Abstract
Background:
Nowadays, non-alcoholic fatty liver disease (NAFLD) is one of the most common chronic liver diseases in children. Our recent clinical trial demonstrated that dietary and VSL#3-based interventions may improve fatty liver by ultrasound and body mass index (BMI) after 4 months.
Objectives:
As in this short-term trial, as in others, it is impracticable to monitor response to therapy or treatment by liver biopsy, we aimed to identify a panel of potential non-invasive metabolic biomarkers by a urinary metabolic profiling.
Methods:
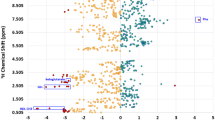
Urine samples from a group of 31 pediatric NAFLD patients, enrolled in a VSL#3 clinical trial, were analyzed by high-resolution proton nuclear magnetic resonance spectroscopy in combination with analysis of variance-Simultaneous Component Analysis model and multivariate data analyses. Urinary metabolic profiles were interpreted in terms of clinical patient feature, treatment and chronology pattern correlations.
Results:
VSL#3 treatment induced changes in NAFLD urinary metabolic phenotype mainly at level of host amino-acid metabolism (that is, valine, tyrosine, 3-amino-isobutyrate or β-aminoisobutyric acid (BAIBA)), nucleic acid degradation (pseudouridine), creatinine metabolism (methylguanidine) and secondarily at the level of gut microbial amino-acid metabolism (that is, 2-hydroxyisobutyrate from valine degradation). Furthermore, some of these metabolites correlated with clinical primary and secondary trial end points after VSL#3 treatment: tyrosine and the organic acid U4 positively with alanine aminotransferase (R=0.399, P=0.026) and BMI (R=0.36, P=0.045); BAIBA and tyrosine negatively with active glucagon-like-peptide 1 (R=−0.51, P=0.003; R=-0.41, P=0.021, respectively).
Conclusions:
VSL#3 treatment-dependent urinary metabotypes of NAFLD children may be considered as non-invasive effective biomarkers to evaluate the response to treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Sallis JF, Hinckson EA . Reversing the obesity epidemic in young people: building up the physical activity side of energy balance. Lancet Diabetes Endocrinol 2014; 2: 190–191.
Giorgio V, Prono F, Graziano F, Nobili V . Pediatric non alcoholic fatty liver disease: old and new concepts on development, progression, metabolic insight and potential treatment targets. BMC Pediatr 2013; 13: 40.
Alisi A, Feldstein AE, Villani A, Raponi M, Nobili V . Pediatric nonalcoholic fatty liver disease: a multidisciplinary approach. Nat Rev Gastroenterol Hepatol 2012; 9: 152–161.
Wree A, Broderick L, Canbay A, Hoffman HM, Feldstein AE . From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nat Rev Gastroenterol Hepatol 2013; 10: 627–636.
Miele L, Marrone G, Lauritano C, Cefalo C, Gasbarrini A, Day C et al. Gut-liver axis and microbiota in NAFLD: insight pathophysiology for novel therapeutic target. Curr Pharm Des 2013; 19: 5314–5324.
Frasinariu OE, Ceccarelli S, Alisi A, Moraru E, Nobili V . Gut-liver axis and fibrosis in nonalcoholic fatty liver disease: an input for novel therapies. Dig Liver Dis 2013; 45: 543–551.
Compare D, Coccoli P, Rocco A, Nardone OM, De Maria S, Cartenì M et al. Gut—liver axis: the impact of gut microbiota on non alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis 2012; 22: 471–476.
Alisi A, Manco M, Devito R, Piemonte F, Nobili V . Endotoxin and plasminogen activator inhibitor-1 serum levels associated with nonalcoholic steatohepatitis in children. J Pediatr Gastroenterol Nutr 2010; 50: 645–649.
Giorgio V, Miele L, Principessa L, Ferretti F, Villa MP, Negro V et al. Intestinal permeability is increased in children with non-alcoholic fatty liver disease, and correlates with liver disease severity. Dig Liver Dis 2014; 46: 556–560.
Alisi A, Ceccarelli S, Panera N, Nobili V . Causative role of gut microbiota in non-alcoholic fatty liver disease pathogenesis. Front Cell Infect Microbiol 2012; 2: 132.
Kelishadi R, Farajian S, Mirlohi M . Probiotics as a novel treatment for non-alcoholic Fatty liver disease; a systematic review on the current evidences. Hepat Mon 2013; 13: e7233.
Li Z, Yang S, Lin H, Huang J, Watkins PA, Moser AB et al. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology 2003; 37: 343–350.
Mencarelli A, Cipriani S, Renga B, Bruno A, D'Amore C, Distrutti E et al. VSL#3 resets insulin signaling and protects against NASH and atherosclerosis in a model of genetic dyslipidemia and intestinal inflammation. PLoS ONE 2012; 7: e45425.
Mencarelli A, Distrutti E, Renga B, D'Amore C, Cipriani S, Palladino G et al. Probiotics modulate intestinal expression of nuclear receptor and provide counter-regulatory signals to inflammation-driven adipose tissue activation. PLoS One 2011; 6: e22978.
Alisi A, Bedogni G, Baviera G, Giorgio V, Porro E, Paris C et al. Randomised clinical trial: the beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2014; 39: 1276–1285.
Dumas ME, Kinross J, Nicholson JK . Metabolic phenotyping and systems biology approaches to understanding metabolic syndrome and fatty liver disease. Gastroenterology 2014; 146: 46–62.
Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41: 1313–1321.
Brasili E, Mengheri E, Tomassini A, Capuani G, Roselli M, Finamore A et al. Lactobacillus acidophilus La5 and Bifidobacterium lactis Bb12 induce different age-related metabolic profiles revealed by 1H-NMR spectroscopy in urine and feces of mice. J Nutr 2013; 143: 1549–1557.
Fan TWM, Lane AN . Structure-based profiling of metabolites and isotopomers by NMR. Progr Nucl Magn Res Spectrosc 2008; 52: 69–117.
Martin FP, Sprenger N, Yap IK, Wang Y, Bibiloni R, Rochat F et al. Panorganismal gut microbiome-host metabolic crosstalk. J Proteome Res 2009; 8: 2090–2105.
Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu Y et al. HMDB 3.0 – The Human Metabolome Database in 2013. Nucleic Acids Res 2013 41: D801–D807.
Smilde AK, Jansen JJ, Hoefsloot HCJ, Lamers R, Van der Greef J, Timmerman ME . ANOVA-simultaneous component analysis (ASCA): a new tool for analyzing designed metabolomics data. Bioinformatics 2005; 21: 3043–3048.
Brasili E, Praticò G, Marini F, Valletta A, Capuani G, Sciubba F et al. A non-targeted metabolomics approach to evaluate the effects of biomass growth and chitosan elicitation on primary and secondary metabolism of Hypericum perforatum in vitro roots. Metabolomics 2014; 10: 1186–1196.
Wiklund PK, Pekkala S, Autio R, Munukka E, Xu L, Saltevo J et al. Serum metabolic profiles in overweight and obese women with and without metabolic syndrome. Diabetol Metab Syndr 2014; 6: 40.
Würtz P, Mäkinen VP, Soininen P, Kangas AJ, Tukiainen T, Kettunen J et al. Metabolic signatures of insulin resistance in 7,098 young adults. Diabetes 2012; 61: 1372–1380.
Holst JJ . The physiology of glucagon-like peptide 1. Physiol Rev 2007; 87: 1409–1439.
Ofengand J . Ribosomal RNA pseudouridines and pseudouridine synthases. FEBS Lett 2002; 514: 17–25.
Charette M, Gray MW . "Pseudouridine in RNA: what, where, how, and why". IUBMB Life 2000; 49: 341–335.
Topp H, Schöch G . Whole-body degradation rates of transfer-, ribosomal-, and messenger ribonucleic acids and resting metabolic rate in 3- to 18-year-old humans. Pediatr Res. 2000; 47: 163–168.
Schoch G, Topp H, Held A, Heller-Schoch G, Ballauff A, Manz F, Sander G . Interrelation between whole-body turnover rates of RNA and protein. Eur J Clin Nutr 1990; 44: 647–658.
Schoch G, Topp H . Interrelations between degradation rates of RNA and protein and the energy turnover rates. In: Raiha NCR (eds). Protein Metabolism During Infancy. Raven Press: New York, NY, USA, 1994. pp 49–52.
Nobili V, Parola M, Alisi A, Marra F, Piemonte F, Mombello C et al. Oxidative stress parameters in paediatric non-alcoholic fatty liver disease. Int J Mol Med 2010; 26: 471–476.
Topp H, Fusch G, Schöch G, Fusch C . Noninvasive markers of oxidative DNA stress, RNA degradation and protein degradation are differentially correlated with resting metabolic rate and energy intake in children and adolescents. Pediatric Res 2008; 64: 246–250.
Sabaté JM, Jouët P, Harnois F, Mechler C, Msika S, Grossin M et al. High prevalence of small intestinal bacterial overgrowth in patients with morbid obesity: a contributor to severe hepatic steatosis. Obes Surg 2008; 18: 371–377.
Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009; 49: 1877–1887.
Calvani R, Miccheli A, Capuani G, Tomassini Miccheli A, Puccetti C, Delfini M et al. Gut microbiome-derived metabolites characterize a peculiar obese urinary metabotype. Int J Obes 2010; 34: 1095–1098.
Li M, Wang B, Zhang M, Rantalainen M, Wang S, Zhou H et al. Symbiotic gut microbes modulate human metabolic phenotypes. Proc Natl Acad Sci USA 2008; 105: 2117–2122.
Rohwerder T, Müller RH . Biosynthesis of 2-hydroxyisobutyric acid (2-HIBA) from renewable carbon. Microb Cell Fact 2010; 9: 13.
Yadav H, Lee JH, Lloyd J, Walter P, Rane SG . Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J Biol Chem 2013; 288: 25088–25097.
Everard A, Cani PD . Gut microbiota and GLP-1. Rev Endocr Metab Disord 2014; 15: 189–196.
Pollitt RJ, Green A, Smith R . Excessive excretion of beta-alanine and of 3-hydroxypropionic, R- and S-3-aminoisobutyric, R- and S-3-hydroxyisobutyric and S-2-(hydroxymethyl)butyric acids probably due to a defect in the metabolism of the corresponding malonic semialdehydes. J Inherit Metab Dis 1985; 8: 75–79.
Roberts LD, Boström P, O'Sullivan JF, Schinzel RT, Lewis GD, Dejam A et al. β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab 2014; 19: 96–108.
Acknowledgements
We are indebted to Professor Claudio De Simone who provided VSL#3 with verified composition and indistinguishable placebo and intellectual contribution to the study. This study was funded by the Italian Ministry of Health (Fondi di Ricerca Corrente and 5X1000) to Professor Valerio Nobili.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Rights and permissions
About this article
Cite this article
Miccheli, A., Capuani, G., Marini, F. et al. Urinary 1H-NMR-based metabolic profiling of children with NAFLD undergoing VSL#3 treatment. Int J Obes 39, 1118–1125 (2015). https://doi.org/10.1038/ijo.2015.40
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2015.40
This article is cited by
-
NMR microsystem for label-free characterization of 3D nanoliter microtissues
Scientific Reports (2020)
-
The Role of Gut Microbiome-Targeted Therapy in Nonalcoholic Fatty Liver Disease
Current Hepatology Reports (2020)
-
Probiotics for dietary management of non-alcoholic fatty liver disease
Environmental Chemistry Letters (2019)
-
Timing for the second fecal microbiota transplantation to maintain the long-term benefit from the first treatment for Crohn’s disease
Applied Microbiology and Biotechnology (2019)
-
Exploration of serum biomarkers for predicting the response to Inchinkoto (ICKT), a Japanese traditional herbal medicine
Metabolomics (2017)