Abstract
Background/Objectives:
The use of bisphenol A (BPA) in consumer products and food packaging has been associated under certain conditions with a risk of negative health outcomes. This prompted its removal from many products and replacement with structural analogs. Bisphenol S (BPS) is one such analog, but its metabolic effects have not been fully characterized. The objective of our study was to determine whether BPS functions similarly to BPA at inducing adipogenesis.
Methods:
Murine 3T3-L1 preadipocytes were used to evaluate and compare the adipogenic potential of BPS to BPA. Cells were treated with 0.01–50 μM BPS or 0.01–50 μM BPA and adipogenic effects were measured. Further, their ability to activate peroxisome proliferator-activated receptor gamma (PPARγ), an adipogenic transcription factor, was also determined.
Results:
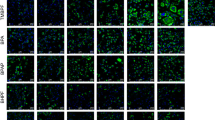
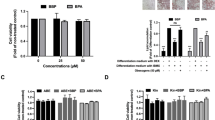
Our results indicate that treatment of 3T3-L1 cells with BPS induced lipid accumulation and increased mRNA and protein expression of key adipogenic markers (1–50 μM; P<0.05). BPS treatment resulted in a higher expression of adipogenic markers as well as greater lipid accumulation when compared with BPA treatment. We showed that BPS can upregulate lipoprotein lipase, adipocyte protein 2, PPARγ, perilipin, adipsin and CCAAT/enhancer-binding protein alpha mRNA expression levels. Furthermore, using transcriptional assays, we showed that BPS and BPA can modestly activate PPARγ using a PPRE (PPARγ response element)-dependent luciferase construct by 1.5-fold (P<0.05). However, BPS but not BPA was able to competitively inhibit rosiglitazone (ROSI)-activated PPARγ, suggesting that BPS interacts with PPARγ distinctly from BPA. Co-treatment of cells with the selective PPARγ antagonist GW9662 inhibits BPS-, BPA-, ROSI- but not dexamethasone-dependent adipogenic differentiation.
Conclusions:
Both BPA and BPS can enhance 3T3-L1 adipocyte differentiation in a dose-dependent manner and require PPARγ to induce adipogenesis. Through direct comparison, we show that BPS is a more potent adipogen than BPA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Rubin B, Bisphenol A . An endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol 2011; 127: 27–34.
Calafat AM, Weuve J, Ye X, Jia LT, Hu H, Ringer S et al. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ Health Perspect 2009; 117: 639–644.
Liao C, Liu F, Guo Y, Moon HB, Nakata H, Wu Q et al. Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: implications for human exposure. Environ Sci Technol 2012; 46: 9138–9145.
Vandenberg LN, Chahoud I, Padmanabhan V, Paumgartten FJ, Schoenfelder G . Biomonitoring studies should be used by regulatory agencies to assess human exposure levels and safety of bisphenol A. Environ Health Perspect 2010; 118: 1051–1054.
Takeuchi T, Tsutsumi O, Ikezuki Y, Takai Y, Taketani Y . Positive relationship between androgen and the endocrine disruptor, bisphenol A, in normal women and women with ovarian dysfunction. Endocr J 2004; 51: 165–169.
Ranciere F, Lyons JG, Loh VH, Botton J, Galloway T, Wang T et al. Bisphenol A and the risk of cardiometabolic disorders: a systematic review with meta-analysis of the epidemiological evidence. Environ Health 2015; 14: 46.
Watkins DJ, Peterson KE, Ferguson KK, Mercado-Garcia A, Tamayo YOM, Cantoral A et al. Relating phthalate and BPA exposure to metabolism in peripubescence: the role of exposure timing, sex, and puberty. J Clin Endocrinol Metab 2016; 101: 79–88.
Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB et al. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA 2008; 300: 1303–1310.
Shankar A, Teppala S, Sabanayagam C . Urinary bisphenol a levels and measures of obesity: results from the national health and nutrition examination survey 2003-2008. ISRN Endocrinol 2012; 2012: 965243.
Masuno H, Iwanami J, Kidani T, Sakayama K, Honda K . Bisphenol a accelerates terminal differentiation of 3T3-L1 cells into adipocytes through the phosphatidylinositol 3-kinase pathway. Toxicol Sci 2005; 84: 319–327.
Somm E, Schwitzgebel VM, Toulotte A, Cederroth CR, Combescure C, Nef S et al. Perinatal exposure to bisphenol a alters early adipogenesis in the rat. Environ Health Perspect 2009; 117: 1549–1555.
Liao C, Kannan K . A survey of bisphenol A and other bisphenol analogues in foodstuffs from nine cities in China. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2014; 31: 319–329.
Rochester JR, Bolden AL . Bisphenol S and F: a systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ Health Perspect 2015; 123: 643–650.
Liao C, Liu F, Alomirah H, Loi VD, Mohd MA, Moon HB et al. Bisphenol S in urine from the United States and seven Asian countries: occurrence and human exposures. Environ Sci Technol 2012; 46: 6860–6866.
Zhou X, Kramer JP, Calafat AM, Ye X . Automated on-line column-switching high performance liquid chromatography isotope dilution tandem mass spectrometry method for the quantification of bisphenol A, bisphenol F, bisphenol S, and 11 other phenols in urine. J Chromatogr B Analyt Technol Biomed Life Sci 2014; 944: 152–156.
Farmer SR . Transcriptional control of adipocyte formation. Cell Metab 2006; 4: 263–273.
Koutnikova H, Cock TA, Watanabe M, Houten SM, Champy MF, Dierich A et al. Compensation by the muscle limits the metabolic consequences of lipodystrophy in PPAR gamma hypomorphic mice. Proc Natl Acad Sci USA 2003; 100: 14457–14462.
Rosen ED . Molecular mechanisms of adipocyte differentiation. Ann Endocrinol (Paris) 2002; 63 (2 Pt 1): 79–82.
Rosen ED, Hsu CH, Wang X, Sakai S, Freeman MW, Gonzalez FJ et al. C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev 2002; 16: 22–26.
Atlas E, Pope L, Wade MG, Kawata A, Boudreau A, Boucher JG . Bisphenol A increases aP2 expression in 3T3L1 by enhancing the transcriptional activity of nuclear receptors at the promoter. Adipocyte 2014; 3: 170–179.
Sargis RM, Johnson DN, Choudhury RA, Brady MJ . Environmental endocrine disruptors promote adipogenesis in the 3T3-L1 cell line through glucocorticoid receptor activation. Obesity (Silver Spring) 2010; 18: 1283–1288.
Li X, Ycaza J, Blumberg B . The environmental obesogen tributyltin chloride acts via peroxisome proliferator activated receptor gamma to induce adipogenesis in murine 3T3-L1 preadipocytes. J Steroid Biochem Mol Biol 2011; 127: 9–15.
Greenspan P, Mayer EP, Fowler SD . Nile red: a selective fluorescent stain for intracellular lipid droplets. J Cell Biol 1985; 100: 965–973.
Kim JB, Wright HM, Wright M, Spiegelman BM . ADD1/SREBP1 activates PPARgamma through the production of endogenous ligand. Proc Natl Acad Sci USA 1998; 95: 4333–4337.
Heim M, Frank O, Kampmann G, Sochocky N, Pennimpede T, Fuchs P et al. The phytoestrogen genistein enhances osteogenesis and represses adipogenic differentiation of human primary bone marrow stromal cells. Endocrinology 2004; 145: 848–859.
Lea-Currie YR, Wen P, McIntosh MK . Dehydroepiandrosterone reduces proliferation and differentiation of 3T3-L1 preadipocytes. Biochem Biophys Res Commun 1998; 248: 497–504.
Boucher JG, Boudreau A, Atlas E . Bisphenol A induces differentiation of human preadipocytes in the absence of glucocorticoid and is inhibited by an estrogen-receptor antagonist. Nutr Diabetes 2014; 4: e102.
Caprio M, Feve B, Claes A, Viengchareun S, Lombes M, Zennaro MC . Pivotal role of the mineralocorticoid receptor in corticosteroid-induced adipogenesis. FASEB J 2007; 21: 2185–2194.
Feve B, Antras J, Lasnier F, Hilliou F, Pairault J . The antiglucocorticoid RU38486 is a potent accelerator of adipose conversion of 3T3-F442A cells. Mol Cell Endocrinol 1989; 67: 17–27.
Helies-Toussaint C, Peyre L, Costanzo C, Chagnon MC, Rahmani R . Is bisphenol S a safe substitute for bisphenol A in terms of metabolic function? An in vitro study. Toxicol Appl Pharmacol 2014; 280: 224–235.
Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS et al. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell 1999; 4: 611–617.
Kroker AJ, Bruning JB . Review of the structural and dynamic mechanisms of PPARgamma partial agonism. PPAR Res 2015; 2015: 816856.
Bruning JB, Chalmers MJ, Prasad S, Busby SA, Kamenecka TM, He Y et al. Partial agonists activate PPARgamma using a helix 12 independent mechanism. Structure 2007; 15: 1258–1271.
Kallenberger BC, Love JD, Chatterjee VK, Schwabe JW . A dynamic mechanism of nuclear receptor activation and its perturbation in a human disease. Nat Struct Biol 2003; 10: 136–140.
Riu A, Grimaldi M, le Maire A, Bey G, Phillips K, Boulahtouf A et al. Peroxisome proliferator-activated receptor gamma is a target for halogenated analogs of bisphenol A. Environ Health Perspect 2011; 119: 1227–1232.
Cao Z, Umek RM, McKnight SL . Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev 1991; 5: 1538–1552.
Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS . Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci USA 2000; 97: 12729–12734.
Joyner JM, Hutley LJ, Cameron DP . Glucocorticoid receptors in human preadipocytes: regional and gender differences. J Endocrinol 2000; 166: 145–152.
Yi KW, Shin JH, Seo HS, Lee JK, Oh MJ, Kim T et al. Role of estrogen receptor-alpha and -beta in regulating leptin expression in 3T3-L1 adipocytes. Obesity (Silver Spring) 2008; 16: 2393–2399.
Rosenmai AK, Dybdahl M, Pedersen M, Alice van Vugt-Lussenburg BM, Wedebye EB, Taxvig C et al. Are structural analogues to bisphenol a safe alternatives? Toxicol Sci 2014; 139: 35–47.
Sharma G, Hu C, Brigman JL, Zhu G, Hathaway HJ, Prossnitz ER . GPER deficiency in male mice results in insulin resistance, dyslipidemia, and a proinflammatory state. Endocrinology 2013; 154: 4136–4145.
Sharma G, Prossnitz ER . Mechanisms of estradiol-induced insulin secretion by the G protein-coupled estrogen receptor GPR30/GPER in pancreatic beta-cells. Endocrinology 2011; 152: 3030–3039.
Hugo ER, Brandebourg TD, Woo JG, Loftus J, Alexander JW, Ben-Jonathan N . Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ Health Perspect 2008; 116: 1642–1647.
Isensee J, Meoli L, Zazzu V, Nabzdyk C, Witt H, Soewarto D et al. Expression pattern of G protein-coupled receptor 30 in LacZ reporter mice. Endocrinology 2009; 150: 1722–1730.
Martensson UE, Salehi SA, Windahl S, Gomez MF, Sward K, Daszkiewicz-Nilsson J et al. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology 2009; 150: 687–698.
Ijichi N, Ikeda K, Horie-Inoue K, Yagi K, Okazaki Y, Inoue S . Estrogen-related receptor alpha modulates the expression of adipogenesis-related genes during adipocyte differentiation. Biochem Biophys Res Commun 2007; 358: 813–818.
Cipelli R, Harries L, Okuda K, Yoshihara S, Melzer D, Galloway T . Bisphenol A modulates the metabolic regulator oestrogen-related receptor-alpha in T-cells. Reproduction 2014; 147: 419–426.
Rochester JR . Bisphenol A and human health: a review of the literature. Reprod Toxicol 2013; 42: 132–155.
Acknowledgements
We thank Dr M Wade and Dr E Tung for reviewing this manuscript. Grant funding was provided by the Health Canada Chemical Management Plan. SA is funded by the Natural Sciences and Engineering Research Council of Canada Visiting Scientist Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ahmed, S., Atlas, E. Bisphenol S- and bisphenol A-induced adipogenesis of murine preadipocytes occurs through direct peroxisome proliferator-activated receptor gamma activation. Int J Obes 40, 1566–1573 (2016). https://doi.org/10.1038/ijo.2016.95
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2016.95
This article is cited by
-
Bisphenol A substitutes and childhood obesity at 7 years: a cross-sectional study in Shandong, China
Environmental Science and Pollution Research (2023)
-
Toxicity of bisphenol A (BPA) and its derivatives in divers biological models with the assessment of molecular mechanisms of toxicity
Environmental Science and Pollution Research (2023)
-
Bisphenol A enhances adipogenic signaling pathways in human mesenchymal stem cells
Genes and Environment (2020)
-
Dechlorane Plus increases adipogenesis in 3T3-L1 and human primary preadipocytes independent of peroxisome proliferator-activated receptor γ transcriptional activity
International Journal of Obesity (2019)
-
Dexamethasone induced miR-155 up-regulation in differentiating 3T3-L1 preadipocytes does not affect adipogenesis
Scientific Reports (2018)