controls immune surveillance of luminal antigens in the colon. Mucosal Immunol. 8, 198–210 (2015).
Kesimer, M. et al. Tracheobronchial air-liquid interface cell culture: a model for innate mucosal defense of the upper airways? Am. J. Physiol. Lung Cell. Mol. Physiol. 296, L92–L100 (2009).
Radicioni, G. et al. The innate immune properties of airway mucosal surfaces are regulated by dynamic interactions between mucins and interacting proteins: the mucin interactome. Mucosal Immunol. (e-pub ahead of print 13 April 2016).
Corthesy, B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front. Immunol. 4, 185 (2013).
Lee, S.H. et al. Increased expression of vascular endothelial growth factor and hypoxia inducible factor-1alpha in lung tissue of patients with chronic bronchitis. Clin. Biochem. 47, 552–559 (2014).
Ehre, C. et al. Overexpressing mouse model demonstrates the protective role of Muc5ac in the lungs. Proc. Natl Acad. Sci. USA 109, 16528–16533 (2012).
Acknowledgements
We thank Troy Rogers for assistance with mucociliary clearance studies; E. Jane Kelly and Danielle L. Waxer for technical assistance with mouse breeding, phenotyping, and morphometric analyses; Dr Michael Chua for assistance with imaging; Carlton W. Anderson of the UNC CGIBD’s Advanced Analytics Core for performing the Multiplex assay; and Alexandra C. Infanzon for editorial assistance.
This work was funded by the Cystic Fibrosis Research Development Program grant RDP CFF R026-CR11 to WKO and the CFF RDP BOUCHE15R0 to RCB, the National Institute of Health (NIH) P30-DK065988, P50-HL060280, P50-HL084934, P50-HL107168, P01-HL108808, P01-HL110873 and UH2-HL123645 (to RCB). The UNC CGIBD's Advanced Analytics Core is supported by NIH grant P30 DK34987.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Drs Boucher, Grubb, and O’Neal are inventors in US Patent US 7,772,458 B2 for the Scnn1b-Tg mouse model. All other authors have no conflict of interest to disclose.
Additional information
Author contributions
A.L.-B., W.K.O., and R.C.B. designed the study; K.J.W. and A.S.V. maintained mouse colonies, generated experimental animals, and collected body weight and survival data; A.L.B. and K.J.W. performed phenotyping experiments involving BAL analyses (differential cell counts, microbiology, etc.) and tissue harvest; K.A.B. provided histological specimens; A.L.-B. performed mucin agarose western blots, histopathological, immunohistochemistry/immunofluorescence, and morphometric analyses; B.R.G. performed and analyzed the data for the MCC assay; C.M.E., provided Muc5ac−/− and Muc5b−/− mice and reviewed the manuscript; A.L.-B. analyzed the rest of the data, and wrote the manuscript. B.R.G., W.K.O., and R.C.B. contributed to interpretation of the data and reviewed the manuscript.
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Supplementary information
Rights and permissions
About this article
Cite this article
Livraghi-Butrico, A., Grubb, B., Wilkinson, K. et al. Contribution of mucus concentration and secreted mucins Muc5ac and Muc5b to the pathogenesis of muco-obstructive lung disease. Mucosal Immunol 10, 395–407 (2017). https://doi.org/10.1038/mi.2016.63
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2016.63
This article is cited by
-
MUC5B regulates goblet cell differentiation and reduces inflammation in a murine COPD model
Respiratory Research (2022)
-
Engineering nano-drug biointerface to overcome biological barriers toward precision drug delivery
Journal of Nanobiotechnology (2022)
-
Lung macrophages drive mucus production and steroid-resistant inflammation in chronic bronchitis
Respiratory Research (2021)
-
Neutrophil extracellular traps are present in the airways of ENaC-overexpressing mice with cystic fibrosis-like lung disease
BMC Immunology (2021)
-
Engineering precision nanoparticles for drug delivery
Nature Reviews Drug Discovery (2021)