Abstract
A hallmark of atopic asthma is development of chronic airways hyper-responsiveness (AHR) that persists in the face of ongoing exposure to perennial aeroallergens. We investigated underlying mechanisms in sensitized rats focusing on a strain expressing the high-allergen–responder phenotype characteristic of human atopic asthmatics, and find that their high susceptibility to aeroallergen-induced persistent AHR is associated with deficiencies in the immunoregulatory and mucosal trafficking properties of inducible T-regulatory cells (iTregs). Counterintuitively, AHR susceptibility was inversely related to aeroallergen exposure level, high exposures conferring protection. We demonstrate that underlying this AHR-susceptible phenotype is reduced capacity of airway mucosal dendritic cells (AMDCs) for allergen sampling in vivo; this defect is microenvironmentally acquired, as allergen uptake by these cells in vitro is normal. Moreover, intranasal transfer of in vitro aeroallergen-loaded AMDC from naïve animals into AHR-susceptible animals during prolonged aerosol challenge markedly boosts subsequent accumulation of iTregs in the airway mucosa and rapidly resolves their chronic AHR, suggesting that compromised antigen surveillance by AMDC resulting in defective functional programming of iTreg may be causally related to AHR susceptibility.
Similar content being viewed by others
Introduction
Asthma is a chronic inflammatory disease affecting mainly small/central airways.1 In the most common form of this disease, allergic/atopic asthma, the underlying mechanisms involve exaggerated immune responses to aeroallergens, including a major T-helper type 2 (Th2)-associated component.2, 3, 4, 5 However, <25% of sensitized subjects develop significant asthma,6 suggesting that mechanisms downstream of allergic sensitization, presumably involving control of expression of Th2 immunity in airway tissue, play a central role in the disease.
In this context, an increasingly important focus of recent studies on asthma pathogenesis has been the endogenous control mechanisms that modulate immune inflammatory processes in the airway mucosa.7, 8, 9, 10 Of particular interest are T-regulatory (Treg) populations that control T-effector responses, and deficiencies in capacity to generate mucosal-homing Tregs are widely believed to be important determinants of asthma risk.11, 12, 13 The potential relevance of these cells as drug targets is supported by indirect evidence from a variety of association studies demonstrating induction of Treg during various treatment protocols that attenuate asthmatic responses, but objective proof-of-concept data with linked mechanistic evidence is lacking, and this represents the focus of the present investigation.
Maintaining the normal balance between T-effector and Treg populations at airway mucosal surfaces is critically dependent on efficient immune surveillance by local airway mucosal dendritic cell (AMDC) subpopulations,2, 14, 15 and hence in this study, we have focused directly upon the mechanisms by which these cells control Treg activity in the airways.
Results
Atopic asthma manifestations in BN rats
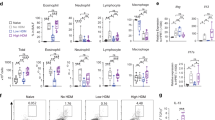
We have developed an animal model that mimics essential features of chronic atopic asthma in humans sensitized to perennial allergens, which are present in the environment on a daily basis, focusing on the BN rat strain that are hypersusceptible to allergic airways disease. Notably, compared with sensitized PVG rats (Figure 1a, b, left panels), in which daily aeroallergen exposure provokes a transient self-resolving cycle of moderate lung eosinophilia and airways hyper-responsiveness (AHR), corresponding responses in BN rats (Figure 1a, b, right panels) are more intense and persist over at least 14 consecutive exposure days. We previously demonstrated that after withdrawal of allergen, methacholine responsiveness in PVG rats rapidly normalizes, and reinstituting exposure reactivates another transient self-limiting cycle of AHR expression,10 reflecting the repeated cycles of disease exacerbation seen in human atopic asthmatics. In contrast, return to baseline was not achieved in BN rats under these conditions, and after 2 exposure-free weeks, they still expressed significant AHR (Supplementary Figure 1a). Moreover, resumption of exposure at this time resulted in accelerated/intensified exacerbation of AHR relative to their first exposure cycle (Supplementary Figure 1a). Similar to AHR, the eosinophilic response also followed a cyclic pattern reflecting antigen exposure/withdrawal (Supplementary Figure 1b).
Airway responses of sensitized and aeroallergen-challenged rats. Data are shown as box plots (mean, interquartile range, and minimum/maximum values) for Piebald Virol Glaxo (PVG) (open bars) and Brown Norway (BN) (shaded bars) for (a) airway resistance (concentration of methacholine that increased Raw by 200% (EC200Raw)) for normal untouched control rats (NC) or sensitized and exposed to a cycle of daily ovalbumin (OVA) aerosols as indicated (1 or 6 exposures); n>10 rats per group. (b) Total eosinophils in bronchoalveolar lavage (BAL) of rats as per panel a; n⩾8 rats per group. Data were collected from at least two independent experiments. ND, not determined. *BN vs. PVG, P<0.01.
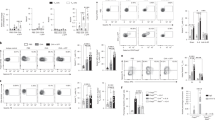
We next investigated the T-cell response in the airway mucosa following aeroallergen exposure of normal control (NC) or sensitized animals. The total number of T cells found in tracheal digests was not significantly different in BN compared with PVG rats (Figure 2a). Further experiments then focused on determining how T-effector and Treg subpopulations contributed to the patterns of disease expression in these rat strains. As illustrated in Supplementary Figure 2, expression of Foxp3 and CD25 within the CD4+ population are closely linked, indicating that Tregs (Foxp3hi) and T-helper/effectors partition principally to CD25hi and CD25lo/neg subsets, respectively. To enumerate Tregs in tissue digest preparations, we gated on Foxp3hi (Supplementary Figure 2c showing representative fluorescence-activated cell sorting plots for tracheal digest cells). To address questions related to Treg functions, we sorted according to the level of CD25 expression as illustrated in Supplementary Figure 3, using isolated CD4+CD25hi cells (Supplementary Figures 2 and 3) as an enriched source of Foxp3+ Treg cells for functional assays. Employing these criteria, we then determined if the differences in the AHR and eosinophilia responses of PVG and BN rats were associated with underlying differences in the respective Treg population response profiles. In our model, we focused on the time point (following six daily exposures) at which disease expression was resolved in PVG but is ongoing in BN rats to determine underlying differences between the two strains. Total cell numbers per tracheal digest did not differ between strains (data not shown). Enumeration of CD25 expression on T cells in tracheal digests demonstrated that while the frequency of total T cells was similar between PVG and BN rats (Figure 2a), there was significantly more CD25hi Treg in PVG (Figure 2b). In clear support of this is the finding that the number of Foxp3 Treg in the airway mucosa of BN rats during ongoing challenge accumulates at a considerably reduced rate relative to PVG (Figure 2c). Of note, Foxp3hi expression clearly localizes to CD25hi cells, and gene expression levels are comparable in the two strains (Figure 2d). Overall, a significant numerical imbalance between Treg (CD3+CD4+CD25hi) and T-effector (CD3+CD4+CD25lo/neg) populations was seen in BN, indicative of a marked deficiency in Tregs in this tissue (Figure 2e, f).
Aeroallergen-induced airway mucosal T-cell infiltration. (a) Data were shown as box plots for percent T-cell receptor (TCR)-expressing cells in tracheal digests in Piebald Virol Glaxo (PVG) (open bars) and Brown Norway (BN) (filled bars) rats. (b) Ratio of CD25hi and CD25low cells among total CD4 expressing tracheal digest cells in sensitized PVG (open bars) and BN (filled bars) rats following six daily ovalbumin (OVA) aerosol challenges. (c) Foxp3+CD4+TCR+ cells in tracheal digest preparations from sensitized PVG (open circles) and BN (filled circles) rats post aerosol exposure; data represent pooled group means (n=5 animals pooled per group for each experiment) performed on at least two independent occasions. (d) Foxp3 gene expression levels determined by quantitative reverse transcription-PCR (qRT-PCR) in cells sorted on the basis of the level of CD25 expression (neg, low, or hi) from airway draining lymph node (ADLN) of sensitized PVG and BN rats following six daily OVA challenges. (e) Ratio of tracheal digest cells that are Foxp3+ among total CD25+ cells in PVG and BN rats following six daily OVA aerosol exposures. Data were derived from pools of animals (n=5 per group analysed on five independent occasions). (f) Ratio of CD25hi among total CD25 expressing tracheal digest cells as per panel e.
The numerical insufficiency of Treg in the airways of BN rats was not associated with a corresponding deficiency in the airway draining lymph node (ADLN) Treg compartment (Figure 3a). Moreover, the magnitude of the overall T-cell response in BNs was significantly increased (Figure 3b), but importantly the ratio of Foxp3 to CD25 total cells was similar between strains (Figure 3c), unlike the situation described above in the airway mucosa (Figure 2). Next, we examined Treg functions (using sorted CD25hi cells) and found several deficiencies associated with functionality in BN Treg populations induced in the ADLN. These included reduced ability to suppress in vitro antigen-specific T-cell proliferation (Figure 3d) and diminished expression of genes known to be associated with immunoregulatory functions, notably IL -10 (Figure 3e),11, 13, 16 LAG-3 (Figure 3f),17 and TIM-2 (Figure 3g),18, 19 all of which are markedly attenuated in BN Tregs. It is relevant to note that while it was not possible to simultaneously evaluate exact numbers of Foxp3hi cells in the CD25hi sorted populations used for the in vitro suppression assays, we did determine in independent experiments that the CD25hi fraction from PVG and BN rats contain a similar frequency of Foxp3hi-expressing cells (Supplementary Figure 4).
Airway draining lymph node (ADLN) response and analysis of function-associated markers in ADLN CD25+ cells. Open vs. filled circles/bars show data from Piebald Virol Glaxo (PVG) and Brown Norway (BN) rats, respectively. (a) ADLN percent Foxp3+ cells in sensitized rats following ovalbumin (OVA) challenge as indicated (0, 1, or 6). (b) ADLN total mononuclear cells in sensitized rats following OVA challenge as per panel a. (c) Ratio of total Foxp3+/total CD25+ cells in ADLN of sensitized rats following six daily OVA aerosols. (d) Suppression of in vitro OVA-specific CD4+ T-cell proliferation by CD4+CD25hi cells sorted from ADLN of sensitized PVG and BN rats following six daily OVA aerosol exposures. Data were derived from individual replicate experiments conducted on independent occasions using n=5 animals pooled per group for each series, analyzed in triplicate. (e) IL-10 gene expression levels determined by quantitative reverse transcription-PCR (qRT-PCR) in cells sorted on the basis of the level of CD25 expression (neg, low, or hi) from ADLN of sensitized PVG and BN rats following six daily OVA challenges. (f) Expression levels of LAG-3 as per panel e. (g) Expression of TIM-2 gene as per panel e, determined by microarray analysis. (h, i) Expression levels of CCR4 and CCR8 genes, respectively, as per panel e. Data were shown as box plots derived from experiments using n=5 rats pooled per group on at least three independent occasions. Treg, T-regulatory cell; ND, not determined.
A pertinent question arising from these data is whether the markedly diminished Treg frequency in the airway mucosa of BN rats, while being normal or slightly elevated in ADLN, is attributable to selective dysregulation of mucosal trafficking functions of Tregs. Expression of chemokine receptors on Treg, in particular CCR4 and CCR8, which have been associated with airways homing of Th2 cells5, 20 and Tregs,21, 22, 23 both of which respond to their respective ligands CCL17/CCL22,5, 20, 22, 24 was not deficient in ADLN Treg (Figure 3h,i for CCR4 and CCR8, respectively) from sensitized six times challenged BN compared with PVG rats. In addition, in these same animals, expression of S1PR1, which is thought to be essential for egress of lymphocytes from LNs,25, 26 was not deficient compared with levels found in sufficient PVG CD25hi Treg cells (data not shown). These findings collectively suggest that the imbalance between Treg and T-helper/effector populations recruited into BN airway mucosal tissues following aeroallergen challenge is unlikely to be a reflection exclusively of reduced mucosal homing of Tregs. In line with the skewed numerical ratio in favor of CD4+CD25lo T effectors in BN airways, we also found significant elevation in the expression of CCR4 in these cells compared with PVG (Figure 3h).
Accompanying changes in AMDC subpopulations
The major subpopulations of DC in rats comprise myeloid DC (mDC) expressing major histocompatibility complex (MHC) Class II, CD11b, CD11c, CD103, and CD172, which are CD4+(majority) or CD4neg, together with plasmacytoid DC (pDC) that are MHC Class II+(low), CD11b−, CD103−, CD172+, and CD4+27, 28, 29 (Supplementary Figure 5). These populations are both found in resting airways tissue; however, pDC represent only a very minor population of total AMDC in both rat strains. As demonstrated in Figure 4a, aerosol exposure triggers a large expansion of the overall AMDC population comprising predominantly mDC in both rat strains. While the pDC population was also expanded following multiple challenges, they remained a very minor component of the response in PVG and BN rats. Further experiments were performed concentrating on the mAMDC population.
Airway mucosal dendritic cell (AMDC) responses to aeroallergen challenge. (a) Total number of myeloid AMDC (mAMDC) (open bars) and plasmacytoid AMDC (pAMDC) (filled bars) in normal untouched control (NC) Piebald Virol Glaxo (PVG) and Brown Norway (BN) rats or following sensitization and a cycle of daily exposure to ovalbumin (OVA) aerosol (0, 1, or 6 exposures) as indicated; *P<0.05 for pAMDC total number in airway mucosa vs. NC rats. No significant differences was found in mAMDC subset frequency between strains. (b) Percentage of tracheal digest mAMDC expressing CD86 in rats as per panel a; mean fluorescence intensity (MFI) of CD86+ cells did not differ significantly between relevant groups. Data were derived using n=5 rats pooled per group on three independent occasions.
This expanded mAMDC population displayed rapid CD86 upregulation following aerosol challenge, which persists in BNs (Figure 4b and Supplementary Figure 6) with continuous exposure while resolving in PVGs, mirroring respective patterns of airway eosinophilia and AHR (Figure 1a, b). The Tregs induced in sensitized animals via aerosol challenge have been shown previously in this and other models10, 30 to attenuate airway responses via inhibition of CD86 upregulation on AMDC. The persistent nature of mAMDC activation observed here in BN rats during ongoing aeroallergen exposure is potentially explicable by the reduced numbers of Treg in airway tissues.
Immune surveillance functions of AMDC in Treg-insufficient BN rats
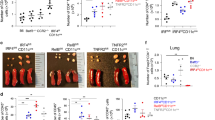
The antigen sampling activities of AMDC are central to the regulation of adaptive immunity to inhaled antigens,2, 15 and antigen dose has been associated with the differential induction of immunity. Similarly, sampling efficiency of AMDC subsets may influence overall responsiveness to aeroallergen challenge, and to investigate this possibility, we directly compared the process in the two strains. Initial analysis of the antigen uptake capacity of mAMDC from the two strains demonstrated comparable endocytic activity in vitro on the basis of percent ovalbumin (OVA)-positive mAMDC (Figure 5a) or mean fluorescence intensity (not shown). Confocal imaging of whole trachea was used to visualize in situ uptake of fluorescent-tagged OVA by AMDC at 2 h following intranasal delivery into the airways. Figure 5b, c shows en face and cross-sectional projections of MHC Class II+(red) AMDC located in the airway epithelium (green) and extending dendrites into the luminal space (endocytosed OVA in AMDC is pink). Figure 5d is a confocal image of AMDC as per Figure 5b, c, modified by surface rendering software techniques to enable clear visualization of OVA (blue) within AMDC. Qualitatively, while uptake was easily observed in PVG rats via confocal imaging, it was more difficult to visualize in BN tissue samples. Quantitative data derived via flow cytometric analysis of cell digest preparations (Figure 5e) following same exposure protocols revealed that in stark contrast to in vitro uptake, mAMDC subsets from BN rats have markedly reduced capacity for antigen uptake/processing in situ compared with PVGs. These data clearly suggest that the expression of the attenuated functional phenotype in AMDC from BN rats is dependent on microenvironmental factors operative in vivo. As shown in Figure 5f, the attenuated uptake of antigen in situ by BN mAMDC is mirrored in their reduced capacity to present proliferation-inducing signals to T-memory cells upon in vitro culture of isolated AMDC 2 h post-intranasal exposure. In contrast, and further supporting a role for microenvironmental modulation of antigen uptake, the ex vivo supply of antigen to same AMDC preparations in parallel cultures (Figure 5f) resulted in a comparable ability to induce memory T-cell proliferation. Taken together, these data indicate that the functional capacity of mAMDC from both strains is not intrinsically different; however, specific microenvironmental factors operating within the airway mucosa of BN rats that develop chronic allergic asthma act to modify AMDC function (reduce antigen uptake capacity). It is relevant to note that antigen uptake in this model by airway pDC remained consistently low and <10% of the pDC population become OVA+ following intranasal delivery of fluorescently tagged OVA (i.e., <0.5% of total AMDC, data not shown).
Deficiency in dendritic cell (DC) function in the airway mucosa of Brown Norway (BN) rats. (a) Percentage of total airway mucosal dendritic cell (AMDC) in tracheal digests freshly isolated from non-sensitized and non-exposed rats that acquire DQ-ovalbumin (OVA) added at increasing concentration as shown during a 2 h in vitro culture. (b) Photomicrograph of Piebald Virol Glaxo (PVG) rat trachea showing immunofluorescence staining for major histocompatibility complex (MHC) Class II (red), cytokeratin (green), and OVA-Alexa647 (blue) delivered by per nasal inoculation 2 h previously, as detailed in Methods section. The image is a projection along the z axis (“top view”) from a stack of optical sections acquired at 1-μm increments. (c) Side view reconstruction as per panel b. (d) Reconstructed image after surface rendering. Images clearly show projection of DC cellular extensions to the apical surface and internalization of OVA-Alexa647. (e) Percentage of total AMDC in tracheal digests from non-sensitized and non-exposed rats isolated 2 h post-intranasal delivery of OVA-Alexa647 that capture OVA-Alexa647 in situ. (f) In vitro OVA-specific CD4+T-cell proliferation induced by myeloid AMDC (mAMDC) sorted from tracheal digests from PVG (open bars) and BN (filled bars) rats 2 h after OVA exposure in vivo. Data show proliferation induced by in situ OVA uptake and following further addition of OVA ex vivo to the cultures. Data were derived from individual replicate experiments conducted on at least three independent occasions using n=5 animals pooled per group for each series, analyzed in triplicate; *P<0.01 vs. other responses.
Functional consequences of attenuated antigen sampling by AMDC
We questioned the hypothesis that the hyper-responsiveness of BN rats to aeroallergen challenge was directly related to inefficient delivery of antigen from the airway mucosa to the immune system by firstly varying antigen exposure doses. We chose a regimen that induced intense/sustained AHR in BN rats (60-min exposure daily/1% OVA aerosol/3 days) and demonstrated that if the OVA dose was increased 10 times the AHR resolved by day 3 (Figure 6a). We then focused more directly on AMDC delivery of antigen, utilizing in vitro AMDC loading as per Figure 5a to bypass the in vivo attenuation in antigen uptake in this strain. For these experiments, sensitized BN rats with ongoing AHR induced by five daily aerosols were treated with either (i) saline or (ii) a single dose of freshly isolated mAMDC (sorted based on phenotypic expression of specific cell surface markers as illustrated in Supplementary Figure 5) from airways of naïve control rats and optimally loaded in vitro with OVA or (iii) mAMDC exposed to phosphate-buffered saline only (i.e., not loaded with OVA). The mAMDCs were administered directly into the airways using a protocol employed elsewhere for delivery of DC-associated immunogen to ADLN;31 12–18 h later, they were then given a sixth aerosol exposure and AHR assessed 24 h thereafter. As shown in Figure 6b, only the recipients of allergen-loaded mAMDC displayed rapid resolution of AHR, and this was associated with influx of Tregs in their airway mucosae (Figures 6c, d). Finally, we demonstrate that adoptive transfer of a bolus of ADLN-derived Tregs into BNs with ongoing AHR results in rapid resolution of their AHR (Figure 6e), likely via boosting Treg numbers at the site of inflammation.
Reversal of susceptibility to chronic disease. Data shown are from experiments relating to the Brown Norway (BN) strain. (a) Airway resistance (concentration of methacholine that increased Raw by 200% (EC200raw)) relative to normal untouched control (NC) and in sensitized/ovalbumin (OVA) aerosol-challenged rats at OVA dose as indicated (n>6 rats per group) after three daily challenges. (b) Relative airway resistance in sensitized/OVA-challenged rats after six daily exposures, with intranasal administration of saline, ex vivo in vitro OVA-loaded, or -unloaded airway mucosal dendritic cell (AMDC) (1 × 105) after fifth aerosol exposure (n>8 rats per group, data collected over at least two independent occasions). (c) Foxp3+ cells in tracheal digest preparations from rats equivalent to test groups in panel b above (data are mean±s.e.m. from individual animals collected over two independent occasions; n=6 rats per group). (d) Foxp3+ cells as percent CD4+ cells in tracheal digest preparations from groups as per panel c. (e) Relative airway resistance in sensitized/OVA aerosol-challenged rats after six daily aerosols, after nil additional treatment, or intravenous (i.v.) administration of 1 × 106 CD4+CD25− or CD4+CD25hi cells sorted from airway draining lymph node (ADLN) of sensitized/ × 6 OVA aerosol-exposed donors.
Discussion
The findings reported here are consistent with a general model,10, 11, 30 in which the intensity/duration of immunoinflammatory responses underlying airway eosinophilia/AHR associated with allergic asthma is linked to the activity of mucosal-homing Tregs that control local activation of Th2-memory cells via attenuating AMDC functions. The stark variation in the efficiency of this process between BN and PVG rat strains that develop persistent vs. resolving eosinophilia/AHR, respectively, provides unique opportunities to probe the mechanisms underlying these genetically determined differences in asthma-associated clinical phenotypes, which mirror the symptom spectrum observed among atopic humans.
We10, 32 and others33, 34 have demonstrated previously that the response to aeroallergen is initiated by migration of allergen-bearing AMDC to ADLN, and the ensuing activation/differentiation of T-helper/effector and Treg populations and their trafficking back to the airway mucosa are crucial in the outcome of the response. These studies revealed that while the respective ratios of CD4+CD25hiFoxp3+Tregs:CD4+CD25loFoxp3-T-helper/effectors are equivalent in ADLN of PVG vs. BN rats, this pattern is not mirrored in airway mucosae. AHR-susceptible BN rats show a T-cell influx into the airways that is significantly skewed towards T helper/effectors during ongoing aerosol challenge. The “balance of power” between T helper/effectors and Tregs has been suggested to depend on this ratio,35 and this relative numerical deficiency in Tregs in BN airway tissue may explain their AHR-susceptibility phenotype. In addition, and similar to findings in human allergic asthmatics,8, 11 Tregs from the BNs were also less potent on a per cell basis in suppressing T-cell activation, and this is reflected by diminished expression of IL-10,16 LAG3,17 and TIM-218, 19 genes, which have been associated with Treg function.
This relative imbalance within the BN airway mucosal T-cell population is suggestive of impaired Treg trafficking. However, analysis of expression of CCR4 and CCR8, both associated with selective migration of Treg and T-helper/effector cells, demonstrated comparable expression on Tregs from the two strains. In contrast, T helper/effectors from BN exhibited significantly enhanced expression of both chemokine receptors, consistent with their increased trafficking to the airway mucosa relative to Tregs.
With regard to the potential underlying mechanisms: DCs are recognized to play a central role in determining the balance between Treg and T-helper/effector populations in mucosal immune responses,2, 15, 36 and in controlling the trafficking of the T-cells involved,1, 5, 37 and hence we reasoned that AMDC represent plausible candidates for the site of the principal defect underlying the AHR-susceptible phenotype. In addressing this possibility, we focused on the primary function of these cells, which is surveillance of the mucosal surface for incoming antigen. This focus was driven by our earlier findings in mouse and rat models that the generation of tolerance to inhaled allergen, which is AMDC-dependent and Treg-mediated,15, 38, 39, 40 required log-fold higher levels of exposure to OVA41 or dust mite allergen42 in animal strains exemplified by BNs that are hypersusceptible to allergic disease.
Our finding that in situ aeroallergen uptake by AMDC from BN rats was constitutively attenuated suggests suboptimal transmission of immunogenic signals to T cells locally and in the ADLN. Moreover, our demonstration that rapid resolution of persistent AHR in these animals can be achieved via manipulation of antigen exposure/delivery argues that this defect in AMDC surveillance functions is causally related to their AHR-susceptible phenotype. The strength of TCR signal during T-cell activation is recognized to play an important role in determining outcomes of T-cell differentiation following antigen presentation by antigen-presenting cells.43 Notably, antigen dose can also influence Th1/Th2 differentiation.44 We argue based on these findings that such outcomes include functional and trafficking properties relevant to mucosal inflammation.
These results from this rat model appear at variance with findings from mice. Firstly, two recent studies have reported that the acute triggering of experimental asthma in sensitized mice is favored by enhanced allergen capture and processing by antigen-presenting cells, in particular mDC;36, 45 i.e., increased antigen uptake (dose) is apparently associated with increased risk of AHR. However, these studies investigated only the sequelae of acute aeroallergen exposure and did not examine events following prolonged/repeated exposure as occurs in humans, and which is the defining feature of this model. It is pertinent to note in this regard that the high AHR-susceptibility phenotype does not manifest unless aeroallergen exposure persists (Figures 1a, b and 4b), and longer exposures of sensitized mice may reveal similar patterns. In the context of our current findings, it is of interest to note that mouse models commonly utilized to study chronic airways inflammation have been developed on the principle of using low antigen doses, e.g., ref. 46. Furthermore, prolonged antigen exposure using particle-based vaccination has been shown to prevent allergic responses in sensitized mice.47
The most prominent difference between the species appears to be the relative contribution of pDC to baseline and post-challenge AMDC populations. Several mouse studies have reported that triggering of Th2-memory cells by aeroallergen is mediated by mDC,14, 36, 48, 49 whereas a parallel (and numerically comparable) pDC response, including enhanced trafficking of antigen-bearing pDC to ADLN, induces Tregs that attenuate airway inflammation.36 In contrast, the corresponding response in sensitized rats is dominated by mAMDC, and we find no evidence of significant aeroallergen uptake by the minor pDC subpopulation. It is important to note that rat pDC from other tissues have been demonstrated to be functionally capable of inducing Treg cells.50
It is pertinent in this regard to note relevant reports from human systems. In particular, resting DC populations in the nasal and bronchial mucosae in atopics are typically >90% mDC.32, 51, 52 Moreover, aeroallergen challenge of atopic asthmatics elicits rapid recruitment of DC into the bronchial mucosa that is largely restricted to mDCs.51 However, pDC do increase in numbers in the nasal mucosa of individuals with rhinitis following several days of allergen exposure,32 and in the sputum of atopic asthmatics 24 h following bronchial challenge,53 suggesting that the kinetics and/or trafficking patterns of this subset may differ from mDCs. It remains plausible that both DC subsets play significant roles in the regulation of immunological homeostasis in the airways, but it is unlikely that their roles are as strictly compartmentalized as suggested by current mouse models.
An important unresolved issue concerns the mechanism(s) underlying attenuation of the antigen-sampling properties of AMDC in AHR-susceptible animals, and the applicability of these findings to human asthmatics. Comparable inverse relationships have been observed between aeroallergen exposure and symptomatic respiratory allergy in humans,54, 55, 56 indicating that the overall phenomenon is not species-restricted. These mechanisms may also be relevant to immunotherapy for allergic disease targeted at expansion of specific Treg populations via repeated mucosal exposure to allergens.13, 57 Current treatments based on this approach exemplified by sublingual immunotherapy have proven clinical efficacy, but modest effect size,57 and these findings suggest that improved delivery systems aimed at increasing allergen loading of mucosal mDC may be a valid approach towards optimization of these therapies.
Methods
Animals, treatments, and allergen exposures. The 8- to 12-week-old specified-pathogen-free PVG and BN rats were used. All experimental protocols were given approval by our Institutional Animal Ethics Committee. OVA sensitization was performed by intraperitoneal inoculation of 100 μg OVA/200 μl aluminum hydroxide and rats utilized for challenge experiments 14–21 days later. Aerosol challenge10, 58 was carried out over a 60-min period using 1% OVA in phosphate-buffered saline. Some animals (Figure 1 and Supplementary Figures 1 and 2) were exposed to two cycles of antigen exposure; the first cycle consisted of six daily aerosols, and animals were then held free from exposure for 14 days before re-exposure to a second cycle of aeroallergen as detailed.
Media and reagents. Culture medium and isolation reagents including monoclonal antibodies and immunostaining reagents are as reported previously.10, 58 Briefly, monoclonal antibodies directed against cell surface antigens to identify T cells and dendritic cells included anti-CD3, TCRa/b, CD4, CD25, CD11b/c, CD45R, CD172, MHC Class II, and CD103, and were purchased from BD Pharmingen (Western Australia, Australia). For intracellular staining of Foxp3, an anti-mouse/rat Foxp3FLR staining kit from eBioscience (San Diego, CA) was used. Data were acquired on an LSRII flow cytometer (BD Biosciences, San Jose, CA) and analyzed using the Flowjo software (version 8.8.6; Tree Star Inc., Stanford, CA). OVA (grade V essentially lipopolysaccharide-free), collagenase type IV, and DNase were purchased from Sigma (St Louis, MO).
Cell preparations. Collagenase digests of lymph nodes and tracheal tissue were prepared by standard methods to obtain single-cell suspensions and when required were depleted of macrophages and B cells as described previously.10, 58 Purification of cell populations was by standard Dynabead depletion, MACS bead enrichment, cell sorting, or combinations of these techniques. BALF cells were obtained by standard methods 24 h following the last aeroallergen challenge and stained using DiffQuik (Lab Aids, Narrabeen, NSW, Australia) for differential counting. Cell culture methodology was as described earlier.10, 58 Unless stated otherwise, all other tissue samples were harvested 2 h following final aerosol exposure.
Antigen uptake. For in situ uptake studies, 50 μg of OVA-Alexa647 was instilled in 75 μl intranasally. Trachea were harvested 2 h after exposure; whole tissue mounts were stained and analyzed by confocal microscopy or single-cell digest preparations were stained as above and analyzed via flow cytometry. Determination of in vitro antigen uptake was by short-term culture of freshly isolated tracheal digest preparations at 37 °C (vs. 4 °C controls) with OVA-Alexa647 or DQ-OVA (at 0, 1, 10, 100, 1,000, and 10,000 ng ml−1) for 2 h. Cells were then stained for flow cytometric analysis as above.
Transfer experiments. For AMDC transfers, total mAMDC (MHC Class IIhi, CD11b/c+, refer to Supplementary Figure 5) sorted from naïve control (NC) rats were placed in short-term culture (as per in vitro antigen uptake experiments above) in the presence or absence of OVA (50 μg ml−1) for 2 h before per nasal delivery at 105 phosphate-buffered saline or OVA-loaded AMDC per animal. For Treg transfers, 106 CD4+CD25hi or CD4+CD25− cells sorted from ADLN of sensitized/aerosol-exposed animals were injected intravenously into sensitized recipients that were exposed to five daily OVA aerosols previously. Recipients were exposed to a sixth OVA aerosol the following day and utilized for experiments 24 h later. Each experiment included AHR measurements on groups of naïve controls and sensitized/ × 6-exposed animals to enable direct comparisons. Data were collected over at least three separate occasions.
In vitro suppression. OVA-primed LN cells were obtained from OVA-sensitized rats using same strain responders/sorted Treg and cultured at 1 × 105 ml in RPMI-5% fetal calf serum. CD4+CD25hi Treg or CD4+CD25− cells isolated from ADLN of sensitized rats exposed to six daily aerosols were added at various ratios as indicated. OVA was added to the cultures at 50 μg ml−1; after 48 h, cultures were pulsed with 3H-thymidine for a further 18 h. Cell proliferation was determined as mean c.p.m. from triplicate cultures and expressed as percent suppression of maximal proliferation in the absence of Treg. Experiments performed over at least three different occasions were pooled to determine mean suppressive activity.
Confocal microscopy. The methodology was as described previously.10, 32 Briefly, whole trachea were excised from rats and fixed in 70% ethanol overnight. Samples were immunostained using anti-MHC Class II monoclonal antibody and cytokeratin purchased from BD Pharmingen. Samples were embedded using Vectashield mounting medium and analyzed using a Leica TCS SP2 AOBS Multiphoton Confocal microscope (Leica Microsystems, New South Wales, Australia).
Lung function and methacholine hyper-responsiveness. The forced oscillation techniques employed were as described previously10 and utilized a computer-controlled piston ventilator (Flexiventâ; Scireq, Montreal, QC, Canada). The constant-phase model was fitted to give estimates of airway (Raw) and tissue mechanics (not shown). A methacholine concentration–response curve was constructed and the concentration associated with a doubling of RAW (EC200Raw) was calculated.
RNA extraction and quantitative reverse transcription-PCR. Total RNA was isolated from sorted T-cell populations by the TRIzol reagent (Invitrogen, Victoria, Australia) and purified using the RNeasy kit (Qiagen, Victoria, Australia), following the manufacturer's instructions, and quantified spectrophotometrically by the Nanodrop (Thermo Scientific, Western Australia, Australia). Complementary DNA was synthesized using the QuantiTect reverse transcription kit (Qiagen) according to the manufacturer's instructions. All genes studied by quantitative reverse transcription-PCR had primers purchased as QuantiTect Primer assays (Qiagen), with exception to the following: Eef1a1 (sense: 5′-AATTGGCTACAACCCTGACACA-3′; antisense: 5′-ATCCCTTGAACCACGGCATA-3′) and IL-10 (sense: 5′-TCATCGATTTCTCCCCTGTGA-3′; antisense: 5′-CACGTAGGCTTCTATGCAGTTGA-3′). All PCR master mixes and complementary DNA samples were dispensed using the Packard Multiprobe II robot (Perkin-Elmer, Victoria, Australia), with reactions set up in 20 ml volumes. Specific targets were quantified using QuantiTect SYBR Green (Qiagen) on an ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Victoria, Australia) and relative standard curves generated from serially diluted PCR products. Data were normalized to the reference gene, Eef1a1.
Statistical analysis. The experiments were carried out using groups of animals (n as indicated) or pooled tissue using five animals per group, and replicated independently on ≥ 3 occasions, unless otherwise stated. As indicated, two-tailed unpaired Student's t-test was performed to determine statistical significance for difference between mean data; a=0.05, b=0.8, or Mann–Whitney was used for non-parametric analysis, as appropriate.
References
Holgate, S.T. Pathogenesis of asthma. Clin. Exp. Allergy 38, 872–897 (2008).
Holt, P.G., Strickland, D.H., Wikstrom, M.E. & Jahnsen, F.L. Regulation of immunological homeostasis in the respiratory tract. Nat. Rev. Immunol. 8, 142–152 (2008).
Kim, H.Y., DeKruyff, R.H. & Umetsu, D.T. The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nat. Immunol. 11, 577–584 (2010).
Galli, S.J., Tsai, M. & Piliponsky, A.M. The development of allergic inflammation. Nature 454, 445–454 2008.
Medoff, B.D., Thomas, S.Y. & Luster, A.D. T cell trafficking in allergic asthma: the ins and outs. Annu. Rev. Immunol. 26, 205–232 (2008).
Hollams, E.M. et al. Elucidation of asthma phenotypes in atopic teenagers through parallel immunophenotypic and clinical profiling. J. Allergy Clin. Immunol. 124, 463–470 (2009).
Curotto de Lafaille, M.A. et al. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity 29, 114–126 2008.
Hartl, D. et al. Quantitative and functional impairment of pulmonary CD4(+)CD25(hi) regulatory T cells in pediatric asthma. J. Allergy Clin. Immunol. 119, 1258–1266 (2007).
Kearley, J., Barker, J.E., Robinson, D.S. & Lloyd, C.M. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J. Exp. Med. 202, 1539–1547 (2005).
Strickland, D.H. et al. Reversal of airway hyperresponsiveness by induction of airway mucosal CD4+CD25+ regulatory T cells. J. Exp. Med. 203, 2649–2660 (2006).
Lloyd, C.M. & Hawrylowicz, C.M. Regulatory T cells in asthma. Immunity 31, 438–449 (2009).
Ray, A., Khare, A., Krishnamoorthy, N., Qi, Z. & Ray, P. Regulatory T cells in many flavors control asthma. Mucosal Immunol. 3, 216–229 (2010).
Akdis, M. & Akdis, C.A. Therapeutic manipulation of immune tolerance in allergic disease. Nat. Rev. Drug Discov. 8, 645–660 (2009).
Lambrecht, B.N., Salomon, B., Klatzmann, D. & Pauwels, R.A. Dendritic cells are required for the development of chronic eosinophilic airway inflammation in response to inhaled antigen in sensitized mice. J. Immunol. 160, 4090–4097 (1998).
Lambrecht, B.N. & Hammad, H. Biology of lung dendritic cells at the origin of asthma. Immunity 31, 412–424 (2009).
Hawrylowicz, C.M. & O'Garra, A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat. Rev. Immunol. 5, 271–283 (2005).
Huang, C.-T. Role of LAG-3 in regulatory T cells. Immunity 21, 503–513 (2004).
Knickelbein, J.E., de Souza, A.J., Tosti, R., Narayan, P. & Kane, L.P. Cutting edge: inhibition of T cell activation by TIM-2. J. Immunol. 177, 4966–4970 (2006).
Rodriguez-Manzanet, R., DeKruyff, R., Kuchroo, V.K. & Umetsu, D.T. The costimulatory role of TIM molecules. Immunol. Rev. 229, 259–270 (2009).
Panina-Bordignon, P. et al. The C–C chemokine receptors CCR4 and CCR8 identify airway T cells of allergen-challenged atopic asthmatics. J. Clin. Invest. 107, 1357–1364 (2001).
Wei, S., Kryczek, I. & Zou, W. Regulatory T-cell compartmentalization and trafficking. Blood 108, 426–431 (2006).
Iellem, A. et al. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J. Exp. Med. 194, 847–853 (2001).
Soler, D. et al. CCR8 expression identifies CD4 memory T cells enriched for FOXP3+ regulatory and Th2 effector lymphocytes. J. Immunol. 177, 6940–6951 (2006).
Ying, S. et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of TH2-attracting chemokines and disease severity. J. Immunol. 174, 8183–8190 (2005).
Matloubian, M. et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360 (2004).
Schwab, S.R. & Cyster, J.G. Finding a way out: lymphocyte egress from lymphoid organs. Nat. Immunol. 8, 1295–1301 (2007).
Hubert, F.X., Voisine, C., Louvet, C., Heslan, M. & Josien, R. Rat plasmacytoid dendritic cells are an abundant subset of MHC class II+ CD4+CD11b−OX62− and type I IFN-producing cells that exhibit selective expression of Toll-like receptors 7 and 9 and strong responsiveness to CpG. J. Immunol. 172, 7485–7494 (2004).
Hubert, F.X. et al. Differential pattern recognition receptor expression but stereotyped responsiveness in rat spleen dendritic cell subsets. J. Immunol. 177, 1007–1016 (2006).
Turnbull, E.L., Yrlid, U., Jenkins, C.D. & Macpherson, G.G. Intestinal dendritic cell subsets: differential effects of systemic TLR4 stimulation on migratory fate and activation in vivo. J. Immunol. 174, 1374–1384 (2005).
Lewkowich, I.P. et al. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J. Exp. Med. 202, 1549–1561 (2005).
Hammad, H. et al. Differential capacity of CD8+ alpha or CD8− alpha dendritic cell subsets to prime for eosinophilic airway inflammation in the T-helper type 2-prone milieu of the lung. Clin. Exp. Allergy 34, 1834–1840 (2004).
Jahnsen, F.L. et al. Accelerated antigen sampling and transport by airway mucosal dendritic cells following inhalation of a bacterial stimulus. J. Immunol. 177, 5861–5867 (2006).
Jakubzick, C., Tacke, F., Llodra, J., van Rooijen, N. & Randolph, G.J. Modulation of dendritic cell trafficking to and from the airways. J. Immunol. 176, 3578–3584 (2006).
Vermaelen, K.Y., Carro-Muino, I., Lambrecht, B.N. & Pauwels, R.A. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J. Exp. Med. 193, 51–60 (2001).
Akdis, M. et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J. Exp. Med. 199, 1567–1575 (2004).
Lewkowich, I.P. et al. Allergen uptake, activation, and IL-23 production by pulmonary myeloid DCs drives airway hyperresponsiveness in asthma-susceptible mice. PLoS One (Electronic Resource) 3, e3879 (2008).
Johansson-Lindbom, B. et al. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J. Exp. Med. 202, 1063–1073 (2005).
Holt, P.G. & Sedgwick, J.D. Suppression of IgE responses following antigen inhalation: a natural homeostatic mechanism which limits sensitization to aeroallergens. Immunol. Today 8, 14–15 (1987).
McMenamin, C. & Holt, P.G. The natural immune response to inhaled soluble protein antigens involves major histocompatibility complex (MHC) class I-restricted CD8+ T cell-mediated but MHC class II-restricted CD4+ T cell-dependent immune deviation resulting in selective suppression of immunoglobulin E production. J. Exp. Med. 178, 889–899 (1993).
van Rijt, L.S. et al. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J. Exp. Med. 201, 981–991 (2005).
Sedgwick, J.D. & Holt, P.G. Suppression of IgE responses in inbred rats by repeated respiratory tract exposure to antigen: responder phenotype influences isotype specificity of induced tolerance. Eur. J. Immunol. 14, 893–897 (1984).
Stewart, G.A. & Holt, P.G. Immunogenicity and tolerogenicity of a major house dust mite allergen, Der p I from Dermatophagoides pteronyssinus, in mice and rats. Int. Arch. Allergy Appl. Immunol. 83, 44–51 (1987).
Lanzavecchia, A. & Sallusto, F. Progressive differentiation and selection of the fittest in the immune response. Nat. Rev. Immunol. 2, 982–987 (2002).
Constant, S., Pfeiffer, C., Woodard, A., Pasqualini, T. & Bottomly, K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J. Exp. Med. 182, 1591–1596 (1995).
von Garnier, C. et al. Allergic airways disease develops after an increase in allergen capture and processing in the airway mucosa. J. Immunol. 179, 5748–5759 (2007).
Herbert, C. et al. Alveolar macrophages stimulate enhanced cytokine production by pulmonary CD4+ T-lymphocytes in an exacerbation of murine chronic asthma. Am. J. Pathol. 177, 1657–1664 (2010).
Thunberg, S. et al. Prolonged antigen-exposure with carbohydrate particle based vaccination prevents allergic immune responses in sensitized mice. Allergy 64, 919–926 (2009).
Akbari, O., DeKruyff, R.H. & Umetsu, D.T. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat. Immunol. 2, 725–731 (2001).
Hammad, H. et al. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat. Med. 15, 410–416 (2009).
Ouabed, A. et al. Differential control of T regulatory cell proliferation and suppressive activity by mature plasmacytoid vs. conventional spleen dendritic cells. J. Immunol. 180, 5862–5870 (2008).
Jahnsen, F.L. et al. Rapid dendritic cell recruitment to the bronchial mucosa of patients with atopic asthma in response to local allergen challenge. Thorax 56, 823–826 (2001).
Chand, H.S. et al. Anti-IgE therapy results in decreased myeloid dendritic cells in asthmatic airways. J. Allergy Clin. Immunol. 125, 1157–1158 e1155 (2010).
Dua, B., Watson, R.M., Gauvreau, G.M. & O'Byrne, P.M. Myeloid and plasmacytoid dendritic cells in induced sputum after allergen inhalation in subjects with asthma. J. Allergy Clin. Immunol. 126, 133–139 (2010).
Jeal, H. et al. Modified Th2 responses at high-dose exposures to allergen: using an occupational model. Am. J. Resp. Crit. Care Med. 174, 21–25 (2006).
Platts-Mills, T., Vaughan, J., Squillace, S., Woodfolk, J. & Sporik, R. Sensitisation, asthma, and a modified Th2 response in children exposed to cat allergen: a population-based cross-sectional study. Lancet 357, 752–756 (2001).
Woodcock, A. et al. Early life environmental control: effect of symptoms, sensitization and lung function at age 3 years. Am. J. Respir. Crit. Care Med. 170, 433–439 (2004).
Durham, S.R. Sublingual immunotherapy: what have we learnt from the ‘big trials’? Curr. Opin. Allergy Clin. Immunol. 8, 577–584 (2008).
Huh, J.C. et al. Bidirectional interactions between antigen-bearing respiratory tract dendritic cells (DCs) and T-cells precede the late phase reaction in experimental asthma: DC activation occurs in the airway mucosa but not in the lung parenchyma. J. Exp. Med. 198, 19–30 (2003).
Acknowledgements
The National Health and Medical Research Council of Australia funded this work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Supplementary information
Rights and permissions
About this article
Cite this article
Strickland, D., Thomas, J., Mok, D. et al. Defective aeroallergen surveillance by airway mucosal dendritic cells as a determinant of risk for persistent airways hyper-responsiveness in experimental asthma. Mucosal Immunol 5, 332–341 (2012). https://doi.org/10.1038/mi.2012.13
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2012.13
This article is cited by
-
Trained immunity induced by in vivo peptide-based STAT6 inhibition prevents ragweed allergy in mice
Allergy, Asthma & Clinical Immunology (2021)
-
TGF-β-mediated airway tolerance to allergens induced by peptide-based immunomodulatory mucosal vaccination
Mucosal Immunology (2015)
-
Dysregulation of alveolar macrophages unleashes dendritic cell–mediated mechanisms of allergic airway inflammation
Mucosal Immunology (2014)
-
New therapeutic approaches for airway hyperimmune response are required
Indian Pediatrics (2013)