Abstract
Serum uric acid (SUA) is correlated with an increased risk of not only gout but also cardiovascular diseases. The present study aimed to longitudinally evaluate the effects of SUA level on renal function and arterial stiffness in a population-based sample of normotensive subjects. The subjects completed a health checkup in 2002 at baseline and in 2011 or 2012 at the end of the follow-up period. A total of 407 normotensive subjects (171 men and 236 women) aged 26–66 years were enrolled in this study. We measured blood pressure (BP), brachial-ankle pulse wave velocity (baPWV), central BP, intima–media thickness, SUA level and estimated glomerular filtration rate (eGFR). We divided the subjects into four subgroups according to the SUA quartile at baseline and compared renal function and arterial stiffness after the follow-up. The cutoff values were 3.6, 4.4, 5.6 and 9.6 mg dl−1. The SUA levels associated with baPWV (Q1, 1324; Q2, 1457; Q3, 1442; Q4, 1489 cm s−1), systolic BP (SBP) (Q1, 110.9; Q2, 110.1; Q3, 112.8; Q4. 116.1 mm Hg) and eGFR (P for trend <0.001). There was a significant difference in the incidence of arterial stiffness in women. Multivariate regression analyses showed that after adjusting for potential confounders, including age, sex, body mass index, SBP and lipids, SUA was a significant determinant of baPWV (β=0.117; P<0.05) and eGFR (β=−0.335, P<0.001). The results of this study suggest that elevated SUA levels may be associated with a higher risk of increased arterial stiffness and reduced renal function in normotensive subjects.
Similar content being viewed by others
Introduction
The adoption of Westernized diets has led to annual increases in the number of cases of gout and its cause, hyperuricemia. The prevalence of hyperuricemia among adult men in Japan is reported to be 20–25%.1 The Japanese Society of Gout and Nucleic Acid Metabolism has defined hyperuricemia as a serum uric acid (SUA) level of >7.0 mg dl−1 irrespective of sex or age. Hyperuricemia is classified into three types: increased production, in which uric acid is produced excessively; decreased excretion, in which the capability of uric acid excretion is reduced because of decreased renal function; and mixed, which is a combination of both types. In Japan, 85% of all cases of gout and hyperuricemia involve decreased renal excretion of uric acid.2
Excess SUA is related to an increased risk of not only gout but also cardiovascular diseases. Several cohort studies have shown a link between SUA and subsequent cardiovascular disease.3, 4, 5 In addition, the SUA level is closely related to other cardiovascular disease risk factors, such as hypertension, hyperlipidemia and obesity.6, 7, 8, 9 Experimental models have demonstrated that elevated concentrations of SUA increase blood pressure (BP) without affecting kidney morphology,10 and lowering SUA levels can normalize BP.11 Moreover, SUA levels are also related to the onset or progression of chronic kidney disease.2 These effects have been proposed to be due to an increase in BP and endothelial dysfunction by hyperuricemia.
However, most of the results linking SUA levels and cardiovascular risk factors and renal function have been derived from studies of hypertensive patients.6, 7, 8, 12, 13, 14, 15 For example, Alderman et al.12 found an association between SUA levels and subsequent cardiovascular events in a large multiracial population of subjects with essential hypertension. Fang and Alderman8 found that an increased SUA level was independently and significantly associated with a risk of cardiovascular mortality in both sexes. Few studies have addressed this issue in normotensive subjects. If SUA levels are related to novel risk factors of atherosclerosis even in normotensive subjects, then we can intervene to control SUA from an early stage. Eventually, this intervention can lead to the prevention of the development of arteriosclerosis and deterioration of renal function in the future. Thus, the present study aimed to longitudinally evaluate the effect of SUA levels on arterial stiffness and renal function in a population-based sample of normotensive subjects.
Methods
Study subjects
The study subjects were selected from individuals participating in a population-based health study16 held in Minabe, Wakayama Prefecture, Japan. Each subject completed a baseline health checkup in 2002 and another checkup at the end of follow-up period in 2011 or 2012. A total of 407 normotensive subjects (171 men and 236 women) aged 26–66 years (mean age, 48.3±9.2 years) were enrolled in this study. Normotension was defined as a systolic BP (SBP) of <140 mm Hg and a diastolic BP of <90 mm Hg without the use of antihypertensive and antihyperuricemic medication. There were 31 participants taking medication for dyslipidemia and 5 subjects taking medication for diabetes. All participants provided their informed consent before the examinations. The study protocol was approved by the ethical committee of Wakayama Medical University.
Baseline measurements
We collected the medical histories and physical examination data of the participants. Height and weight were measured at each examination, and body mass index (kg m−2) was calculated as the weight in kg divided by the square of the height in meters. SBP and diastolic BP were measured twice in the left arm with the subject in a seated position using a mercury-column sphygmomanometer. The mean of the two readings was used for each BP variable. Blood samples were obtained after 12 h of fasting to determine of the following parameters using standard biochemical techniques: triglycerides, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and SUA that were detected using an enzymatic method (uricase-UV) and hemoglobin A1c (HbA1c). Spot urine was collected in the morning before the examination.
Markers of arterial stiffness
Each subject was placed in the supine position, with special cuffs placed bilaterally on the arms and legs, to investigate their bilateral brachial-ankle pulse wave velocity (baPWV) using a noninvasive arterial atherosclerosis measuring system (form PWV/ABI; Omron Healthcare, Kyoto, Japan). Radial arterial pulse waveforms were measured noninvasively using an automated tonometric system (HEM-9000AI; Omron Healthcare). The measurement was performed using a tonometer probe fastened to the right wrist and a BP cuff placed on the left arm. The late systolic peak of radial arterial waveforms (SBP2) was identified automatically using this device. Carotid arterial stiffness was determined using B-mode ultrasonography (GM-72 P00A: Panasonic, Kanagawa, Japan). Subjects were kept in the supine position with their heads slightly extended. The intima–media thickness (IMT) was measured on the far wall of both sides of the common carotid artery, ∼10 mm proximal to the bifurcation of the carotid artery.
Markers of renal function
We assessed the estimated glomerular filtration rate (eGFR) based on the patient’s serum creatinine level, age and sex using the Japanese equation.17
We adjusted the creatinine clearance to offset the bias based on the relationship between creatinine values measured by the Jaffe and enzymatic methods: (serum creatinine by the Jaffe method (mg dl−1)=serum creatinine by the enzymatic method (mg dl−1)+0.2).18
Statistical analyses
Data are presented as mean±s.d. unless otherwise specified. Logarithmic transformation was performed before analysis if the variables were not normally distributed. We divided the subjects into four subgroups according to the SUA level quartiles at baseline and compared their arterial stiffness and renal function after the follow-up period. The quartile division points were 3.6, 4.4, 5.6 and 9.6 mg dl−1. Differences in the characteristics of subjects between baseline and the end of follow-up were analyzed using paired t-tests. The differences between the SUA groups at baseline were analyzed using the Bonferroni multiple comparison. The effect of SUA levels on arterial stiffness was investigated after controlling for sex, age and BP, and the one on renal function was investigated after controlling for sex, age and eGFR by analysis of covariance. Next, separate analyses were performed for men and women. All participants were divided according to sex-specific SUA quartiles, and the effects of SUA levels on arterial stiffness were investigated as described above. To evaluate the independent determinants of arterial stiffness and renal function, multiple regression analyses were performed. In the analyses, age and sex were forced into the model, and body mass index, SBP, diastolic BP, SUA, eGFR, HbA1c, triglycerides, high-density lipoprotein cholesterol and low-density lipoprotein cholesterol were entered using a stepwise selection method. A P-value of <0.05 was considered significant. Data analyses were performed using SPSS 16.0 for Windows (SPSS Software, Chicago, IL, USA).
Results
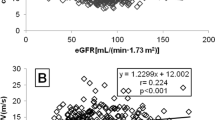
Table 1 shows the characteristics of subjects at both the baseline and follow-up measurements. The mean age of the subjects at their baseline was 48.3±9.2 years (men, 48.3±9.8 years; women, 48.3±8.7 years). The mean SUA level was 4.7±1.4 mg dl−1 at baseline and 4.8±1.4 mg dl−1 at the follow-up examination. At the end of the follow-up period, the subjects had significantly higher SBP, total cholesterol, high-density lipoprotein cholesterol and HbA1c, and significantly lower eGFR than at baseline. Table 2 shows the characteristics of the subjects by SUA quartiles at baseline. Individuals with high SUA levels had higher body mass index, triglycerides and eGFR, but lower high-density lipoprotein cholesterol. In particular, subjects in the higher SUA quartiles had high triglycerides. In addition, 88% of men were in the highest quartile. Table 3 shows the relationships among SUA levels, BP, arterial stiffness and renal function. A gradual association with SUA levels was found for baPWV (P for trend <0.01) and eGFR (P for trend <0.001). However, there was no significant difference in BP, SBP2 or mean IMT among the SUA subgroups.
When the analyses were performed separately by sex, significant differences in arterial stiffness markers were not observed among men. However, there was a significant difference in baPWV among the women. In addition, we found an association between SUA levels and eGFR in both sexes. Table 4 shows the multiple regression analyses for the independent determinants of BP, arterial stiffness and renal function. After adjusting for potential confounding variables, including age, sex, SBP, SUA, eGFR and HbA1c, the SUA level was found to be a significant determinant of baPWV (β=0.117; P<0.05) and eGFR (β=−0.335, P<0.001).
Discussion
The present study demonstrated that subjects in the high SUA quartiles at baseline had high baPWV and low eGFR at the end of the follow-up period. In addition, the SUA level was shown to be a significant determinant of baPWV and eGFR after adjusting for potential confounding variables, including age, sex and BP. Uric acid is the end product of purine metabolism. Epidemiological studies have demonstrated that elevated SUA levels are significantly associated with the progression of high BP.19, 20, 21 In our study, there was no significant difference in BP by SUA subgroups. We found an association between SUA levels and arterial stiffness. The development of atherosclerosis progresses in stages. SUA interacts as an aggravating factor in the early stages of this process.22 Nitric oxide (NO) plays an important role in endothelial function, and high uric acid levels decrease the production of NO.23 This finding suggests the possibility of endothelial dysfunction, caused by uric acid, being involved in the progression of atherosclerosis. In addition, reduced NO levels induce insulin resistance. In insulin-resistant states, the vasodilatory effect of insulin mediated by NO is blunted, resulting in a disruption in arterial blood flow.24 Furthermore, hyperuricemia has also been associated with elevated circulating endothelin levels,25 and one of the major sites of SUA production in the cardiovascular system is the vessel wall, notably in the endothelium.26 Taken together, these findings suggest that high SUA levels could be a marker of impaired hemodynamic reserves and/or disrupted blood flow.
We found a clearer association between SUA levels and arterial stiffness in women as compared with men. Among the recent large-scale prospective studies, a report from the Framingham Heart Study observed that after adjusting for diuretic use and menopausal status, there was a strong relationship between increased SUA levels and cardiovascular mortality in women.7 However, a report from the NHANES I (First National Health and Nutrition Examination Survey) showed a significant and independent association between SUA concentrations and cardiovascular and all-cause mortality in both men and women.8 However, a contradictory association between SUA levels and cardiovascular risk factors might exist because the SUA level is, at least in part, passively increased by various cardiovascular risk factors. First, SUA is higher in men27 and postmenopausal women because estrogen is known to be uricosuric. Second, hyperuricemia frequently occurs in obese subjects with insulin resistance because insulin stimulates sodium and urate reabsorption in the proximal tubule.28 Third, SUA levels are frequently increased in patients with hypertension, presumably because of a decrease in renal blood flow that stimulates urate reabsorption.29 Finally, increased SUA levels are closely associated with decreased renal urate excretion.
Our study revealed a gradual association between SUA levels and eGFR. High SUA levels might result in renal vasoconstriction by inhibiting the NO pathway and by activating the renin–angiotensin system.30, 31 In addition, SUA induces vascular smooth muscle cell proliferation, inflammation and oxidative stress, and elevated levels might lead to irreversible damage to small renal vessels, causing renal microvascular lesions and, subsequently, elevated BP.7 Renal function might also be decreased by these mechanisms. In our study, different outcomes in the two sexes were observed, and one or more of these mechanisms might be involved.
In addition, multivariate regression analyses demonstrated that SUA levels were significant determinants of baPWV and eGFR. The associations were still observed despite adjusting for potential confounding variables, including age, sex, SBP, eGFR and HbA1c values at baseline. When the analyses were performed separately by sex, we could not demonstrate a clear association between SUA levels and arterial stiffness in men. However, the results of multiple regression analyses suggest that elevated SUA levels may contribute to increased arterial stiffness in both sexes. A recent study by Ndrepepa et al.32 showed a stronger association between hyperuricemia and an increased risk of mortality in both sexes, with a stronger association in women, in a population of 13 273 patients. Consistent with the findings obtained in most studies, we found that despite the higher uric acid levels observed in men, a significant association between uric acid and coronary artery disease was observed only in women.33 In the present study, age is a more important determinant of baPWV in women than in men independent of blood pressure variables. Furthermore, baPWV is increased at ∼50–60 years of age in women.34 Although we could not confirm the menopausal status in each individual, these results suggest that menopause is an important factor influencing arterial stiffness in healthy female subjects. Estrogen has beneficial effects on arterial stiffness. The present study suggests that the exhaustion of estrogen enhances age-related arterial stiffing.
Of the several arterial stiffness markers, a significant association of SUA levels was found only in baPWV. The baPWV is a simple and reproducible measurement of arterial stiffness and is widely used in several studies.35 The baPWV reflects arterial wall structural components, such as collagen and elastin, as well as transmural pressure and smooth muscle tone that mainly regulate arterial vessel distensibility and function;36 baPWV has been reported to be a crucial independent determinant of cardiovascular risk.37 However, the SBP2 is the late systolic shoulder of the radial pressure waveform and is linked with central SBP. However, there are some unresolved issues regarding central BP. One problem that has been observed is that central BP is calculated by calibrating the upper arm BP radial artery waveform. The central BP, calculated in this manner, is dependent on the brachial pressure, making it difficult to determine whether these are different issues. In addition, the IMT is a marker of systemic and coronary atherosclerosis, and does not reflect the early stage of atherosclerosis. Although the reason why SUA levels were not associated with IMT still remains unclear, it can be speculated that our study subjects consist of healthy normotensive individuals and there were a few subjects who demonstrated abnormal levels of IMT.
There are several limitations in the present study. First, the study population may not be representative of the Japanese population as a whole. The subjects of this study resided in Minabe Town, Wakayama Prefecture, Japan. The National Health Care expenditure for this population is the fourth lowest in Japan, and the response rate to specific health checkups was higher than the rates observed in other towns. Thus, the people in this community appeared healthier than those in other Japanese communities. Furthermore, medical (nurse or co-medical) intervention may have affected our results. However, regardless, we found an association between elevated SUA levels and arterial stiffness, even among participants in this healthy community. Second, there was a lack of information regarding the history of drug use. We obtained the data at baseline and at the end of the follow-up period, but not throughout the study’s duration because of this being a retrospective cohort study. Third, it is possible that uric acid levels are increased as a negative feedback control in response to increased oxidative stress. Unfortunately, we did not measure the levels of plasma NO, antioxidants, lipid peroxides or H2S at baseline or at the end of the follow-up period. Thus, we cannot describe the association between uric acid and oxidative stress. Fourth, alcohol intake is strongly associated with uric acid levels, but in this study, we did not investigate alcohol intake.
Conclusion
This longitudinal study assessed the effects of SUA levels on arterial stiffness and renal function among a population-based sample of normotensive subjects. The results of this study suggest that elevated SUA levels may be associated with increased arterial stiffness and reduced renal function among normotensive subjects over a 10-year period.
References
Taniguchi A . Treatment of hyperuricemia and gout based on the guideline for the management of hyperuricemia and gout. Folia Pharmacol Jpn 2010; 136: 330–334.
Imamura S, Nakamura T . Characteristic features in pathophysiology of hyperuricemia with overproduction in gout. Purine Pyrimidine Metab 1994; 18: 132–143.
Feig DI, Kang DH, Johnson RJ . Uric acid and cardiovascular risk. N Engl J Med 2008; 359: 1811–1821.
Vučak J, Katić M, Bielen I, Vrdoljak D, Lalić DI, Kranjčević K, Marković BB . Association between hyperuricemia, prediabetes, and prehypertension in the Croatian adult population-a cross-sectional study. BMC Cardiovasc Disord 2012; 12: 117.
Kiyohara Y . Trends and current problem of lifestyle-related diseases: the Hisayama Study. J Jpn Soc Nutr Food Sci 2010; 63: 299–305.
Freedman DS, Williamson DF, Gunter EW, Byers T . Relation of serum uric acid to mortality and ischemic heart disease. The NHANES I Epidemiologic Follow-up Study. Am J Epidemiol 1995; 141: 637–644.
Culleton BF, Larson MG, Kannel WB, Levy D . Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med 1999; 131: 7–13.
Fang J, Alderman MH . Serum uric acid and cardiovascular mortality: the NHANES I epidemiologic follow-up study, 1971–1992. JAMA 2000; 283: 2404–2410.
Moriarity JT, Folsom AR, Iribarren C, Nieto FJ, Rosamond WD . Serum uric acid and risk of coronary heart disease: Atherosclerosis Risk in Communities (ARIC) Study. Ann Epidemiol 2000; 10: 136–143.
Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, Lan HY, Kivlighn S, Johnson RJ . Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension 2001; 38: 1101–1106.
Feig DI, Nakagawa T, Karumanchi SA, Oliver WJ, Kang DH, Finch J, Johnson RJ . Hypothesis: uric acid, nephron number, and the pathogenesis of essential hypertension. Kidney Int 2004; 66: 281–287.
Alderman MH, Cohen H, Madhavan S, Kivlighn S . Serum uric acid and cardiovascular events in successfully treated hypertensive patients. Hypertension 1999; 34: 144–150.
Verdecchia P, Schillaci G, Reboldi G, Santeusanio F, Porcellati C, Brunetti P . Relation between serum uric acid and risk of cardiovascular disease in essential hypertension: the PIUMA study. Hypertension 2000; 36: 1072–1078.
Franse LV, Pahor M, Di Bari M, Shorr RI, Wan JY, Somes GW, Applegate WB . Serum uric acid, diuretic treatment and risk of cardiovascular events in the Systolic Hypertension in the Elderly Program (SHEP). J Hypertens 2000; 18: 1149–1154.
Kohagura K, Tana T, Higa A, Yamazato M, Ishida A, Nagahama K, Sakima A, Iseki K, Ohya Y . Effects of xanthine oxidase inhibitors on renal function and blood pressure in hypertensive patients with hyperuricemia. Hypertens Res 2016; 39: 593–597.
Ministry of Health, Labour and Welfare Specific Health Checkups and Specific Health Guidance. Ministry of Health, Labour and Welfare [serial online]. Available at http://www.mhlw.go.jp/english/wp/wp-hw3/dl/2-007.pdf Accessed on 6 Feb 2016.
Imai E, Yasuda Y, Makino H . Japan Association of Chronic Kidney Disease Initiatives (J-CKDI). Japan Med Assoc J 2011; 54: 403–405.
Ando Y, Minami H, Saka H, Ando M, Sakai S, Shimokata K . Adjustment of creatinine clearance improves accuracy of Calvert's formula for carboplatin dosing. Br J Cancer 1997; 76: 1067–1071.
Krishnan E, Kwoh CK, Schumacher HR, Kuller L . Hyperuricemia and incidence of hypertension among men without metabolic syndrome. Hypertension 2007; 49: 298–303.
Mellen PB, Bleyer AJ, Erlinger TP, Evans GW, Nieto FJ, Wagenknecht LE, Wofford MR, Herrington DM . Serum uric acid predicts incident hypertension in a biethnic cohort the atherosclerosis risk in communities study. Hypertension 2006; 48: 1037–1042.
Sundström J, Sullivan L, D’Agostino RB, Levy D, Kannel WB, Vasan RS . Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension 2005; 45: 28–33.
Yamamoto T . Uric acid as a risk factor for arteriosclerosis. Hyperuricemia Gout 2010; 18: 38–42.
Zharikov S, Krotova K, Hu H, Baylis C, Johnson RJ, Block ER, Patel J . Uric acid decreases NO production and increases arginase activity in cultured pulmonary artery endothelial cells. Am J Physiol Cell Physiol 2008; 295: C1183–C1190.
Kooy NW, Royall JA . Agonist-induced peroxynitrite production from endothelial cells. Arch Biochem Biophys 1994; 310: 352–359.
Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD . Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest 1994; 94: 1172–1179.
Becker BF . Towards the physiological function of uric acid. Free Radic Biol Med 1993; 14: 615–631.
Mikkelsen WM, Dodge HJ, Valkenburg H, Himes S . The distribution of serum uric acid values in a population unselected as to gout or hyperuricemia: Tecumseh, Michigan 1959–1960. Am J Med 1965; 39: 242–251.
Quiñones GA, Natali A, Baldi S, Frascerra S, Sanna G, Ciociaro D, Ferrannini E . Effect of insulin on uric acid excretion in humans. Am J Physiol Endocrinol Metab 1995; 268: E1–E5.
Messerli FH, Frohlich ED, Dreslindki GR, Suarez DH, Aristimuno GG . Serum uric acid in essential hypertension: an indicator of renal vascular involvement. Ann Intern Med 1980; 93: 817–821.
Mazzali M, Kanellis J, Han L, Feng L, Xia YY, Chen Q, Kang DH, Gordon KL, Watanabe S, Nakagawa T, Lan HY, Johnson RJ . Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol 2002; 282: F991–F997.
Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, Krotova K, Block ER, Prabhakar S, Johnson RJ . Hyperuricemia induces endothelial dysfunction. Kidney Int 2005; 67: 1739–1742.
Ndrepepa G, Cassese S, Braun S, Fusaro M, King L, Tada T, Schömig A, Kastrati A, Schmidt R . A gender-specific analysis of association between hyperuricaemia and cardiovascular events in patients with coronary artery disease. Nutr Metab Cardiovasc Dis 2013; 23: 1195–1201.
Barbieri L, Verdoia M, Schaffer A, Marino P, Suryapranata H, De Luca G, Novara Atherosclerosis Study Group. Impact of sex on uric acid levels and its relationship with the extent of coronary artery disease: a single-centre study. Atherosclerosis 2015; 241: 241–248.
Tomiyama H, Yamashina A, Arai T, Hirose K, Koji Y, Chikamori T, Hori S, Yamamoto Y, Doba N, Hinohara S . Influences of age and gender on results of noninvasive brachial-ankle pulse wave velocity measurement- a survey of 12517 subjects. Atherosclerosis 2003; 166: 303–309.
Doba N, Tokuda Y, Tomiyama H, Goldstein NE, Kushiro T, Hinohara S . Changes in ankle brachial pulse wave velocity during a five-year follow-up period in older Japanese adults: sub-analysis results of the health research volunteer study in Japan. Intern Med 2013; 52: 21–27.
Avolio A, Jones D, Tafazzoli-Shadpour M . Quantification of alterations in structure and function of elastin in the arterial media. Hypertension 1998; 32: 170–175.
Blacher J, Asmar R, Djane S, London GM, Safar ME . Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension 1999; 33: 1111–1117.
Acknowledgements
We thank the staff of the Minabe Division of Health and Welfare for their cooperation and Ms T Hori for her secretarial assistance with this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Nagano, S., Takahashi, M., Miyai, N. et al. Association of serum uric acid with subsequent arterial stiffness and renal function in normotensive subjects. Hypertens Res 40, 620–624 (2017). https://doi.org/10.1038/hr.2017.10
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2017.10
Keywords
This article is cited by
-
High serum uric acid is a risk factor for arterial stiffness in a Chinese hypertensive population: a cohort study
Hypertension Research (2024)
-
Gender-Specific Association between Serum Uric Acid and Incident Fundus Arteriosclerosis in Chinese Population: A Retrospective Cross-Sectional Study
Scientific Reports (2020)
-
Age-differential association between serum uric acid and incident hypertension
Hypertension Research (2019)
-
Differential effects of arterial stiffness and fluid overload on blood pressure according to renal function in patients at risk for cardiovascular disease
Hypertension Research (2019)
-
Vascular Aging and Disease of the Large Vessels: Role of Inflammation
High Blood Pressure & Cardiovascular Prevention (2019)