Abstract
Autophagy is activated in hypertension-induced cardiac hypertrophy. However, the mechanisms and significance of an activated autophagy are not clear. This study was designed to determine the role of atorvastatin (ATO) in cardiac autophagy and associated benefits on cardiac remodeling and left ventricular function in spontaneously hypertensive rats (SHRs). Twenty-eight male SHRs at 8 weeks of age were randomized to treatment with vehicle (saline solution; SHR+V) or ATO (SHR+ATO; 50 mg kg−1 per day) for 6 or 12 months. Age-matched male Wistar-Kyoto (WKY) rats were used as normotensive controls. Cardiac magnetic resonance was used to evaluate cardiac function and structure. Compared with WKY rats, SHRs showed significant left ventricle (LV) dysfunction, remodeling and increases in cardiomyocyte size, which were all attenuated by 6 and 12 months of ATO treatment. Compared with WKY rats, autophagy was activated in the hearts of SHRs and this effect was amplified by chronic ATO treatment, particularly following 12 months of treatment. Protein expression levels of microtubule-associated protein-1 light chain 3-II and beclin-1, the biomarkers of an activated cardiac autophagy, were significantly elevated in ATO-treated versus vehicle-treated SHRs and control WKY rats. Cardiac Akt and phosphorylated mammalian target of rapamycin (mTOR) expression were also increased in the hearts of SHR versus WKY rats, and this effect was attenuated by ATO treatment. These findings suggest that ATO-mediated improvements in LV function and structure in SHRs may be, in part, through its regulation of cardiac autophagy via the Akt/mTOR pathway.
Similar content being viewed by others
Introduction
Hypertension is a leading risk factor of mortality globally. In response to the hemodynamic burden of hypertension, the heart initially compensates with an increase in crossbridge formation, adaptive hypertrophy and neurohormonal mechanisms that lead to increases in contractility.1, 2, 3 Although compensatory hypertrophy offsets the adverse effects of pressure overload, it ultimately leads to contractile dysfunction and heart failure (HF). Cardiac hypertrophy alone is a major independent predictor of HF and a risk factor for future lethal cardiovascular events. Although extensive research has focused on the progression from hypertrophy to failure, the exact mechanisms governing the transition from hypertrophy to failure are still poorly understood.
Hypertension-induced cardiac hypertrophic remodeling involves more than the addition of proteins and molecular signaling pathways that mediate the hypertrophic response triggered by mechanical stress. Recent data show that autophagy is involved in the development of cardiac hypertrophy and its transition to HF.4, 5 Autophagy is a highly conserved cellular process for degradation and recycling of protein aggregates and organelles. It is thought to be a response to extra- or intracellular stresses, that is initiated by a variety of factors, such as starvation, growth factor deprivation, ER stress and pathogenic infections. In cardiovascular diseases, autophagy is altered during ischemic stress, cardiac hypertrophy and HF. Although autophagy is activated during the progression of hypertension-induced cardiac hypertrophy,6, 7 the experimental studies showed inconsistent results regarding the roles of autophagy in hypertension and cardiac hypertrophy.8, 9 Initially, it was supposed that increased autophagy contributed to cell death during stress.10 However, recent evidence suggests that autophagy may have a cardioprotective role.11, 12, 13 Activated autophagy in failing hearts might be an adaptive response for protecting cells from hemodynamic stress and ischemic preconditioning.9, 14, 15 Autophagy-deficient hearts display an increased sensitivity to cardiomyopathies. During autophagic degeneration, degraded membrane lipids and proteins within autophagosomes are recruited to maintain ATP levels, which is thought to be one mechanism that contributes to cardiomyocyte protection. However, the exact role of autophagy in hypertension-induced cardiac hypertrophy, that is, whether it mediates cell survival or cell death and whether it up- or downregulates cellular function, remains poorly understood.
Statins, the inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase, are widely used to decrease plasma cholesterol and treat atherosclerotic disease.16, 17 In addition to their lipid-lowering effect, statins may also have direct cardioprotective action as suggested by the improved outcomes observed in HF patients.18, 19, 20 The underlying mechanisms for these cardioprotective effects remain unclear. Given that statins induce autophagy in cancer cells, as well as in some normal cells,21, 22, 23 there is reason to believe that statin-mediated cardiac benefits may be through autophagy-related mechanisms.
Beclin-1 is an essential autophagy and tumor suppressor protein, and a target of the protein kinase Akt.24 Activation of the Akt pathway inhibits apoptotic and autophagic programmed cell death. Among the downstream effectors of Akt, mammalian target of rapamycin (mTOR) is particularly well-characterized. Activated Akt stimulates the mTOR pathway, which is required for the initiation of protein synthesis. Inhibition of mTOR prevents cardiac hypertrophy, suggesting that mTOR and its downstream molecules regulate the development of cardiac hypertrophy.25 Autophagy might be involved in the cardioprotective effects of mTOR inhibition. Based on these findings, we hypothesized that statins affect autophagy and protect the heart through its regulation on Akt–mTOR pathways.
Methods
Animals and experimental protocols
All rats were treated in accordance with the recommendations from the Guide for Animal Management Rules of the Ministry of Health, China (2001). The experimental protocol was accepted by the Institutional Animal Care Committee of Shandong University. Eight-week-old male Wistar-Kyoto (WKY) and spontaneously hypertensive rats (SHR) were purchased from Charles River(Portage, MI, USA), USA, and housed at room temperature, with a 12 h light–dark cycle. Animals were given rodent diet and tap water ad libitum throughout the experiments. Twenty-eight male SHRs, weighing ~200 g, were randomly divided into four groups: vehicle (saline solution; SHR+V) or atorvastatin (SHR+ATO; 50 mg kg−1 per day) for 6 or 12 months (n=7 in each group). Seven WKY rats were used as age-matched, normotensive controls. ATO was purchased from Novartis Pharmaceutical Company, Beijing, China, and administered daily by gavage.
Cardiac magnetic resonance studies
The rat cardiac magnetic resonance examinations were performed under 1.5–2.5% isoflurane anesthesia, while body temperature was maintained at 37 °C. All imaging experiments were implemented on a 3T magnetic resonance system (Philips Medical System, Best, The Netherlands) with a custom-built quadrature cylindrical radiofrequency volume coil of ID 70 mm length. All scans were performed using electrocardiogram gating. The animals were placed in a cradle with four neonatal ECG electrodes attached to the paws in order to record an ECG for triggering of image data acquisition. All images were acquired in the short-axis cine imaging plane with multiple (8–10) slices for postprocessing functional analyses. Epicardial and endocardial left ventricular (LV) borders at end-diastole and end-systole were manually contoured. End-diastolic and end-systolic volumes, ejection fraction, LV wall thickness, interventricular septal thickness diastole and wall motion were calculated.
Histopathology staining
Paraffin-embedded slides (5 μm thickness) were stained with hematoxylin–eosin for morphologic examination or Masson's trichrome for interstitial fibrosis determination. LV diameter was measured at the level of the papillary muscle as previously described.26 LV wall thickness, myofilament density, cardiomyocyte size and collagen volume fraction were measured and analyzed using Image Pro Plus software (Media Cybernetics, Rockville, MD, USA). Collagen volume fraction was calculated as area of Masson's trichrome-stained connective tissue divided by total area of the image as described previously.27
Immunohistochemistry
Tissue sections were deparaffinized and exposed to 3% hydrogen peroxide for 15 min to block endogenous peroxidase activity. For heat-induced epitope retrieval, the sections were placed in a 0.01 mol l−1 citrate buffer and heated at 120 °C for 15 min. The nonspecific binding was blocked by preincubation with 5% normal goat serum in phosphate-buffered saline (blocking buffer) for 60 min at room temperature. Individual slides were then incubated overnight at 4 °C with an anti-LC3 antibody (diluted 1:6400; Cell Signaling, Danvers, MA, USA) at a final concentration of 2 μg ml−1 in the blocking buffer. The slides were washed with phosphate-buffered saline and then incubated with a peroxidase-labeled polymer conjugated to goat anti-rabbit immunoglobulin G (Vector Laboratories, Burlingame, CA, USA) for 45 min at room temperature. After extensive washing with phosphate-buffered saline, the color reaction was developed using 2% 3,3′-diaminobenzidine in 50 mmol l−1 Tris buffer (pH 7.6) containing 0.3% hydrogen peroxide for 5–10 min. The sections were then counterstained with Meyer's hematoxylin, dehydrated and mounted. Images were captured using Olympus BX51 microscope and software (Olympus, Tokyo, Japan).
Western blot analysis
Myocardium tissue was homogenized in RIPA buffer (150 mm NaCl, 50 mm Tris-HCl, pH 7.4, 1 mm EDTA, 1% Triton X-100, 1% sodium deoxycholate and 0.1% SDS) with protease inhibitors. After centrifugation at 12 000 g for 20 min, the supernatant was transferred to new 1.5-ml microcentrifuge tubes and protein was quantified using coomassie blue reagent. Protein was then heat denatured for 4 min in boiling water and loaded on an 12% SDS–polyacrylamide gel followed by transfer to a nitrocellulose membrane at 80 V and 4 °C for 120 min. The membrane was incubated in blocking buffer (5% milk, 1 × tris-buffered saline, 0.1% Tween 20) for 2 h at room temperature, and then with anti-LC3B polyclonal antibody (1:2000 dilution, Cell Signaling) overnight at 4 °C. After washing with TBST (1 × tris-buffered saline with 0.1% Tween 20), the membrane was incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:5000 dilution) at room temperature for 1 h. The reactions were developed with enhanced chemiluminescence reagents, and the images were obtained by the exposure to x-ray films. The films were digitized and quantified with the ImageJ software (Bethesda, MD, USA). The same protocol was used for antibodies against caspase-3 (1:3000 dilution, Cell Signaling) total Akt and phosphorylated Akt (Ser473; 1:1000 and 1:1000 dilution, Santa Cruz Biotechnology, Dallas, TX, USA), total and phosphorylated AMPK (1:2000 dilution, Santa Cruz Biotechnology) and total and phosphorylated S6K (Ser235/236; 1:1000 diluted each; Cell Signaling).
Electron microscopy
Cardiac tissue was carefully and quickly dissected from sacrificed SHRs. Left ventricles were cut into 1 mm3 cubes, fixed with 2% glutaraldehyde in phosphate-buffered saline overnight at 4 °C. The fixed samples were then post-fixed with 1% buffered osmium tetroxide, embedded and analyzed using transmission electron microscopy (JEM-1200EX, Tokyo, Japan).
Statistical analysis
Results were expressed as the mean±s.d. Data were analyzed using SPSS 11.5 software (SPSS Inc., Chicago, IL, USA) under Windows 7. All statistics were analyzed by analysis of variance, with a P-value of <0.05 considered significant.
Results
Body weight, heart weight and blood pressure changes during the experiment
Blood pressure (BP), body weight and heart weight of SHR and WKY rats are summarized in Table 1. Body weight was significantly increased in SHRs compared with WKY rats, whereas the body weights decreased significantly following ATO treatment for both 6 and 12 months. Heart rate among SHR groups and WKY rats was not different (Table 1). Consistent with our previous study,28 systolic BP was significantly elevated in SHRs compared with WKY rats of the same age. In SHR group, both systolic BP and heart weight increased during aging, while WKY rats remained unchanged. After treatment with ATO, systolic BP and heart weight in SHRs significantly decreased (P<0.05 vs vehicle-treated rats). The heart weight-to-body weight ratios were higher in vehicle-treated SHRs versus WKY rats, while ATO treatment for 6 or 12 months decreased the heart weight-to-body weight ratios when compared with vehicle-treated SHR counterparts.
Effects of ATO on cardiac remodeling of SHRs
Consistent with our previous studies,28 ATO showed protective effects on cardiac remodeling. Compared with WKY rats, vehicle-treated SHRs showed increased LV mass, LV wall thickness, interventricular septal diameter and LV posterior wall at diastole (Table 2 and Figure 1a). ATO limited these adverse effects of hypertension on LV structure and also attenuated increases in interventricular septal diameter and LV posterior wall thickness in diastole. However, LV fractional shortening, LV end-diastolic diameter, LV end-systolic diameter and LV ejection fraction were not different among groups (Table 2). Moreover, SHRs with vehicle treatment had increased cardiomyocyte size determined by hematoxylin–eosin staining, when compared with WKY rats or ATO-treated SHRs (Figure 1b). Masson's trichrome staining demonstrated that the increased LV interstitial fibrosis in SHRs was attenuated by ATO treatment (Figure 1b). LV content of hydroxyproline, an indicator of collagen deposit, significantly increased in SHRs compared with WKY rats, which was also attenuated by ATO treatment (Figure 1c).
(a) Representative mid-papillary MR images of the heart in WKY rats and SHRs treated with vehicle (SHR+V) or atorvastatin (SHR+ATO). It shows significant hypertrophy of the SHR+V heart compared with WKY and SHR+ATO. (b) Representative images of hematoxylin and eosin staining and Masson’s trichrome staining in WKY rats and SHRs treated with vehicle (SHR+V) or atorvastatin (SHR+ATO). (c) Hydroxyproline content was analyzed in WKY, SHR+V and SHR+ATO groups. Data are shown as mean±s.d. *P<0.05 vs. WKY; #P<0.05 vs. SHR+V. MR, magnetic resonance; SHR, spontaneous hypertensive rats; WKY, Wistar-Kyoto rats. A full color version of this figure is available at Hypertension Research online.
Autophagy in the heart of SHR and the effects of ATO
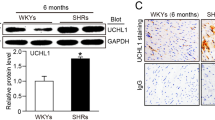
To determine the autophagic activity and the effects of ATO during the progression of hypertension in SHR, we assessed the expression of autophagosome markers, light chain 3B (LC3B; including LC3-I and LC3-II), beclin-1 and caspase-3, which are key proteins involved in the regulation of autophagy. Consistent with the results of the previous study,29 immunohistochemistry staining revealed that the number of cardiac LC3-positive autophagic vacuoles increased during the progression of hypertension in SHRs, while it was higher in SHRs compared with the WKY rats of the same ages (Figure 2a). Interestingly, ATO treatment significantly increased LC3 compared with vehicle-treated SHRs (Figure 2a).
(a) Representative images of immunohistochemistry staining for LC3, a marker of autophagy, in the heart of WKY rats and SHRs treated with vehicle (SHR+V) or atorvastatin (SHR+ATO). (b) Representative electron micrographs of autophagic vacuoles in cardiomyocytes of WKY rats and SHRs treated with vehicle (SHR+V) or atorvastatin (SHR+ATO). Arrows indicate autophagic vacuoles. Bar=1 μm. (c) Representative electronic micrographs and summarized data showing the number of autophagosomes/cell in different groups (a random number of 30 cells were selected for each group). *P<0.05 vs WKY; #P<0.05 vs SHR+V. LC3, light chain 3; SHR, spontaneous hypertensive rats; WKY, Wistar-Kyoto rats. A full color version of this figure is available at Hypertension Research online.
Electron microscopic examination revealed that both autophagic vacuoles and lysosomes were abundant within cardiomyocytes in the SHR hearts at 12 months when compared with age-matched WKY rats (Figure 2b), and this electron-microscopic portrayal of autophagy was even more exaggerated in SHRs treated with ATO (Figure 2c).
Western blot analysis showed that Beclin-1 expression was upregulated by ATO in hearts of SHRs (Figure 3). Beclin-1 expression significantly increased in SHR+ATO compared with age-matched SHR+V and WKY (P=0.007 and 0.012 versus SHR+V and WKY, respectively). Moreover, the value of the LC3-II/LC3-I ratio was significantly increased in SHR+ATO rats compared with other groups (P<0.001). However, cardiac caspase-3 expression in ATO-treated rats was not different from vehicle-treated SHRs or WKYs (P=0.18; Figure 3).
(a) Representative images of western blot for the autophagy-related proteins, LC3-I, LC3-II, Beclin-1 and caspase-3 in the hearts of WKY rats and SHRs treated with vehicle (SHR+V) or atorvastatin (SHR+ATO). (b) The signal densities of western blot were quantified using ImageJ. Values are mean±s.d.; *P<0.05 vs WKY; #P<0.05 vs SHR+V. SHR, spontaneous hypertensive rats; WKY, Wistar-Kyoto rats.
Activation of Akt/mTOR signal pathway
To determine the signaling pathways involved in the ATO-induced cardiac autophagy in SHRs, we assessed the activation of the main autophagy repressor mTOR and its upstream inhibitor AMPK and the activator Akt. Western blot analysis showed that ATO treatment decreased Akt phosphorylation (Figure 4). Cardiac p-mTOR increased in SHRs compared with WKY rats, while it was significantly reduced by ATO compared with vehicle treatment. In addition, the changes of p-S6K expression, a direct downstream target of mTOR, paralleled the changes of p-mTOR (Figure 4). Both kinases were activated in SHRs compared with WKY rats, and significantly reduced by ATO treatment. We also evaluated AMPK protein expression, which showed that the cardiac p-AMPK levels were higher in SHRs versus WKY rats, and this was not affected by ATO treatment.
(a) Western blot analysis of phospho-akt (p-AKT), total AKT and phospho-AMPK (p-AMPK), and total AMPK, phospho-mTOR (p-mTOR), total mTor, phospho-S6K (p-S6K) and total S6K in hearts of WKY rats and SHRs treated with vehicle (SHR+V) or atorvastatin (SHR+ATO). (b) The signal densities of western blot were quantified using ImageJ. Values are mean±s.d.; *P<0.05 vs WKY; #P<0.05 vs SHR+V at the same time. SHR, spontaneous hypertensive rats; WKY, Wistar-Kyoto rats.
Discussion
The main findings in the present study suggest that the cardioprotective effects of ATO in SHR are associated with the activation of autophagy and AKT/mTOR pathways.
LV remodeling is the process by which ventricular size, shape and function are regulated by mechanical, neurohormonal and genetic factors.30, 31 When stress persists, the compensatory remodeling can evolve into a decompensated state, as depicted by increases in cardiomyocyte length and/or width, collagen volume, contractile dysfunction and extracellular remodeling, ultimately leading to HF, arrhythmias and sudden cardiac death.32, 33 In the present study, SHRs at 6 and 12 months of age, were used to emulate the progression of LV hypertrophy from a compensated to decompensated state. Compared with WKY rats of the same age, SHRs exhibited increased cardiomyocyte size, LV mass, interventricular septal diameter and LV posterior wall at diastole. This hypertrophic phenotype was most pronounced in the 12-month-old SHRs. Although it is controversial regarding the effects of statin in attenuating cardiac function and improving survival rate in HF,18, 19, 20, 28 the present study has shown that chronic ATO, at the dose of 50 mg kg−1 per day, attenuated cardiac remodeling in SHR. This result is consistent with our previous report, and that of others, using the same dose of ATO.28, 34
Statin was originally touted as a drug for the treatment of high cholesterol. However, many beneficial effects independent of its action on lipid regulation have been recognized in recent years, including improvements in endothelial function, reductions in oxidative stress, less platelet adhesion and atherosclerotic plaque stabilization.16, 17, 24, 35, 36, 37, 38, 39, 40, 41 Finding from the current study further suggests that ATO’s beneficial effects on limiting the adverse effects of hypertension on cardiac structure may be through its regulation on autophagy. Both in vitro and in vivo studies have suggested that autophagy is involved in the development of cardiomyocyte hypertrophy.42, 43
Several recent studies have shown that statins induced autophagy in mammalian cells, such as human rhabdomyosarcoma cells and PC3 cells.44, 45 More recently, simvastatin was demonstrated to increase autophagy in human airway smooth muscle cells and coronary arterial myocytes.46, 47 In this study, our original hypothesis was that the autophagy activation might be decreased in hypertrophic heart. However, our results have shown the opposite, which might reflect a compensatory mechanism in the hypertensive heart. That is, autophagy was increased in the hypertensive heart and ATO augmented this effect with associated reductions in cardiac hypertrophy.
Nutritional starvation in the hypertrophic heart might stimulate cardiac autophagy. Nutritional stress induces autophagy, which might be due to a compensatory mechanism that generates nutrients by degradation of cytoplasmic components to sustain cell survival.48 Autophagy is the basic catabolic mechanism that involves degradation of dysfunctional cellular components through the action of lysosome, as well as supplying energy and compounds for the synthesis of essential biomacromolecules. Although excessive autophagy can cause cell death, enhanced and controlled autophagy is protective by providing essential substrates during starvation and by removing damaged materials. In the SHR heart, chronic cardiac stress results in progressive myocardial hypertrophy that eventually exceeds the capacity of the coronary vasculature to adequately perfuse the myocardial mass. This pathophysiological process leads to multiple foci of myocardial ischemia, particularly in the subendocardium, which simulates starvation, a powerful inducer of autophagy. Several recent studies have shown that statins induced formation of autophagosomes.44, 45, 47 However, the exact mechanisms are still unclear.
One of the characteristics of autophagy is the formation of the autophagosome, followed by its fusion with a lysosome, termed autophagolysosome.48 Microtubule-associated protein 1A/1B-LC3, a soluble protein in mammalian tissues and cultured cells, is normally dispersed diffusely throughout the cytoplasm in healthy cells and accumulates within the autophagosomal membrane in a form covalently linked to phosphatidylethanolamine during stimulation of autophagy.49 Our data show that statin regulates LC3 expression and subsequently might influence autophagy in SHR hearts. The elevation of autophagic proteins activates or facilitates the autophagic process in the heart. In the cardiovascular system, mTOR is believed to be an important negative regulator of autophagy.9 Indeed, inhibition of the mTOR signaling pathway has been shown to be involved in the autophagy induced by ATO.50 Our findings are consistent with the recent studies showing that statin inhibits mTOR and its substrate S6K.47, 51 To our knowledge, the present study provides the first evidence that ATO limits hypertensive LV remodeling possibly by inducing autophagy via mTOR inhibition.
mTOR is a serine/threonine protein kinase that regulates many cellular activities including autophagy, proliferation, survival and metabolism in mammalian cells.52 Activation of mTOR signaling is implicated in diseases such as cancer and tissue hypertrophy and inhibitors of mTOR signaling are used clinically to inhibit cancer growth and prevent graft rejection and restenosis after coronary angioplasty.53 mTOR is regulated by and communicates with multiple signaling pathways,54, 55, 56 such as activated by Akt (protein kinase B). Akt has become among the most studied signal-transduction molecules in cardiac biology in recent years, which is activated in response to hypertension, pressure overload and chronic neurohormonal signaling.57, 58, 59 Knockdown of Akt by small interfering RNA blunts agonist-dependent hypertrophy, whereas in vivo cardiac-specific expression of Akt causes a brief phase of cardiac hypertrophy, followed by chamber dilation and impaired systolic function and death.60, 61 In hypertrophic cardiomyocytes, one major event is increased protein turnover, where enhanced protein synthesis is accompanied by increased removal of deleterious proteins. Many pathways that mediate protein turnover depend on mTOR. Specifically, Akt/mTOR signals are essential for the regulation of autophagy.62, 63, 64 Our results suggest that regulation of the Akt/mTOR pathway might be responsible for the effect of statin on autophagy in SHRs.
Limitations and future studies
Although the dose of ATO used in this study was based on other studies involving rat models,28, 37, 38, 39, 40, 41, 65 we are aware that it exceeded doses used in clinical studies, indicating that sensitivities to ATO might be different among species. High-dose ATO (50 mg kg−1 per day) improved insulin sensitivity, endothelial function, anti-oxidant effects and lowered BP and diet-induced elevations in blood cholesterol in various rat models.28, 37, 38, 39, 40, 41, 65 The present study demonstrated that chronic, high-dose ATO also attenuated hypertension-induced cardiac remodeling in SHRs and this advantageous effect was associated with an upregulation in autophagy-related gene expression with concomitant inhibition of the Akt/mTOR pathway in the hearts of these rats. The effects of high dose of ATO on other tissues in rats, such as liver functions and serum creatine kinase, will be the subject of future studies. As cardiac autophagy was increased in SHRs versus normotensive WKY rats, further investigations are needed to determine whether this is simply a compensatory mechanism that offsets the deleterious effects of longstanding hypertension. Finally, the cardioprotective effects of ATO might be through multiple pathways in both cardiomyocytes and non-cardiomyocytes. Whether ATO directly or indirectly regulates autophagy activity was not determined in this study. Further in vitro and in vivo studies are in our future plans to determine the exact mechanisms by which statins regulate autophagy and modulate cardiac hypertrophy and HF.
References
Lorell BH, Carabello BA . Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation 2000; 102: 470–479.
Weber KT, Brilla CG . Pathologic hypertrophy and cardiac interstitium: fibrosis and renin-angiotensin-aldosterone system. Circulation 1991; 83: 1849–1865.
Matsuo T, Carabello BA, Nagatomo Y, Koide M, Hamawaki M, Zile MR, McDermott PJ . Mechanisms of cardiac hypertrophy in canine volume overload. Am J Physiol 1998; 275: H65–H74.
Wang ZV, Rothermel BA, Hill JA . Autophagy in hypertensive heart disease. J Biol Chem 2010; 285: 8509–8514.
Rothermel BA, Hill JA . Autophagy in load-induced heart disease. Circ Res 2008; 103: 1363–1369.
Porrello ER, D'Amore A, Curl CL, Allen AM, Harrap SB, Thomas WG, Delbridge LM . Angiotensin II type 2 receptor antagonizes angiotensin II type 1 receptor-mediated cardiomyocyte autophagy. Hypertension 2009; 53: 1032–1040.
Rothermel BA, Hill JA . Myocyte autophagy in heart disease: friend or foe? Autophagy 2007; 3: 632–634.
Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, Le V, Levine B, Rothermel BA, Hill JA . Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest 2007; 117: 1782–1793.
Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K . The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med 2007; 13: 619–624.
Mizushima N, Levine B, Cuervo AM,, Klionsky DJ . Autophagy fights disease through cellular self-digestion. Nature 2008; 451: 1069–1075.
Huang C, Yitzhaki S, Perry CN, Liu W, Giricz Z, Mentzer RM Jr, Gottlieb RA . Autophagy induced by ischemic preconditioning is essential for cardioprotection. J Cardiovasc Trans Res 2010; 3: 365–373.
Gottlieb RA, Carreira RS . Autophagy in health and disease. 5. Mitophagy as a way of life. Am J Physiol Cell Physiol 2010; 299: 203–210.
Sciarretta S, Zhai P, Shao D, Maejima Y, Robbins J, Volpe M, Condorelli G, Sadoshima J . Rheb is a critical regulator of autophagy during myocardial ischemia: pathophysiological implications in obesity and metabolic syndrome. Circulation 2012; 125: 1134–1146.
Shiomi M, Miyamae M, Takemura G, Kaneda K, Inamura Y, Onishi A, Koshinuma S, Momota Y, Minami T, Figueredo VM . Induction of autophagy restores the loss of sevoflurane cardiac preconditioning seen with prolonged ischemic insult. Eur J Pharmacol 2013; 724C: 58–66.
Petrovski G, Das S, Juhasz B, Kertesz A, Tosaki A, Das DK . Cardioprotection by endoplasmic reticulum stress-induced autophagy. Antioxid Redox Signal 2011; 14: 2191–2200.
Stone NJ, Robinson JG, Lichtenstein AH, Goff DC Jr, Lloyd-Jones DM, Smith SC Jr, Blum C, Schwartz JS . 2013 ACC/AHA Cholesterol Guideline Panel. Treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: synopsis of the 2013 American College of Cardiology/American Heart Association cholesterol guideline. Ann Intern Med 2014; 160: 339–343.
Rabkin SW, Langer A, Ur E, Calciu CD, Leiter LA . Inflammatory biomarkers CRP, MCP-1, serum amyloid alpha and interleukin-18 in patients with HTN and dyslipidemia: impact of diabetes mellitus on metabolic syndrome and the effect of statin therapy. Hypertens Res 2013; 36: 550–558.
Horwich TB, MacLellan WR, Fonarow GC . Statin therapy is associated with improved survival in ischemic and non-ischemic heart failure. J Am Coll Cardiol 2004; 43: 642–648.
Tehrani F, Morrissey R, Phan A, Chien C, Schwarz ER . Statin therapy in patients with diastolic heart failure. Clin Cardiol 2010; 33: E1–E5.
Thambidorai SK, Deshmukh AR, Walters RW, Turner PD, Monaghan MS, Mooss AN, Hunter CB, Esterbrooks DJ, Mohiuddin SM . Impact of statin use on heart failure mortality. Int J Cardiol 2011; 147: 438–443.
Andres AM, Hernandez G, Lee P, Huang C, Ratliff EP, Sin J, Thornton CA, Damasco MV, Gottlieb RA . Mitophagy is Required for Acute Cardioprotection by Simvastatin. Antioxid Redox Signal 2013; 21: 1960–1973.
Peng X, Li W, Yuan L, Mehta RG, Kopelovich L, McCormick DL . Inhibition of proliferation and induction of autophagy by atorvastatin in PC3 prostate cancer cells correlate with downregulation of Bcl2 and upregulation of miR-182 and p21. PLoS One 2013; 8: e70442.
Toepfer N, Childress C, Parikh A, Rukstalis D, Yang W . Atorvastatin induces autophagy in prostate cancer PC3 cells through activation of LC3 transcription. Cancer Biol Ther 2011; 12: 691–699.
Wang RC, Wei Y, An Z, Zou Z, Xiao G, Bhagat G, White M, Reichelt J, Levine B . Akt-mediated regulation of autophagy and tumorigenesis through Beclin 1 phosphorylation. Science 2012; 338: 956–959.
Shioi T, McMullen JR, Tarnavski O, Converso K, Sherwood MC, Manning WJ, Izumo S . Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation 2003; 107: 1664–1670.
Li Z, Zhang T, Dai H, Liu G, Wang H, Sun Y, Zhang Y, Ge Z . Endoplasmic reticulum stress is involved in myocardial apoptosis of streptozocin-induced diabetic rats. J Endocrinol 2008; 196: 565–572.
Tanaka Y, Nagai M, Date T, Okada T, Abe Y, Seki S, Taniguchi M, Taniguchi I, Mochizuki S . Effects of bradykinin on cardiovascular remodeling in renovascular hypertensive rats. Hypertens Res 2004; 27: 865–875.
Geng J, Zhao Z, Kang W, Wang W, Zhang Y, Zhiming GE . Atorvastatin reverses cardiac remodeling possibly through regulation of protein kinase D/myocyte enhancer factor 2D activation in spontaneously hypertensive rats. Pharmacol Res 2010; 61: 40–47.
Li MH, Zhang YJ, Yu YH, Yang SH, Iqbal J, Mi QY, Li B, Wang ZM, Mao WX, Xie HG, Chen SL . Berberine improves pressure overload-induced cardiac hypertrophy and dysfunction through enhanced autophagy. Eur J Pharmacol 2014; 728: 67–76.
Pfeffer MA, Braunwald E . Ventricular remodeling after myocardial infarction: experimental observations and clinical implications. Circulation 1990; 81: 1161–1172.
Rouleau JL, de Champlain J, Klein M, Bichet D, Moyé L, Packer M, Dagenais GR, Sussex B, Arnold JM, Sestier F . Activation of neurohumoral systems in postinfarction left ventricular dysfunction. J Am Coll Cardiol 1993; 22: 390–398.
Diwan A, Dorn GW . Decompensation of cardiac hypertrophy: cellular mechanisms and novel therapeutic targets. Physiology (Bethesda) 2007; 22: 56–64.
Cohn JN, Ferrari R, Sharpe N . Cardiac remodeling–concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol 2000; 35: 569–582.
Xu X, Zhang L, Liang J . Rosuvastatin prevents pressure overload-induced myocardial hypertrophy via inactivation of the Akt, ERK1/2 and GATA4 signaling pathways in rats. Mol Med Rep 2013; 8: 385–392.
Ray KK, Cannon CP . The potential relevance of the multiple lipid-independent (pleiotropic) effects of statins in the management of acute coronary syndromes. J Am Coll Cardiol 2005; 46: 1425–1433.
Istvan ES . Structural mechanism for statin inhibition of 3-hydroxy-3- methylglutaryl coenzyme A reductase. Am Heart J 2002; 144: S27–S32.
Mondo CK, Yang WS, Zhang N, Huang TG . Anti-oxidant effects of atorvastatin in dexamethasone-induced hypertension in the rat. Clin Exp Pharmacol Physiol 2006; 33: 1029–1034.
Kishi T, Hirooka Y, Mukai Y, Shimokawa H, Takeshita A . Atorvastatin causes depressor and sympatho-inhibitory effects with upregulation of nitric oxide synthases in stroke-prone spontaneously hypertensive rats. J Hypertens 2003; 21: 379–386.
Shafiei M, Nobakht M, Moazzam AA . Lipid-lowering effect of Rhus coriaria L. (sumac) fruit extract in hypercholesterolemic rats. Pharmazie 2011; 66: 988–992.
Guimarães DA, Rizzi E, Ceron CS, Pinheiro LC, Gerlach RF, Tanus-Santos JE . Atorvastatin and sildenafil lower blood pressure and improve endothelial dysfunction, but only atorvastatin increases vascular stores of nitric oxide in hypertension. Redox Biol 2013; 1: 578–585.
Alamgeer A, Ghuffar A, Ahmad T, Mushtaq MN . Antihyperlipidemic effect of Berberis orthobotrys in hyperlipidemic animal models. Bangladesh J Pharmacol 2014; 9: 377–382.
Oyabu J, Yamaguchi O, Hikoso S, Takeda T, Oka T, Murakawa T, Yasui H, Ueda H, Nakayama H, Taneike M, Omiya S, Shah AM, Nishida K, Otsu K . Autophagy -mediated degradation is necessary for regression of cardiac hypertrophy during ventricular unloading. Biochem Biophys Res Commun 2013; 441: 787–792.
Xu X, Roe ND, Weiser-Evans MC, Ren J . Inhibition of mammalian target of rapamycin with rapamycin reverses hypertrophic cardiomyopathy in mice with cardiomyocyte-specific knockout of PTEN. Hypertension 2014; 63: 729–739.
Araki M, Motojima K . Hydrophobic statins induce autophagy in cultured human rhabdomyosarcoma cells. Biochem Biophys Res Commun 2008; 367: 462–467.
Parikh A, Childress C, Deitrick K, Lin Q, Rukstalis D, Yang W . Statin-induced autophagy by inhibition of geranylgeranyl biosynthesis in prostate cancer pc3 cells. Prostate 2010; 70: 971–981.
Ghavami S, Mutawe MM, Sharma P, Yeganeh B, McNeill KD, Klonisch T, Unruh H, Kashani HH, Schaafsma D, Los M, Halayko AJ . Mevalonate cascade regulation of airway mesenchymal cell autophagy and apoptosis: A dual role for p53. PLoS One 2011; 6: e16523.
Wei YM, Li X, Xu M, Abais JM, Chen Y, Riebling CR, Boini KM, Li PL, Zhang Y . Enhancement of autophagy by simvastatin through inhibition of Rac1-mTOR signaling pathway in coronary arterial myocytes. Cell Physiol Biochem 2013; 31: 925–937.
Vellai T . Autophagy genes and ageing. Cell Death Differ 2009; 16: 94–102.
Tanida I, Ueno T, Kominami E . LC3 conjugation system in mammaliam autophagy. Int J Biochem Cell Biol 2004; 36: 2503–2518.
Toepfer N, Childress C, Parikh A, Rukstalis D, Yang W . Atorvastatin induces autophagy in prostate cancer PC3 cells through activation of LC3 transcription. Cancer Biol Ther 2011; 12: 691–699.
Misirkic M, Janjetovic K, Vucicevic L, Ristic B, Vilimanovich U, Harhaji-Trajkovic L, Sumarac-Dumanovic M, Micic D, Bumbasirevic V, Trajkovic V . Inhibition of ampk-dependent autophagy enhances in vitro antiglioma effect of simvastatin. Pharmacol Res 2012; 65: 111–119.
Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, Massey DC, Menzies FM, Moreau K, Narayanan U, Renna M, Siddiqi FH, Underwood BR, Winslow AR, Rubinsztein DC . Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev 2010; 90: 1383–1435.
Wang X, Proud CG . Mtorc1 signaling: what we still don't know. J Mol Cell Biol 2011; 3: 206–220.
Fingar D, Blenis J . Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 2004; 23: 3151–3171.
Bhaskar P, Hay N . The two TORCs and Akt. Dev Cell 2007; 12: 487–502.
Sarbassov D, Guertin D, Ali S, Sabatini DM . Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005; 307: 1098–1101.
Iaccarino G, Ciccarelli M, Sorriento D, Cipolletta E, Cerullo V, Iovino GL, Paudice A, Elia A, Santulli G, Campanile A, Arcucci O, Pastore L, Salvatore F, Condorelli G, Trimarco B . AKT participates in endothelial dysfunction in hypertension. Circulation 2004; 109: 2587–2593.
Kemi OJ, Ceci M, Wisloff U, Grimaldi S, Gallo P, Smith GL, Condorelli G, Ellingsen O . Activation or inactivation of cardiac Akt/mTOR signaling diverges physiological from pathological hypertrophy. J Cell Physiol 2008; 214: 316–321.
Katare R, Caporali A, Emanueli C, Madeddu P . Benfotiamine improves functional recovery of the infarcted heart via activation of pro-survival G6PD/Akt signaling pathway and modulation of neurohormonal response. J Mol Cell Cardiol 2010; 49: 625–638.
Chaanine AH, Hajjar RJ . AKT signalling in the failing heart. Eur J Heart Fail 2011; 13: 825–829.
Huang Y, Wu D, Zhang X, Jiang M, Hu C, Lin J, Tang J, Wu L . Cardiac-specific Traf2 overexpression enhances cardiac hypertrophy through activating AKT/GSK3β signaling. Gene 2014; 536: 225–231.
Saiki S, Sasazawa Y, Imamichi Y, Kawajiri S, Fujimaki T, Tanida I, Kobayashi H, Sato F, Sato S, Ishikawa K, Imoto M, Hattori N . Caffeine induces apoptosis by enhancement of autophagy via PI3K/Akt/mTOR/p70S6K inhibition. Autophagy 2011; 7: 176–187.
van der Vos KE, Eliasson P, Proikas-Cezanne T, Vervoort SJ, van Boxtel R, Putker M, van Zutphen IJ, Mauthe M, Zellmer S, Pals C, Verhagen LP, Groot Koerkamp MJ, Braat AK, Dansen TB, Holstege FC, Gebhardt R, Burgering BM, Coffer PJ . Modulation of glutamine metabolism by the PI(3)K-PKB-FOXO network regulates autophagy. Nat Cell Biol 2012; 14: 829–837.
Li Y, Zhu H, Zeng X, Fan J, Qian X, Wang S, Wang Z, Sun Y, Wang X, Wang W, Ju D . Suppression of autophagy enhanced growth inhibition and apoptosis of interferon-beta in human glioma cells. Mol Neurobiol 2013; 47: 1000–1010.
Wong V, Stavar L, Szeto L, Uffelman K, Wang CH, Fantus IG, Lewis GF . Atorvastatin induces insulin sensitization in Zucker lean and fatty rats. Atherosclerosis 2006; 184: 348–355.
Acknowledgements
This work was funded in whole or part by National Natural Science Foundation of China Grant (No.81270175, No.81170087, No.81470404), the National Natural Science Funds for Young Scholar Grant 81200211 and Doctoral Research Grant of Shandong Province BS2010YY005 (to ZZ).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, W., Wang, H., Geng, QX. et al. Augmentation of autophagy by atorvastatin via Akt/mTOR pathway in spontaneously hypertensive rats. Hypertens Res 38, 813–820 (2015). https://doi.org/10.1038/hr.2015.85
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2015.85
Keywords
This article is cited by
-
Ibandronate promotes autophagy by inhibiting Rac1–mTOR signaling pathway in vitro and in vivo
Cell Death Discovery (2022)
-
Altered mTOR and Beclin-1 mediated autophagic activation during right ventricular remodeling in monocrotaline-induced pulmonary hypertension
Respiratory Research (2017)
-
2,5-Dimethylcelecoxib prevents pressure-induced left ventricular remodeling through GSK-3 activation
Hypertension Research (2017)