Abstract
Left ventricular hypertrophy (LVH) is a marker of prolonged exposure to high blood pressure and a predictor of cardiovascular disease risk. The objective of the current study was to investigate its association with cognitive function. Following standard guidelines, pairs of independent reviewers screened 2359 articles to search for studies that addressed the research question, extracted data and evaluated the quality of the studies using the Newcastle-Ottawa scale; authors were contacted for additional data. A random-effects meta-analysis and a meta-regression analysis were performed. Eighteen eligible studies using various methodologies and of varying quality were identified. However, both cross-sectional and prospective studies were indicative of a positive association between LVH and cognitive impairment or cognitive performance and decline in both population-based and patient-based subjects. The meta-analysis showed an increased risk of cognitive impairment among subjects with LVH in population-based studies (9 studies; 28 648 subjects; odds ratio (OR): 1.40, 95% confidence interval (CI): 1.18–1.66) and studies exclusively on hypertensive subjects (3 studies; 1262 subjects; OR: 2.14, 95% CI: 1.39–3.30). The effect was stronger when assessing LVH by echocardiography rather than electrocardiogram and was retained in the sensitivity analyses of prospective and highest quality studies and studies adjusting for hypertension or blood pressure levels. No heterogeneity or publication bias was documented, whereas the presence of hypertension seemed to reinforce the reported association, as derived from the meta-regression analysis. There is evidence suggesting an independent association of LVH with cognitive impairment. Because of the highly heterogeneous methodologies, future large prospective studies with clinically defined dementia outcomes are needed to replicate the findings.
Similar content being viewed by others
Introduction
The dramatic increase in the average life expectancy during the last century has resulted in a global rise in cognitive impairment and dementia rates; the prevalence of mild cognitive impairment among the elderly worldwide is estimated to be 20–30%,1 whereas 5–7% of individuals older than 60 years suffer from dementia.2 Although cognitive decline has been conventionally attributed to neurodegenerative etiology, cumulative evidence suggests that vascular pathology is a major contributor.3 Indeed, the presence of traditional cardiovascular disease (CVD) risk factors at midlife, including diabetes mellitus,4 hypertension5 and hypercholesterolemia,6 is associated with an increased risk of cognitive decline later in life.
Particularly, numerous epidemiological studies have been published on the effect of blood pressure (BP) on cognition, with the findings being more consistent towards an association of midlife high BP levels and cognitive impairment.7, 8, 9 Similarly, efficient hypertension control through treatment appears to attenuate the effect of this association.10 These observations are thought to be primarily mediated by the effect of chronic hypertension on the cerebral vasculature; specifically, exposure to high BP levels for years results in an acceleration of atherosclerosis in cerebral large vessels, along with a thickening of cerebral perforating arterioles, leading to white matter hypoperfusion and ischemic rarefaction.11 However, single BP measurements, particularly in the elderly, do not correspond to the chronic hemodynamic burden posed by the exposure to high BP; thus, more objective markers of the duration of hypertension are needed to better predict CVD risk.
Chronic exposure to hypertension leads to damage in target organs, including heart, kidney, retina and brain. Markers of damage in any of these organs could be used as proxies for a prolonged exposure to high BP, whereas their recognition could provide a window for preclinical diagnosis of damage to the other organs.12 Indeed, microalbuminuria, considered an early marker of renal small vessel disease, and retinal vascular damage are associated with increased risk of silent cerebral microvascular lesions and cognitive dysfunction.13, 14, 15, 16 Left ventricular mass (LVM) is strongly associated with long-term high BP levels17, 18 and comprises a predictor of hypertension development in non-hypertensive individuals.19 Left ventricular hypertrophy (LVH) is the consequence of high arterial loading of the heart, is linearly related with arterial stiffness,20 and comprises a good predictor of CVD risk prospectively.21
In this context, the cardio-cerebral axis has received scientific attention with a number of studies declaring an association of LVH with stroke22, 23 and silent cerebrovascular lesions.24 Likewise, previous studies have examined whether presence of LVH is associated with cognitive decline and dementia.25, 26, 27 To our knowledge, no systematic evaluation of these studies has been attempted so far. Thus, the aim of the current study was to conduct a systematic review of the literature on the association between LVH and cognitive function and, if possible, quantitatively synthesize the results of the published studies.
Methods
This systematic review and meta-analysis was conducted based on the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines,28 as detailed in Supplementary Table 1. Accordingly, studies were identified through a systematic literature search, as detailed in the 'Search strategy' described in the Supplementary Materials.
Eligibility criteria
Cohort, case-control and cross-sectional studies quantifying an association of LVH with either measures of cognitive function or risk of dementia were eligible for inclusion. Case series, case reports, in vitro and animal studies were excluded, as were studies including solely children or adolescents. We included studies both on the general population and studies examining a specific patient-based population, including individuals suffering from hypertension, diabetes mellitus, chronic kidney disease and CVD. Studies on different populations were thereafter independently examined.
The exposure variable was either a continuous measure of LVM, assessed via transthoracic echocardiogram (TTE) or LVH (dichotomous variable), assessed by electrocardiogram (ECG) or TTE. LVM should be calculated via the method of Devereux et al,29 indexed or not to the body surface area of the participants (left ventricular mass index, LVMI). TTE-assessed LVH should be preferably defined as LVMI ⩾115 g m−2 in males and ⩾95 g m−2 in females,30 but different cutoff points were also considered for eligibility. The definition of LVH by studies using ECG should be based on a standard 7-lead or 12-lead ECG using a validated calculation (for example, Sokolow-Lyon indices or Cornell voltage criteria).31, 32, 33, 34, 35 Given the significantly lower sensitivity of ECG in the detection of LVH compared to TTE36 and their independence of one another’s association with CVD outcomes,37 these indices were also independently examined.
Two major outcomes were explored: dementia/cognitive impairment (dichotomous variable) and cognitive function (continuous variable). Diagnosis of dementia or major dementia subtypes, namely Alzheimer’s disease (AD) or vascular dementia (VaD), by diagnostic criteria or of cognitive impairment via validated neuropsychological tests was required. With regards to cognitive function, studies should assess via validated instruments either global cognitive function or individual cognitive domains; eligible studies provided an association of LVH with either cognitive performance at a single measurement or scoring decline between two distinct time points.
Data extraction and quality assessment
Publication details (year, authors, title and journal), study information (region, study period, design, follow-up, inclusion-exclusion criteria and cohort-size), characteristics of participants (age, gender, smoking, body mass index, presence of diabetes, hypertension and CVD, BP levels), LVH/LVM assessment, cognitive measures, number of dementia cases and statistical analysis (effect estimate, effect size and adjustments) were abstracted in a pre-piloted spreadsheet. Maximally adjusted effect estimates were preferred, whereas authors were contacted or previous publications were sought in case of missing data.
The nine-item Newcastle-Ottawa Scale (NOS)38 was used for quality assessment of case-control and cohort studies, whereas its eight-item adapted version was used for cross-sectional studies.39 Detailed studies of NOS in the context of this review are provided in the respective section of the Supplementary Materials.
Two pairs of reviewers (four reviewers total) worked independently and masked each other from the study selection, data abstraction and quality assessment; disagreements were resolved by consensus.
Statistical analysis
Due to the heterogeneity of studies on cognitive function, a meta-analysis was feasible only for the effect of LVH on the risk of cognitive impairment. Effect estimates along with their 95% confidence intervals (CI) by LVH, were pooled using random-effects models.40 Although both cross-sectional and longitudinal cohort studies were included in the quantitative synthesis, only odds ratios (ORs) were included in the analysis to avoid statistical heterogeneity. Particularly, two out of the five longitudinal studies presented ORs derived from logistic regression analysis in the published articles, whereas we self-calculated the respective ORs from 2 × 2 tables for the remaining three studies. Thus, no analysis for hazard ratios was feasible. Two different analyses were performed; the first analysis was performed on population-based studies and the second analysis was performed on exclusively hypertensive patient-based studies. All analyses were stratified by the method of LVH assessment (TTE or ECG). In addition to the dichotomous evaluation of LVH, no analysis by LVMI increments (for example, by 1SD increase) was possible due to the non-availability of the required data by the individual studies. Sub-analyses by etiology and severity of cognitive impairment (all-cause dementia, AD, VaD and mild cognitive impairment), assessment of cognitive impairment (clinical diagnosis or screening cognitive test), level of adjustment (adjusted for hypertension or not), study design (prospective and cross-sectional) and quality score were performed. Heterogeneity was assessed via the I2 estimation and the Cochran Q statistic, whereas potential publication bias was evaluated by funnel plots. Analyses were performed using the STATA Software (v13.0, Stata Corporation, College Station, TX, USA).
Results
Results of the search strategy
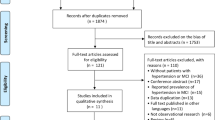
A total of 2359 results were retrieved from the literature search, whereas 7 additional articles were identified using the 'snowball' procedure. We excluded studies based on an examination of the title or abstract of 2295 articles, resulting in 71 articles, requiring an evaluation of their full text. Out of these 71 articles, 16 studies were identified to meet our eligibility criteria, 15 studies were excluded due to specific reasons and the authors of 40 articles, which were not eligible based on their published version, were contacted for additional information. For 6 of the latter articles, we obtained the required data, and these studies were also included. From the total of 22 eligible studies, 4 studies had to be excluded due to overlapping populations; therefore, a final group of 18 studies was deemed eligible for inclusion in this systematic review.25, 26, 27, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55 The study selection process is graphically summarized in Figure 1; details on how each individual study was processed and the full reference list are provided in Supplementary Table 2.
Description of studies and quality evaluation
Study design and populations
Twelve of the 18 studies were based on community subjects from the general population, whereas 6 were patient-based, including solely subjects with hypertension, who manifested arterial disease or chronic kidney disease; one of the population-based studies examined only individuals with subjective memory complaints (Table 1). Only eight studies had a prospective design examining the association of LVH with incident cognitive impairment or cognitive decline over follow-up periods ranging from 1 to 10 years. The mean/median age of the participants was >55 years in all studies and exceeded 65 years in 10 of the studies.
Assessment of exposure variable
The exposure variable was LVH in 15 of the studies and LVM in 3 studies. Highly heterogeneous methodologies were used for the definition of LVH, with six studies using ECG, and nine studies assessing it using TTE-based definitions; in particular, the desired ⩾115 g m−2 for males and ⩾95 g m−2 for females were adopted only by four studies, whereas higher cutoff points of 125 g m−2 for males and 110 g m−2 for females were used in three studies. Scuteri et al. compared the highest LVM quartile, whereas one study did not clarify the cutoff point used.
Assessment of the cognitive outcomes
A variety of outcomes was examined by eligible studies; these studies could be further divided into continuous outcomes of cognitive performance or cognitive decline in a specific domain and dichotomous outcomes of cognitive impairment or dementia (all-cause, AD, VaD). Six studies examined cognitive function, four of the studies as cognitive performance at a single time-point (cross-sectional studies), whereas two studies assessed the decline in score between temporally different measures. Some studies examined solely global cognitive function, but cognitive sub-domains, which were generally categorized to executive function/attention, processing speed and verbal memory, were also assessed in some studies using composite scores of a variety of instruments; two studies assessed global cognition using a single MMSE measurement.
Out of the 14 studies examining dichotomous outcomes, 9 studies defined cognitive impairment by the MMSE, using a variety of cutoff points (ranging from 21 to 27; maximum: 30). Likewise, one study defined cognitive impairment as a decline of Six-Item Screener scoring (maximum: 6) under 5. Another study prospectively ascertained cognitive decline by a worsening (0.5 points or more) of the Clinical Dementia Rating over times, whereas 1 study defined mild cognitive impairment based on clinical and neuropsychological assessment. Finally, two prospective studies defined all-cause dementia, AD and VaD/multi-infarct dementia using official diagnostic criteria.
Quality assessment
Supplementary Table 3 presents the results of the study quality evaluation. The eight longitudinal studies scored generally high, as only two of them lost >2 points in NOS, mostly due to the lack of comparability between the exposed and unexposed cohort, given the unadjusted associations provided. However, five of the studies were additionally compromised due to the high percentage (>20%) of participants lost to follow-up evaluation.
None of the 10 cross-sectional studies received the maximum of 10 points in NOS and 5 of the studies lost >2 points. The high or non-reported non-response rate was the main limitation of the included studies, followed by the unjustified/low sample size. Studies of the lowest quality were further compromised by the lack of comparability among exposed and unexposed subjects.
Association of left ventricular hypertrophy with cognitive outcomes
Findings from longitudinal cohort studies
Population-based studies
The largest published study (n=23 752 subjects) from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) population showed longitudinally (mean follow-up: 4.1 years) that among individuals with normal cognitive performance at baseline, LVH, assessed by ECG, is associated with increased odds of incident cognitive impairment (Six-Item Screener<5; OR: 1.29). This effect was significant even after adjustment for a variety of potential confounding factors, namely demographic variables, comorbidities, traditional CVD risk factors and systolic BP levels.44 Out of the remaining 4 population-based studies with a prospective design, none of the studies showed a statistically significant association of LVH (2 studies by ECG and 2 by TTE) with cognitive decline, mild cognitive impairment, or incident dementia.41, 49, 50, 54 Nevertheless, 3 of the studies showed positive associations (OR ranging from 1.05 to 1.74),41, 49, 54 whereas the oldest study by Katzman et al,50 demonstrated a non-significant increased risk for VaD (OR: 1.82), but not AD (OR: 0.46). The lack of statistical power (105–650 study subjects) could have precluded the emergence of statistically significant effects; however, only 1 of the studies presented estimates adjusted for major confounders.
Out of the 2 prospective studies examining measures of cognitive function, the Hoorn Study (N=313, mean age 66 years)45 showed an association of increasing LVM with worse performance in a single assessment of the examined cognitive sub-domains (categorized in executive function/attention, verbal memory and processing speed) after a mean follow-up period of 5.9 years, which remained statistically significant after multivariate adjustments, only for attention/executive function and processing speed.45 Similarly, the Whiterhall I study, which included 5810 individuals, showed a significant association of LVH (assessed via ECG) and worsening in global cognition, as defined by a decline of a composite score from various tests in a time period of 10 years.43
Patient-based studies
In the single prospective study, referring to a population of 473 individuals with clinically manifested arterial disease (Second Manifestations of Arterial Disease—Magnetic Resonance Study; mean age 57 years), ECG-assessed LVH, did not show by itself any association with annual prospective decline of executive function and verbal memory scoring over a follow-up period of 4 years.27
Findings from cross-sectional studies
Population-based studies
Four of the cross-sectional studies (N ranging from 50 to 535 subjects) reported positive associations of LVH (assessed by TTE) with cognitive impairment (defined by a single instrument) or clinically defined dementia,26, 41, 42, 47 being statistically significant in 3 studies.26, 41, 47 However, the study by Jefferson et al (Framingham offspring; N=2,535; mean age 58 years) showed a null association (OR: 0.96).52 Only two of the studies presented unadjusted estimates,41, 42 whereas Reitz et al (N=59; mean age 76 years) showed a sizeable increased risk for AD (OR: 4.3; OR for VaD: 1.3), further adjusted for the findings for the APOE genotype.47 Importantly, in a sample of 400 elderly individuals (mean age 79 years), Scuteri et al. showed that the association of LVH with cognitive impairment was independent of the mean BP, use of antihypertensive medications and pulse wave velocity.26 However, in the Framingham Offspring study, (N=1673, mean age 57 years), the negative correlations between LVMI and performance in composite measurements of visuo-spatial ability, verbal memory, and processing speed/ executive function did not reach statistical significance.48
Patient-based studies
Three studies exclusively examined subjects with hypertension in cross-sectional designs, showing an increased risk of cognitive impairment (ORs range: 1.74–2.94; statistically significant only in 1 study), after adjustment for demographic, medical and vascular risk factor confounders.25, 51, 55 More specifically, Delgado et al.51 examined ECG-defined LVH in association with mild cognitive impairment in 701 individuals (mean age 63 years), whereas the remaining 2 studies assessed cognitive impairment by a single MMSE measurement and LVH by TTE.25, 55 Hayakawa et al.25 showed a linear association of LVM with MMSE score but also an association of LVH with the odds of scoring in the lowest MMSE quartile, which was interestingly retained even after adjustment for 24-h BP levels. Finally, LVH or increasing LVM did not show any association with cognitive impairment or MMSE scoring in cross-sectional analyses either of subjects with advanced stage chronic kidney disease in hemodialysis (N=168; mean age 45 years)53 or subjects with atrial fibrillation (N=94; mean age 75 years).46
Meta-analysis
Left ventricular hypertrophy and cognitive impairment in population-based studies
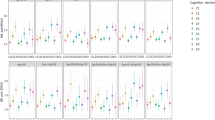
In the pooled analysis (9 studies; 28 648 subjects; 2562 cases), LVH was associated with an increased risk of cognitive impairment (OR: 1.40, 95% CI: 1.18–1.66; Figure 2a). No heterogeneity was documented (I2=0%), whereas the funnel plot was not representative of publication bias (Supplementary Figure 1). This effect was significant in both studies assessing LVH using the ECG criteria and studies using a TTE-based definition, although the effect was stronger in the latter (ECG, OR: 1.27, 95% CI: 1.05–1.54; TTE, OR: 1.88, 95% CI: 1.34–2.64). Figure 3 specifically presents the analysis stratified by the study design. These results were significant in both longitudinal (24 944 subjects; 2169 cases; Figure 3a) and cross-sectional studies (5239 subjects; 658 cases; Figure 3b). Interestingly, the stronger association of TTE-assessed LVH with cognitive impairment was evident only in studies of cross-sectional design, which included a much larger sample size, compared to longitudinal studies (5239 vs. 216 subjects). The sub-analyses confirmed the robustness of these findings (Table 2), as the results remained unchanged in sensitivity analyses of prospective studies, studies adjusting for the presence of hypertension/BP levels and studies of the highest quality. Sub-analyses for clinically diagnosed all-cause dementia, AD or VaD were compromised by the paucity of relevant studies.
Association between left ventricular hypertrophy and cognitive impairment in (a) studies of the general population and (b) studies with hypertensive individuals. Odds ratios of individual studies are indicated by the data markers; shaded boxes around the data markers reflect the statistical weight of the study; 95% confidence intervals (CI) are indicated by the error bars; pooled-effect estimates with its 95% CI are depicted as a diamond. The sub-analyses by method of left ventricular hypertrophy assessment are also provided.
Association between left ventricular hypertrophy and cognitive impairment in population-based (a) longitudinal cohort and (b) cross-sectional studies. Odds ratios of individual studies are indicated by the data markers; shaded boxes around data markers reflect the statistical weight of the study; 95% confidence intervals (CI) are indicated by the error bars; pooled-effect estimates with its 95% CI are depicted as a diamond. The sub-analyses by method of left ventricular hypertrophy assessment are also provided.
Left ventricular hypertrophy and cognitive impairment in hypertensive individuals
Among the studies solely on hypertensive individuals, LVH was associated with more than a twofold risk of cognitive impairment (OR: 2.14, 95% CI: 1.39–3.30), as presented in Figures 2b. A total of 1,262 subjects (195 cases) from three studies were included in the analysis, which was also not characterized by heterogeneity (I2=0). Similar to population-based studies, the pooled effect for studies assessing LVH by TTE was stronger (OR: 2.34), compared to the only study using the ECG definition (OR: 1.74).
Meta-regression analysis
The meta-regression analysis among population-based studies, presented in Supplementary Table 4, revealed no statistically significant modifying effect of mean age, gender, presence of hypertension, mean systolic BP and diastolic BP, presence of diabetes mellitus, method of LVH assessment and publication year on the association with cognitive impairment; it should be noted that the paucity of studies may have limited the power of these analyses.
To further explore the role of hypertension, we performed an overall meta-regression analysis, which also included studies on exclusively hypertensive individuals (presence of hypertension: 100%). It was found that the increasing percentage of hypertensive individuals in this group of 12 studies reinforced the association of LVH with cognitive impairment (exponentiated coefficient by 5% increments of hypertension presence: 1.028, 95% CI: 1.003–1.055, P=0.03; Figure 4).
Results of the random-effects meta-regression analysis regarding the effect of hypertension (expressed in % of population under study) on the association between left ventricular hypertrophy and dementia. The cycles correspond to the individual study estimates (Log (odds ratio)), with their size indicating the study weight.
Discussion
This systematic review supports an increased risk of cognitive impairment among subjects with LVH, which is an effect observed in both the general population and among subjects with hypertension. This finding was evident for both ECG and TTE-assessed LVH, although the effect estimate was higher for the latter method. In addition, this increased risk appears to be independent of hypertension and BP levels, although the presence of hypertension was found to reinforce the findings. The findings were also replicated in the highest quality and prospective studies, but there was a lack of longitudinal studies assessing dementia and its major subtypes using the established diagnostic criteria.
Previous studies have shown that LVH is an independent predictor of cardiovascular outcomes in hypertensive individuals and the general population,56, 57, 58, 59, 60, 61, 62 including stroke22, 23, 63 and cerebral microvascular lesions.24, 64 Its assessment via ECG is currently recommended for the screening of hypertensive individuals for target organ damage.65 Although its regression appears to be associated with an improvement of CVD risk,66, 67 it remains increased compared to individuals who have never had LVH.68 The current systematic review additionally contributes to this knowledge by implicating LVH as a risk factor for cognitive impairment in the general population and among hypertensive individuals. Through an extensive screening of >2000 abstracts and titles using a sound methodological approach based on standard guidelines,28 18 relevant articles were identified. The meta-analysis on community-based population was based on a large sample size of 28 648 subjects and it was possible to explore the robustness of the findings in sensitivity analyses, evaluate potential confounders through meta-regression and explore sources of bias.
Interpretation of findings
LVH is considered a compensatory adjustment of the heart muscle to chronic pressure load, aimed at reducing wall stress and maintaining adequate stroke volume. However, its pathogenic mechanism is multifactorial and complex, including both neurohormonal responses and genetic predisposition.69 LVH is considered a target organ damage in hypertension and its diagnosis has been associated with concurrent vascular damage in the kidney and retina;70, 71 thus, given the effect of high BP on cerebral circulation and cognition,11 these findings may underlie a generalized effect of chronic hypertension on both the heart and brain. This finding is supported by the stronger effect found in hypertensive individuals and the reinforcement of effect by the increasing presence of a hypertensive population in the included studies. Nevertheless, the effect was independent of BP levels, indicating that markers of endured exposure to hypertension rather than single BP measurements prone to several biases are better predictors of disease outcome. Indeed, according to epidemiological studies, only midlife and not later life BP measurements appear to be associated with cognition in the elderly.48, 72, 73, 74, 75, 76 Hypertension is particularly related to concentric cardiac remodeling, and previous studies have shown that concentric hypertrophy is more strongly related to cerebrovascular lesions compared to eccentric remodeling.24, 64 However, none of the studies in this systematic review evaluated the association of LVH type with cognition. Compared to brachial pressures, central BP measurements have been more strongly correlated to left ventricular geometry and LVH in previous studies.77, 78 These findings may particularly underlie the effect of elevated central BP on brain vasculature, given that central BP is linearly related to the risk of brain small vessel disease.79 Another interesting parameter that should be taken into account is blood pressure variability, which has also been positively correlated with LVMI80, 81 and has been shown to be associated with cerebral small vessel disease in both cross-sectional and prospective studies.82, 83
Because the findings were also evident in studies of the general population and remained significant after adjustment for major confounders, including hypertension, LVH could at least partly and independently lead to cognitive impairment via its hemodynamic consequences. In particular, LVH is associated with both systolic and diastolic left ventricular dysfunction,84, 85 resulting in decreased stroke volume and systemic hypoperfusion, which could predispose to cerebral ischemia. Indeed, decreased ejection fraction and diastolic dysfunction have been associated with the presence of white matter hyperintensities.86, 87 In addition, myocardial fibrosis accompanying LVH comprises an appropriate substrate for ventricular arrhythmogenesis,88 but also potentially via the consequent atrial load and hypertrophy for atrial fibrillation.89, 90 Atrial fibrillation is related to cognitive decline primarily via embolic infarction.91
Finally, aging, which comprises the strongest independent predictor of cognitive decline,2 could be a common factor underlying both phenomena. Advancing age leads to increased aortic stiffness, which increases the pressure load in the left ventricle, thereby predisposing to LVH.92 Furthermore, arterial stiffness has been related to cerebral small vessel disease and cognitive impairment.93 Nevertheless, most of the studies adjusted for age, whereas meta-regression for age did not affect the findings. Likewise, in some of the individual studies, LVH was associated with cognition independent of arterial stiffness measures.25, 26
Methodological considerations
The study findings should be interpreted considering the limitations, which were mainly related to the heterogeneity of the included studies. First, there was high variability regarding the LVH assessment. In particular, the studies did not only differ on the use of TTE or ECG but also on the specific criteria applied for each method. Thus, a clinical recommendation based on the studies is not feasible. However, LVH assessed by either method was independently associated with the risk of cognitive impairment, although the effect was stronger for TTE. ECG is less sensitive compared to TTE in the assessment of LVH,36 but both were independently associated with cardiovascular outcomes.37 The higher risk associated with TTE could be related to its higher sensitivity36 and thus better categorization of subjects with less pronounced LVH, who are also at an increased risk of adverse outcomes is needed.94 Importantly, the difference was non-significant in the meta-regression analysis and should be viewed in the context of statistical uncertainty.
Second, most relevant studies were of cross-sectional design, whereas consistent prospective data were available only for ECG-assessed LVH; this was also observed in the sensitivity analysis of the prospective studies, in which despite documenting statistically significant results, the data showed that these were attributed only to studies using ECG. This also does not allow an evaluation of the prognostic significance of LVH regarding dementia development or cognitive decline.
Third, the included sample of studies was characterized by highly heterogeneous measures of cognitive outcomes. The studies did not only differ regarding the neuropsychological tests used but also on the specific cutoff point defining the cognitive impairment. Notably, no heterogeneity was recorded in the meta-analysis though. In addition, due to the paucity of relevant studies, it was not possible to examine via meta-analysis the association of LVH with specific cognitive deficits and most importantly, with the diagnosis of dementia and its major subtypes.
Implications and future perspectives
Currently, LVH assessment is recommended in the clinical setting via ECG for subjects with hypertension65 as a major predictor of negative CVD outcomes.56, 57, 58, 59, 60, 61, 62 Treatment of hypertension in patients with LVH aims at regression of LVH, given the associated decrease in CVD risk.67, 95 On the basis of the current findings, it would be intriguing to assess whether pharmacological LVH regression could also affect the risk of cognitive impairment and delay cognitive decline among individuals with hypertension or even in the general population in future studies. However, future large prospective studies should replicate the findings using established diagnostic criteria for the definition of dementia, AD and VaD, and further assess whether the findings are mediated by cerebral microvascular disease.
Conclusions
In conclusion, there is evidence that LVH, assessed either electrocardiographically or echocardiographically, is independently associated with an increased risk of cognitive impairment. However, there is a lack of large prospective studies examining clinically diagnosed dementia and its subtypes as outcomes. The findings most likely reflect the hemodynamic consequences of chronic hypertension on the cerebral circulation and potentially indicate via LVH assessment a window into the health of brain vasculature.
References
Ward A, Arrighi HM, Michels S, Cedarbaum JM . Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimers Dement 2012; 8: 14–21.
Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP . The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 2013; 9: 63–75 e62.
Snyder HM, Corriveau RA, Craft S, Faber JE, Greenberg SM, Knopman D, Lamb BT, Montine TJ, Nedergaard M, Schaffer CB, Schneider JA, Wellington C, Wilcock DM, Zipfel GJ, Zlokovic B, Bain LJ, Bosetti F, Galis ZS, Koroshetz W, Carrillo MC . Vascular contributions to cognitive impairment and dementia including Alzheimer's disease. Alzheimers Dement 2015; 11: 710–717.
Gudala K, Bansal D, Schifano F, Bhansali A . Diabetes mellitus and risk of dementia: a meta-analysis of prospective observational studies. J Diabetes Invest 2013; 4: 640–650.
Xu W, Tan L, Wang HF, Jiang T, Tan MS, Tan L, Zhao QF, Li JQ, Wang J, Yu JT . Meta-analysis of modifiable risk factors for Alzheimer's disease. J Neurol Neurosurg Psychiatry 2015; 86: 1299–1306.
Anstey KJ, Lipnicki DM, Low LF . Cholesterol as a risk factor for dementia and cognitive decline: a systematic review of prospective studies with meta-analysis. Am J Geriatr Psychiatry 2008; 16: 343–354.
Novak V, Hajjar I . The relationship between blood pressure and cognitive function. Nat Rev Cardiol 2010; 7: 686–698.
Reitz C, Luchsinger JA . Relation of blood pressure to cognitive impairment and dementia. Curr Hypertens Rev 2007; 3: 166–176.
Ryuno H, Kamide K, Gondo Y, Nakama C, Oguro R, Kabayama M, Kawai T, Kusunoki H, Yokoyama S, Imaizumi Y, Takeya M, Yamamoto H, Takeda M, Takami Y, Itoh N, Yamamoto K, Takeya Y, Sugimoto K, Nakagawa T, Ikebe K, Inagaki H, Masui Y, Ishizaki T, Takayama M, Arai Y, Takahashi R, Rakugi H . Differences in the association between high blood pressure and cognitive functioning among the general Japanese population aged 70 and 80 years: the SONIC study. Hypertens Res 2016; 39: 557–563.
Wu L, He Y, Jiang B, Liu M, Wang J, Yang S, Wang Y . The association between the prevalence, treatment and control of hypertension and the risk of mild cognitive impairment in an elderly urban population in China. Hypertens Res 2016; 39: 367–375.
Pires PW, Dams Ramos CM, Matin N, Dorrance AM . The effects of hypertension on the cerebral circulation. Am J Physiol Heart Circ Physiol 2013; 304: H1598–H1614.
Neves MF, Virdis A, Oigman W . Target organ damage in hypertension. Int J Hypertens 2012; 2012: 454508.
Knopman DS, Mosley TH Jr, Bailey KR, Jack CR Jr, Schwartz GL, Turner ST . Associations of microalbuminuria with brain atrophy and white matter hyperintensities in hypertensive sibships. J Neurol Sci 2008; 271: 53–60.
Heringa SM, Bouvy WH, van den Berg E, Moll AC, Kappelle LJ, Biessels GJ . Associations between retinal microvascular changes and dementia, cognitive functioning, and brain imaging abnormalities: a systematic review. J Cereb Blood Flow Metab 2013; 33: 983–995.
Barzilay JI, Gao P, O'Donnell M, Mann JF, Anderson C, Fagard R, Probstfield J, Dagenais GR, Teo K, Yusuf S . Ontarget, Investigators T. Albuminuria and decline in cognitive function: The ONTARGET/TRANSCEND studies. Arch Intern Med 2011; 171: 142–150.
Georgakis M, Dimitriou N, Karalexi M, Mihas C, Nasothimiou E, Tousoulis D, Tsivgoulis G, Petridou E . Albuminuria in association with cognitive function and dementia: a systematic review and meta-analysis. J Am Geriatr Soc 2016; 88: 198–208.
Lauer MS, Anderson KM, Levy D . Influence of contemporary versus 30-year blood pressure levels on left ventricular mass and geometry: the Framingham Heart Study. J Am Coll Cardiol 1991; 18: 1287–1294.
Ruilope LM, Schmieder RE . Left ventricular hypertrophy and clinical outcomes in hypertensive patients. Am J Hypertens 2008; 21: 500–508.
Devereux RB, de Simone G, Koren MJ, Roman MJ, Laragh JH . Left ventricular mass as a predictor of development of hypertension. Am J Hypertens 1991; 4: 603S–607S.
Rabkin SW, Chan SH . Correlation of pulse wave velocity with left ventricular mass in patients with hypertension once blood pressure has been normalized. Heart Int 2012; 7: e5.
Gosse P . Left ventricular hypertrophy as a predictor of cardiovascular risk. J Hypertens Suppl 2005; 23: S27–S33.
Bikkina M, Levy D, Evans JC, Larson MG, Benjamin EJ, Wolf PA, Castelli WP . Left ventricular mass and risk of stroke in an elderly cohort. The Framingham Heart Study. JAMA 1994; 272: 33–36.
McAreavey D, Vidal JS, Aspelund T, Owens DS, Hughes T, Garcia M, Sigurdsson S, Bjornsdottir H, Harris TB, Gudnason V, Launer LJ, Plehn JF . Correlation of echocardiographic findings with cerebral infarction in elderly adults: the AGES-Reykjavik study. Stroke 2010; 41: 2223–2228.
Selvetella G, Notte A, Maffei A, Calistri V, Scamardella V, Frati G, Trimarco B, Colonnese C, Lembo G . Left ventricular hypertrophy is associated with asymptomatic cerebral damage in hypertensive patients. Stroke 2003; 34: 1766–1770.
Hayakawa M, Yano Y, Kuroki K, Inoue R, Nakanishi C, Sagara S, Koga M, Kubo H, Imakiire S, Aoyagi Z, Kitani M, Kanemaru K, Hidehito S, Shimada K, Kario K . Independent association of cognitive dysfunction with cardiac hypertrophy irrespective of 24-h or sleep blood pressure in older hypertensives. Am J Hypertens 2012; 25: 657–663.
Scuteri A, Coluccia R, Castello L, Nevola E, Brancati AM, Volpe M . Left ventricular mass increase is associated with cognitive decline and dementia in the elderly independently of blood pressure. Eur Heart J 2009; 30: 1525–1529.
van der Veen PH, Geerlings MI, Visseren FL, Nathoe HM, Mali WP, van der Graaf Y, Muller M, Group SS. Hypertensive target organ damage and longitudinal changes in brain structure and function: the Second Manifestations of Arterial Disease-Magnetic Resonance Study. Hypertension 2015; 66: 1152–1158.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB . Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283: 2008–2012.
Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N . Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986; 57: 450–458.
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT St, John Sutton M, Stewart W . American Society of Echocardiography's N, Standards C, Task Force on Chamber Q, American College of Cardiology Echocardiography C, American Heart A, European Association of Echocardiography ESoC. Recommendations for chamber quantification. Eur J Echocardiogr 2006; 7: 79–108.
Soliman EZ, Howard G, Prineas RJ, McClure LA, Howard VJ . Calculating Cornell voltage from nonstandard chest electrode recording site in the Reasons for Geographic And Racial Differences in Stroke study. J Electrocardiol 2010; 43: 209–214.
Gosse P, Jan E, Coulon P, Cremer A, Papaioannou G, Yeim S . ECG detection of left ventricular hypertrophy: the simpler, the better? J Hypertens 2012; 30: 990–996.
Casale PN, Devereux RB, Alonso DR, Campo E, Kligfield P . Improved sex-specific criteria of left ventricular hypertrophy for clinical and computer interpretation of electrocardiograms: validation with autopsy findings. Circulation 1987; 75: 565–572.
Casale PN, Devereux RB, Kligfield P, Eisenberg RR, Miller DH, Chaudhary BS, Phillips MC . Electrocardiographic detection of left ventricular hypertrophy: development and prospective validation of improved criteria. J Am Coll Cardiol 1985; 6: 572–580.
Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Dahlof B . Baseline characteristics in relation to electrocardiographic left ventricular hypertrophy in hypertensive patients: the Losartan intervention for endpoint reduction (LIFE) in hypertension study. The Life Study Investigators. Hypertension 2000; 36: 766–773.
Pewsner D, Juni P, Egger M, Battaglia M, Sundstrom J, Bachmann LM . Accuracy of electrocardiography in diagnosis of left ventricular hypertrophy in arterial hypertension: systematic review. BMJ 2007; 335: 711.
Sundstrom J, Lind L, Arnlov J, Zethelius B, Andren B, Lithell HO . Echocardiographic and electrocardiographic diagnoses of left ventricular hypertrophy predict mortality independently of each other in a population of elderly men. Circulation 2001; 103: 2346–2351.
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P The Newcastle-Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-analyses. 2011. Availale at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm Accessed 28 Jun 2015.
Herzog R, Alvarez-Pasquin MJ, Diaz C, Del Barrio JL, Estrada JM, Gil A . Are healthcare workers' intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health 2013; 13: 154.
DerSimonian R, Laird N . Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188.
Scuteri A, Tesauro M, Guglini L, Lauro D, Fini M, Di Daniele N . Aortic stiffness and hypotension episodes are associated with impaired cognitive function in older subjects with subjective complaints of memory loss. Int J Cardiol 2013; 169: 371–377.
Chrysohoou C, Psaltopoulou T, Panagiotakos D, Pitsavos C, Lazaros G, Skoumas J, Oikonomou E, Poulidakis E, Striggou M, Stefanadis C . Aortic elastic properties and cognitive function in elderly individuals: the Ikaria Study. Maturitas 2013; 74: 241–245.
Kaffashian S, Dugravot A, Brunner EJ, Sabia S, Ankri J, Kivimaki M, Singh-Manoux A . Midlife stroke risk and cognitive decline: a 10-year follow-up of the Whitehall II cohort study. Alzheimers Dement 2013; 9: 572–579.
Unverzagt FW, McClure LA, Wadley VG, Jenny NS, Go RC, Cushman M, Kissela BM, Kelley BJ, Kennedy R, Moy CS, Howard V, Howard G . Vascular risk factors and cognitive impairment in a stroke-free cohort. Neurology 2011; 77: 1729–1736.
van den Hurk K, Reijmer YD, van den Berg E, Alssema M, Nijpels G, Kostense PJ, Stehouwer CD, Paulus WJ, Kamp O, Dekker JM, Biessels GJ . Heart failure and cognitive function in the general population: the Hoorn Study. Eur J Heart Fail 2011; 13: 1362–1369.
Wozakowska-Kaplon B, Opolski G, Kosior D, Jaskulska-Niedziela E, Maroszynska-Dmoch E, Wlosowicz M . Cognitive disorders in elderly patients with permanent atrial fibrillation. Kardiologia Polska 2009; 67: 487–493.
Reitz C, Brickman AM, Luchsinger JA, Wu WE, Small SA, Tang MX . Frequency of subclinical heart disease in elderly persons with dementia. Am J Geriat Cardiol 2007; 16: 183–188.
Elias MF, Sullivan LM, Elias PK, D'Agostino RB Sr., Wolf PA, Seshadri S, Au R, Benjamin EJ, Vasan RS . Left ventricular mass, blood pressure, and lowered cognitive performance in the Framingham offspring. Hypertension 2007; 49: 439–445.
Kahonen-Vare M, Brunni-Hakala S, Lindroos M, Pitkala K, Strandberg T, Tilvis R . Left ventricular hypertrophy and blood pressure as predictors of cognitive decline in old age. Aging Clin Exp Res 2004; 16: 147–152.
Katzman R, Aronson M, Fuld P, Kawas C, Brown T, Morgenstern H, Frishman W, Gidez L, Eder H, Ooi WL . Development of dementing illnesses in an 80-year-old volunteer cohort. Ann Neurol 1989; 25: 317–324.
Delgado P, Riba-Llena I, Tovar JL, Jarca CI, Mundet X, Lopez-Rueda A, Orfila F, Llussa J, Manresa JM, Alvarez-Sabin J, Nafria C, Fernandez JL, Maisterra O, Montaner J . Prevalence and associated factors of silent brain infarcts in a Mediterranean cohort of hypertensives. Hypertension 2014; 64: 658–663.
Jefferson AL, Himali JJ, Au R, Seshadri S, Decarli C, O'Donnell CJ, Wolf PA, Manning WJ, Beiser AS, Benjamin EJ . Relation of left ventricular ejection fraction to cognitive aging (from the Framingham Heart Study). Am J Cardiol 2011; 108: 1346–1351.
Ok E, Duman S, Asci G, Tumuklu M, Onen Sertoz O, Kayikcioglu M, Toz H, Adam SM, Yilmaz M, Tonbul HZ, Ozkahya M . Comparison of 4- and 8-h dialysis sessions in thrice-weekly in-centre haemodialysis: a prospective, case-controlled study. Nephrol Dialysis Transplant 2011; 26: 1287–1296.
Muscari A, Giannoni C, Pierpaoli L, Berzigotti A, Maietta P, Foschi E, Ravaioli C, Poggiopollini G, Bianchi G, Magalotti D, Tentoni C, Zoli M . Chronic endurance exercise training prevents aging-related cognitive decline in healthy older adults: a randomized controlled trial. Int J Geriat Psychiatry 2010; 25: 1055–1064.
Triantafyllidi H, Arvaniti C, Lekakis J, Ikonomidis I, Siafakas N, Tzortzis S, Trivilou P, Zerva L, Stamboulis E, Kremastinos DT . Cognitive impairment is related to increased arterial stiffness and microvascular damage in patients with never-treated essential hypertension. Am J Hypertens 2009; 22: 525–530.
Rautaharju PM, Soliman EZ . Electrocardiographic left ventricular hypertrophy and the risk of adverse cardiovascular events: a critical appraisal. J Electrocardiol 2014; 47: 649–654.
Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP . Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990; 322: 1561–1566.
Kannel WB . Left ventricular hypertrophy as a risk factor in arterial hypertension. Eur Heart J 1992; 13: 82–88.
Kannel WB, Dannenberg AL, Levy D . Population implications of electrocardiographic left ventricular hypertrophy. Am J Cardiol 1987; 60: 85I–93I.
Dunn FG, McLenachan J, Isles CG, Brown I, Dargie HJ, Lever AF, Lorimer AR, Murray GD, Pringle SD, Robertson JW . Left ventricular hypertrophy and mortality in hypertension: an analysis of data from the Glasgow Blood Pressure Clinic. J Hypertens 1990; 8: 775–782.
Levy D, Salomon M, D'Agostino RB, Belanger AJ, Kannel WB . Prognostic implications of baseline electrocardiographic features and their serial changes in subjects with left ventricular hypertrophy. Circulation 1994; 90: 1786–1793.
Aronow WS, Ahn C, Kronzon I, Koenigsberg M . Congestive heart failure, coronary events and atherothrombotic brain infarction in elderly blacks and whites with systemic hypertension and with and without echocardiographic and electrocardiographic evidence of left ventricular hypertrophy. Am J Cardiol 1991; 67: 295–299.
Verdecchia P, Porcellati C, Reboldi G, Gattobigio R, Borgioni C, Pearson TA, Ambrosio G . Left ventricular hypertrophy as an independent predictor of acute cerebrovascular events in essential hypertension. Circulation 2001; 104: 2039–2044.
Kohara K, Zhao B, Jiang Y, Takata Y, Fukuoka T, Igase M, Miki T, Hiwada K . Relation of left ventricular hypertrophy and geometry to asymptomatic cerebrovascular damage in essential hypertension. Am J Cardiol 1999; 83: 367–370.
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Redon J, Dominiczak A, Narkiewicz K, Nilsson PM, Burnier M, Viigimaa M, Ambrosioni E, Caufield M, Coca A, Olsen MH, Schmieder RE, Tsioufis C, van de Borne P, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Clement DL, Coca A, Gillebert TC, Tendera M, Rosei EA, Ambrosioni E, Anker SD, Bauersachs J, Hitij JB, Caulfield M, De Buyzere M, De Geest S, Derumeaux GA, Erdine S, Farsang C, Funck-Brentano C, Gerc V, Germano G, Gielen S, Haller H, Hoes AW, Jordan J, Kahan T, Komajda M, Lovic D, Mahrholdt H, Olsen MH, Ostergren J, Parati G, Perk J, Polonia J, Popescu BA, Reiner Z, Ryden L, Sirenko Y, Stanton A, Struijker-Boudier H, Tsioufis C, van de Borne P, Vlachopoulos C, Volpe M, Wood DA . 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34: 2159–2219.
Pierdomenico SD, Cuccurullo F . Risk reduction after regression of echocardiographic left ventricular hypertrophy in hypertension: a meta-analysis. Am J Hypertens 2010; 23: 876–881.
Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Nieminen MS, Snapinn S, Harris KE, Aurup P, Edelman JM, Wedel H, Lindholm LH, Dahlof B, Investigators LS . Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA 2004; 292: 2343–2349.
Angeli F, Reboldi G, Poltronieri C, Stefanetti E, Bartolini C, Verdecchia P, Investigators M . The prognostic legacy of left ventricular hypertrophy: cumulative evidence after the MAVI study. J Hypertens 2015; 33: 2322–2330.
Frohlich ED, Gonzalez A, Diez J . Hypertensive left ventricular hypertrophy risk: beyond adaptive cardiomyocytic hypertrophy. J Hypertens 2011; 29: 17–26.
Shigematsu Y, Hamada M, Mukai M, Matsuoka H, Sumimoto T, Hiwada K . Clinical evidence for an association between left ventricular geometric adaptation and extracardiac target organ damage in essential hypertension. J Hypertens 1995; 13: 155–160.
Blake J, Devereux RB, Herrold EM, Jason M, Fisher J, Borer JS, Laragh JH . Relation of concentric left ventricular hypertrophy and extracardiac target organ damage to supranormal left ventricular performance in established essential hypertension. Am J Cardiol 1988; 62: 246–252.
Desmond DW, Tatemichi TK, Paik M, Stern Y . Risk factors for cerebrovascular disease as correlates of cognitive function in a stroke-free cohort. Arch Neurol 1993; 50: 162–166.
Battersby C, Hartley K, Fletcher AE, Markowe HJ, Brown RG, Styles W, Carne S, Jamieson T, Koppel I, Fraser S . Cognitive function in hypertension: a community based study. J Hum Hypertens 1993; 7: 117–123.
Elias MF, Wolf PA, D'Agostino RB, Cobb J, White LR . Untreated blood pressure level is inversely related to cognitive functioning: the Framingham Study. Am J Epidemiol 1993; 138: 353–364.
van Boxtel MP, Gaillard C, Houx PJ, Buntinx F, de Leeuw PW, Jolles J . Can the blood pressure predict cognitive task performance in a healthy population sample? J Hypertens 1997; 15: 1069–1076.
Breteler MM, Claus JJ, Grobbee DE, Hofman A . Cardiovascular disease and distribution of cognitive function in elderly people: the Rotterdam Study. BMJ 1994; 308: 1604–1608.
Roman MJ, Okin PM, Kizer JR, Lee ET, Howard BV, Devereux RB . Relations of central and brachial blood pressure to left ventricular hypertrophy and geometry: the Strong Heart Study. J Hypertens 2010; 28: 384–388.
Wohlfahrt P, Wichterle D, Seidlerova J, Filipovsky J, Bruthans J, Adamkova V, Cifkova R . Relation of central and brachial blood pressure to left ventricular hypertrophy. The Czech Post-MONICA Study. J Hum Hypertens 2012; 26: 14–19.
Ochi N, Kohara K, Tabara Y, Nagai T, Kido T, Uetani E, Ochi M, Igase M, Miki T . Association of central systolic blood pressure with intracerebral small vessel disease in Japanese. Am J Hypertens 2010; 23: 889–894.
Cipollini F, Arcangeli E, Seghieri G . Left atrial dimension is related to blood pressure variability in newly diagnosed untreated hypertensive patients. Hypertens Res 2016; 39: 583–587.
Madden JM, O'Flynn AM, Fitzgerald AP, Kearney PM . Correlation between short-term blood pressure variability and left-ventricular mass index: a meta-analysis. Hypertens Res 2016; 39: 171–177.
Liu Z, Zhao Y, Zhang H, Chai Q, Cui Y, Diao Y, Xiu J, Sun X, Jiang G . Excessive variability in systolic blood pressure that is self-measured at home exacerbates the progression of brain white matter lesions and cognitive impairment in the oldest old. Hypertens Res 2016; 39: 245–253.
Yamaguchi Y, Wada M, Sato H, Nagasawa H, Koyama S, Takahashi Y, Kawanami T, Kato T . Impact of nocturnal heart rate variability on cerebral small-vessel disease progression: a longitudinal study in community-dwelling elderly Japanese. Hypertens Res 2015; 38: 564–569.
Palmieri V, Wachtell K, Gerdts E, Bella JN, Papademetriou V, Tuxen C, Nieminen MS, Dahlof B . de Simone G, Devereux RB. Left ventricular function and hemodynamic features of inappropriate left ventricular hypertrophy in patients with systemic hypertension: the LIFE study. Am Heart J 2001; 141: 784–791.
Stork T, Mockel M, Danne O, Voller H, Eichstadt H, Frei U . Left ventricular hypertrophy and diastolic dysfunction: their relation to coronary heart disease. Cardiovasc Drugs Ther 1995; 9: 533–537.
Shimizu A, Sakurai T, Mitsui T, Miyagi M, Nomoto K, Kokubo M, Bando YK, Murohara T, Toba K . Left ventricular diastolic dysfunction is associated with cerebral white matter lesions (leukoaraiosis) in elderly patients without ischemic heart disease and stroke. Geriatr Gerontol Int 2014; 14: 71–76.
Vogels RL, van der Flier WM, van Harten B, Gouw AA, Scheltens P, Schroeder-Tanka JM, Weinstein HC . Brain magnetic resonance imaging abnormalities in patients with heart failure. Eur J Heart Fail 2007; 9: 1003–1009.
Kahan T, Bergfeldt L . Left ventricular hypertrophy in hypertension: its arrhythmogenic potential. Heart 2005; 91: 250–256.
Okin PM, Wachtell K, Devereux RB, Harris KE, Jern S, Kjeldsen SE, Julius S, Lindholm LH, Nieminen MS, Edelman JM, Hille DA, Dahlof B . Regression of electrocardiographic left ventricular hypertrophy and decreased incidence of new-onset atrial fibrillation in patients with hypertension. JAMA 2006; 296: 1242–1248.
Kirchhof P, Schotten U . Hypertension begets hypertrophy begets atrial fibrillation? Insights from yet another sheep model. Eur Heart J 2006; 27: 2919–2920.
Thacker EL, McKnight B, Psaty BM, Longstreth WT Jr, Sitlani CM, Dublin S, Arnold AM, Fitzpatrick AL, Gottesman RF, Heckbert SR . Atrial fibrillation and cognitive decline: a longitudinal cohort study. Neurology 2013; 81: 119–125.
Chung CM, Lin YS, Chu CM, Chang ST, Cheng HW, Yang TY, Hsiao JF, Pan KL, Hsu JT . Arterial stiffness is the independent factor of left ventricular hypertrophy determined by electrocardiogram. Am J Med Sci 2012; 344: 190–193.
van Sloten TT, Protogerou AD, Henry RM, Schram MT, Launer LJ, Stehouwer CD . Association between arterial stiffness, cerebral small vessel disease and cognitive impairment: a systematic review and meta-analysis. Neurosci Biobehav Rev 2015; 53: 121–130.
Park JS, Shin JS, Lee YH, Seo KW, Choi BJ, Choi SY, Yoon MH, Hwang GS, Tahk SJ, Shin JH . Left ventricular hypertrophy on long-term cardiovascular outcomes in patients with ST-elevation myocardial infarction. Clin Exp Hypertens 2015; 37: 674–679.
Pierdomenico SD, Lapenna D, Cuccurullo F . Regression of echocardiographic left ventricular hypertrophy after 2 years of therapy reduces cardiovascular risk in patients with essential hypertension. Am J Hypertens 2008; 21: 464–470.
Acknowledgements
We are thankful to the authors of the studies that provided the primary data or analyses based on their data. Specifically, we would like to acknowledge the contributions made by Professor Antonio Muscari and Dr Pilar Delgado Martinez for sending the results of a requested re-analysis of their data, Professor Helen Triantafyllidi and Dr Gulay Asci for providing the raw data of their studies, and Professor Alexa Beiser for conducting a re-analysis of the data from the Framingham Offspring Study (the data collection for the Framingham Study was funded by contracts from the National Heart, Lung and Blood Institute [N01-HC-25195; HHSN268201500001l] and by grants obtained from the National Institute on Aging [AG008122] and the National Institute for Neurological Disorders and Stroke [NS017950]). We would also like to thank all the authors who replied to the request for additional data, as detailed in the Supplementary Table 2. We thank Mr Nikolaos Dimitriou for participating in the selection of studies, and lastly, we thank Dr Prodromos Kanavidis for developing the electronic platform for retrieving the abstracts for blind screening by the reviewers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Hypertension Research website
Supplementary information
Rights and permissions
About this article
Cite this article
Georgakis, M., Synetos, A., Mihas, C. et al. Left ventricular hypertrophy in association with cognitive impairment: a systematic review and meta-analysis. Hypertens Res 40, 696–709 (2017). https://doi.org/10.1038/hr.2017.11
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2017.11
Keywords
This article is cited by
-
Left ventricular hypertrophy, carotid atherosclerosis, and cognitive impairment in peritoneal dialysis patients
BMC Cardiovascular Disorders (2023)
-
Prognostic Significance of Echocardiographic Measures of Cardiac Remodeling in the Community
Current Cardiology Reports (2021)
-
Ambulatory Blood Pressure Monitoring in Pediatrics
Current Hypertension Reports (2019)
-
Hypertension Management at Older Age: An Update
High Blood Pressure & Cardiovascular Prevention (2019)
-
Hypertension-induced cognitive impairment: insights from prolonged angiotensin II infusion in mice
Hypertension Research (2018)