Abstract
The RhoA/Rho-associated kinase (ROCK) pathway has a key physiological role in the pathogenesis of atherosclerosis. Increased ROCK activity is associated with cardiovascular diseases. Endogenous nitric oxide (NO) has an anti-atherosclerotic effect, whereas the exogenous NO-mediated cardiovascular effect still remains controversial. The purpose of this study was to evaluate the effect of exogenous NO on ROCK activity in patients with angina pectoris. This is a prospective, open-label, randomized, controlled study. A total of 30 patients with angina pectoris were randomly assigned to receive 40 mg day−1 of isosorbide mononitrate (n=15, 12 men and 3 women, mean age of 63±12 years, isosorbide mononitrate group) or conventional treatment (n=15, 13 men and 2 women, mean age of 64±13 years, control group) for 12 weeks. ROCK activity in peripheral leukocytes was measured by western blot analysis. ROCK activities at 4 and 12 weeks after treatment were decreased in the isosorbide mononitrate group (0.82±0.33 at 0 week, 0.62±0.20 at 4 weeks, 0.61±0.19 at 12 weeks, n=15 in each group, P<0.05, respectively) but not altered in the control group. ROCK1 and ROCK2 expression levels were similar in all treatment periods in the two groups. These findings suggest that the administration of exogenous NO can inhibit ROCK activity, indicating that the usage of exogenous NO could have a protective effect in patients with angina pectoris.
Similar content being viewed by others
Introduction
For more than 100 years, exogenous nitric oxide (NO), such as organic nitrates, has been used clinically as an anti-anginal agent that dilates the vasculature via the effect of exogenous NO-mediated cyclic 3′5′-guanosine monophosphate on vascular smooth muscle cells (VSMCs).1 Nevertheless, the long-term outcome in patients with coronary artery disease treated with exogenous NO remains controversial.2, 3, 4, 5 According to most of the previous studies, harmful effects of exogenous NO administration were reported in patients with an acute coronary syndrome but not in patients with angina pectoris. Indeed, long-term administration of organic nitrates is widely used in patients with angina syndromes, including vasospastic angina and stable angina on effort in a clinical setting. Presumably, cardiologists worldwide speculate and/or have practical experience and confidence that clinical use of exogenous NO for the treatment of an angina attack does not worsen cardiovascular mortality and morbidity in patients with angina pectoris, although this may not be the case for an acute coronary syndrome.
Increased Rho-associated kinase (ROCK) activity is associated with endothelial dysfunction and cardiovascular diseases.6, 7, 8 Several lines of evidence have shown that the RhoA/ROCK signaling pathway mediates various cellular and physiological functions, including cell proliferation, migration, adhesion, apoptosis and contraction,8, 9, 10 all of which may be involved in the cellular/organ damage and pathogenesis of atherosclerosis. Previous evidence has revealed increases in ROCK activity in patients with spastic angina,11 hypertension,12 stable angina on effort13 and even in smokers.14 Interestingly, recent studies have shown that leukocyte ROCK activity can potentially be used to predict the presence and severity of vasospastic angina, indicating that leukocyte ROCK activity could be a promising biomarker for the treatment of angina patients.13, 15, 16 Previously, we also revealed that ROCK could be a possible upstream target molecule of oxidative stress in humans.17, 18 Taken together, ROCK could be useful, not only as a novel therapeutic target but also as a useful biomarker in patients with cardiovascular diseases.
The activation of the RhoA/ROCK pathway has been shown to mediate eNOS mRNA destabilization and eNOS dephosphorylation at Ser1177, leading to the inhibition of eNOS expression and activation, which results in a subsequent decrease of NO bioavailability.19 On the other hand, NO has been shown to phosphorylate RhoA at Ser188, which can prevent its translocation from the cytosol to membrane, resulting in the inhibition of an RhoA activation.20 However, there is still no information on the inhibitory effect on ROCK activity by exogenous NO in humans. Exogenous NO donors are well known as anti-anginal agents that can dilate arteries and veins by mediating an increase in exogenous NO-mediated 3′5′-guanosine monophosphate.1 Although the cardiovascular protective outcome of long-term treatment with nitrates has been controversial,2, 3, 4, 5 organic nitrates are extensively used for angina syndromes, such as vasospastic angina and stable angina on effort in clinical practice. We hypothesized that this may be due, at least in part, to the beneficial effect of exogenous NO inhibiting ROCK activity. In the present study, we evaluated the role of exogenous NO on ROCK activity in patients with angina pectoris.
Methods
Subjects
This was an investigator-initiated, prospective, open-label, randomized, controlled study conducted at Hiroshima University Hospital between April 2011 and March 2013. We studied 30 patients with angina pectoris on effort. Of the patients with angina pectoris who underwent follow-up care in outpatient cardiology in Hiroshima University Hospital, 30 patients agreed to participate in this study. The diagnosis of angina was based on a classic history of chest pain on exertion and by means of coronary angiography and a thallium exercise scan, demonstrating the presence of coronary artery disease. The subjects were randomly assigned to receive 40 mg day−1 of isosorbide mononitrate (Toa Eiyo, Tokyo, Japan) (n=15, 12 men and 3 women, mean age of 63±12 years, isosorbide mononitrate group) or conventional treatment (n=15, 13 men and 2 women, mean age of 64±13 years, control group) for 12 weeks. None of the patients had a history of isosorbide nitrate treatment before the study. The randomization was performed by the envelope method. The physicians were given randomly treatment allocations within sealed opaque envelopes after a patient consented to enter the study. The study protocol was approved by the Ethics Committee of Hiroshima University Graduate School of Biomedical Sciences. Written informed consent for participation in the study was obtained from all subjects.
Subjects fasted for at least 12 h the night prior to assessment. Thirty minutes after remaining in the supine position, basal leukocyte ROCK activity, and fasting serum concentrations of total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, creatinine, glucose and HbA1c were measured. Measurements of leukocyte ROCK activity were performed at the beginning (0 week) and at 4 weeks and 12 weeks after treatment.
Measurement of ROCK activity
ROCK activity was assayed in peripheral blood leukocytes, based on the amount of phospho-Thr853 in the myosin-binding subunit (p-MBS) of myosin light chain phosphatase. Blood was collected at room temperature in heparinized tubes (20 U ml−1). After adding an equal volume of 2% dextran, the sample was kept at room temperature for 30 min. The supernatant was spun at 1450 r.p.m. for 10 min. Red blood cells in the resulting cell pellet were lysed with the addition of water and spun at 1450 r.p.m. for 10 min after the addition of Hank’s balanced salt solution (Hyclone, Logan, UT, USA). The resulting leukocyte pellet was resuspended in medium 199 (Sigma Chemical, Saint Louis, MO, USA) and counted using a hematocytometer. The cells were fixed in 10% trichloroacetic acid and 10 mmol l−1 dichlorodiphenyltrichloroethane. After centrifugation, the cell pellets were stored at −80 °C for western blot analysis. The cell pellets were dissolved in 10 μl of 1 mol l−1 Tris base and then mixed with 100 μl of extraction buffer (8 moll−1 urea, 2% sodium dodecyl sulfate, 5% sucrose, and 5% 2-mercaptoethanol). Equal amounts of cell extracts were subjected to 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. NIH 3T3 cell lysates were used as a positive control and to standardize the results of western blot analyses from several membranes. After serum starvation for 20 h, confluent cells were stimulated with 10 μmol l−1 lysophosphatidic acid for 10 min and then subsequently fixed and harvested in 10% trichloroacetic acid and 10 mmol l−1 dichlorodiphenyltrichloroethane. Following centrifugation at 1450 r.p.m. for 10 min at 4 °C, the precipitates were dissolved in 10 μl of 1 mol l−1 Tris base and mixed with 100 μl of extraction buffer. An equal volume of positive control cell lysate was used for each gel. The membranes were incubated with rabbit anti–phospho-specific Thr853–MBS polyclonal antibody (Biosource Invitrogen, Carlsbad, CA, USA) or rabbit anti-MBS polyclonal antibody (Covance Laboratories, Evansville, IN, USA), or anti-actin monoclonal antibody (Sigma). The bands were visualized by using the ECL system (Amersham-Pharmacia, London, UK). Images were captured using Adobe Photoshop (Adobe Systems, San Jose, CA, USA), and the band intensities were quantified using National Institutes of Health Image 1.61. ROCK activity was expressed as the ratio of p-MBS in each sample to p-MBS in each positive control divided by the total MBS in each sample per total MBS in each positive control.
Statistical analysis
The sample size calculation was based on the differences in the mean between two groups with equal sample size, prespecified 5% type I error, and 90% power. A sample size of 16 subjects in the isosorbide mononitrate group or the control group could achieve 90% power for detecting a difference of 40% in leukocyte ROCK activity between the null hypothesis that both group difference in mean are 0.00 and the alternative hypothesis that the difference in mean between the 2 group is 40% ROCK activities using a two-sided test.21 The results are presented as the mean±s.d. P-values less than 0.05 were considered to indicate statistical significance. Multigroup comparisons of variables were carried out by one-way analysis of variance followed by Bonferroni correction. The Mann–Whitney U-test was used to evaluate differences between the isosorbide mononitrate group and control group. The data were processed using the software package Stat View V (SAS Institute, Cary, NC, USA).
Results
Clinical characteristics
The baseline clinical characteristics of the isosorbide mononitrate group and control group are summarized in Table 1. Systemic hemodynamics, blood pressure, heart rate and serum levels of lipids and glucose were similar in all treatment periods in the two groups.
Effect of isosorbide mononitrate on ROCK activity
The effects of isosorbide mononitrate on leukocyte ROCK activity before (0 week) and after 4 and 12 weeks of treatment of patients with angina pectoris are shown in Figure 1. Isosorbide mononitrate significantly reduced ROCK activity after 4 weeks of treatment, in comparison with the pretreatment values (0.82±0.33 at 0 week vs 0.62±0.20 at 4 weeks, P=0.006). The ability of isosorbide mononitrate to decrease ROCK activity was maintained throughout the 12-week treatment period (0.61±0.19 at 12 weeks, P=0.002 vs 0 week). ROCK activities after 4 and 12 weeks of treatment were similar (P=N.S.). In the control group, ROCK activities were similar during follow-up periods (0.83±0.31 at 0 week, 0.84±0.35 at 4 weeks and 0.82±0.34 at 12 weeks, P=N.S., respectively). ROCK1 and ROCK2 expression levels were similar in all treatment periods in the two groups (Figure 2).
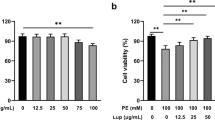
Effect of isosorbide mononitrate on ROCK activity in patients with angina pectoris. ROCK activities in the isosorbide mononitrate and control groups at the beginning of treatment (0 week) and after 4 and 12 weeks of treatment. (a) Representative results of western blot analysis for p-MBS, t-MBS and β-tublin. (b) Quantitative analysis of ROCK activity (p-MBS/t-MBS). n=15 in each group. *P<0.01 compared with 0 week. t-MBS, total MBS.
Effect of isosorbide mononitrate on ROCK1 and ROCK2 expression in patients with angina pectoris. ROCK1 and ROCK2 expression in the isosorbide mononitrate and control groups at the beginning of treatment (0 week) and after 4 and 12 weeks of treatment. (a) Representative results of western blot analysis for ROCK1, ROCK2 and β-tublin. (b) ROCK1 expression. (c) ROCK2 expression. n=15 in each group.
Discussion
In the present study, ROCK activity was significantly decreased in leukocytes isolated from the peripheral whole blood in angina patients treated with isosorbide mononitrate, compared with the control patients. To the best of our knowledge, this is the first study to demonstrate that the administration of exogenous NO can inhibit ROCK activity in humans.
Although ROCK1 and ROCK2 protein expression levels were similar throughout the treatment period, ROCK activities were significantly reduced after nitrate therapy in the isosorbide mononitrate group. ROCK activity has been shown to be regulated not at the protein expression level but instead by a conformation change in ROCK structure.22 In the inactive form, the carboxy terminus of ROCK binds to the amino-terminal region of the enzyme, forming an autoinhibitory loop. The active open kinase conformation is mediated by the interaction of GTP-bound RhoA to the Rho-binding domain of ROCK, or binding of arachidonic acid to the pleckstrin homology motif, or cleaving of the carboxy terminus by caspase-3 which disrupts the interaction between the catalytic and the inhibitory carboxy-terminal region of the enzyme, leading to the elevation of ROCK activity.
Several lines of evidence have indicated that the RhoA/ROCK pathway has a key physiological role in atherosclerotic lesion formation, vascular inflammation,23 vasoconstriction11, 24 and hypertrophy.25 ROCK, therefore, is becoming a key therapeutic target for the prevention of cardiovascular damage. Although there is no oral drug for direct Rho-kinase inhibition in the clinical setting, statin therapy has been demonstrated to reduce ROCK activity through the inhibition of isoprenoid synthesis, which is thought to be a predominant mechanism contributing to the pleiotropic effects of statins.26 In addition, we previously reported that calcium channel blockers also reduced ROCK activity in patients with hypertension by an unknown mechanism.27
In the present study, exogenous NO inhibited ROCK activities in peripheral leukocytes in patients with angina pectoris. Endogenous NO, released from the endothelial cells in response to numerous stimuli, is well known to have protective effects against diverse cardiovascular injuries. Nonetheless, the cardiovascular outcome by the treatment with exogenous NO still remains controversial,2, 3, 4, 5 despite its antioxidant and vasodilatory effects.28, 29 Of great interest, the continuous application of organic nitrates is known to evoke tolerance and subsequent loss of efficiency, which could potentially mediate harmful effects, such as increased oxidative stress, endothelial dysfunction, and sympathetic activation.30 The precise mechanisms underlying nitrate tolerance, however, are not completely understood. The most popular and clinical technique to prevent the tolerance phenomenon is to have a nitrate-free interval. Although intermittent nitrate therapy, which allows a daily nitrate washout interval, cannot provide continuous and uninterrupted therapeutic effects, this regimen has been reported to be effective for preventing nitrate tolerance.31 In this clinical study, patients with angina pectoris received isosorbide mononitrate at a clinical dose of 20 mg twice daily, as recommended. Potentially the clinical effect of exogenous NO, that is NO-mediated ROCK inhibition, might have given cardiologists practical confidence regarding the safety of using exogenous NO for the treatment of angina patients, although exogenous NO has not been recommended for patients with acute coronary syndrome.2, 3, 4 Nevertheless, the exact cardiovascular outcome after treatment with exogenous NO in patients with angina pectoris still remains to be clarified.
Considering the treatment of patients with cardiovascular diseases in which the RhoA/ROCK signaling is involved, the clinical view that intermittent administration of organic nitrates can not only replace the compromised endothelial NO production without nitrate tolerance but also inhibits the RhoA/ROCK pathway, is fairly plausible and attractive. Indeed, ROCK has been shown to be critically involved in patients with vasospastic angina and angina on effort,13, 15, 16 and here, we found that exogenous NO administration could inhibit ROCK activity in angina patients. Interestingly, in vitro, it has been shown that vascular smooth muscle cell relaxation is mediated by 3′5′-guanosine monophosphate-dependent protein kinase type I-induced phosphorylation of RhoA at Ser188, which leads to substantial ROCK inhibition, myosin light chain phosphatase activation and subsequent myosin light chain dephosphorylation. Together, these findings indicate the possible efficacy of organic nitrates, not only in patients with angina pectoris but also those with other cardiovascular diseases in which ROCK is particularly activated. Kikuchi et al.15 previously demonstrated that ROCK activity in peripheral leukocytes was increased and associated with an increased vasospastic activity in patients with vasospastic angina and that the level of ROCK activity was decreased after 3 months of medical treatment, including treatment with calcium channel blockers, nitrates, nicorandil and statins, although a direct inhibitory effect of nitrates on ROCK activity was not investigated in that study. Our findings raise the possibility that nitrate therapy prevents coronary vasospasm and reduces symptoms through the inhibition of the elevated ROCK activity in patients with vasospastic angina.
Conventionally, clinical ROCK activity has been evaluated in the vasculature. For example, vascular ROCK activity can be measured by the vascular response to an intra-arterial infusion of fasudil, a specific ROCK inhibitor, by using strain-gauge plethysmography.11, 12, 17, 18 However, recent studies have shown that the evaluation of leukocyte ROCK activity in the peripheral blood, based on the ratio of phospho-MBS to total MBS, could be a useful and alternative method to assess clinical ROCK activity in patients. Indeed, we have reported that leukocyte ROCK activity can be evaluated noninvasively compared with vascular ROCK activity and that leukocyte ROCK activity has a substantial correlation with vascular ROCK activity.32, 33 Although vascular ROCK activity can be quite accurately evaluated as the dose-dependent forearm vascular response to a ROCK inhibitor, leukocyte ROCK activity is also likely to be reliable. Indeed, the increased leukocyte ROCK activities in patients with hypertension,27 heart failure,34 metabolic syndrome21 and coronary artery disease,35 have previously been reported. In the present study, we found that leukocyte ROCK activity in patients with angina pectoris was significantly reduced after treatment with long-acting nitrates, suggesting that in humans, leukocyte ROCK activity could be a useful biomarker, instead of vascular ROCK activity, due to its noninvasive assessment properties. However, western blot analysis was necessary for the measurement of leukocyte ROCK activity used in this study, and there are currently no other convenient methods to measure ROCK activity in humans. Therefore, it is difficult to assess ROCK activity in daily clinical practice. The development of an easier method for measuring ROCK activity that is applicable to daily practice is needed.
In conclusion, we demonstrated the inhibitory effect of exogenous NO on ROCK activity in patients with angina pectoris. The beneficial effect of organic nitrates inhibiting the ROCK activity may be, at least in part, a rationale for the clinical use of exogenous NO in patients with stable and vasospastic angina. In addition, leukocyte ROCK activity could be a future promising biomarker, not only in patients with angina pectoris but also in patients with cardiovascular diseases in which RhoA/ROCK signaling is involved. Further studies are needed to determine whether the use of exogenous NO for the treatment of an angina attack would affect cardiovascular morbidity and mortality in patients with angina pectoris, which could provide cardiologists worldwide with a strong confidence regarding the safety of using organic nitrates.
References
Abrams J . Clinical practice. Chronic stable angina. N Engl J Med 2005; 352: 2524–2533.
Nakamura Y, Moss AJ, Brown MW, Kinoshita M, Kawai C . Long-term nitrate use may be deleterious in ischemic heart disease: a study using the databases from two large-scale postinfarction studies. Multicenter Myocardial Ischemia Research Group. Am Heart J 1999; 138: 577–585.
Gruppo Italiano per lo Studio della Sopravvivenza nell'infarto Miocardico. GISSI-3: effects of lisinopril and transdermal glyceryl trinitrate singly and together on 6-week mortality and ventricular function after acute myocardial infarction. Gruppo Italiano per lo Studio della Sopravvivenza nell'infarto Miocardico. Lancet 1994; 343: 1115–1122.
ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group. ISIS-4: a randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58,050 patients with suspected acute myocardial infarction. ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group. Lancet 1995; 345: 669–685.
Mahmarian JJ, Moye LA, Chinoy DA, Sequeira RF, Habib GB, Henry WJ, Jain A, Chaitman BR, Weng CS, Morales-Ballejo H, Pratt CM . Transdermal nitroglycerin patch therapy improves left ventricular function and prevents remodeling after acute myocardial infarction: results of a multicenter prospective randomized, double-blind, placebo-controlled trial. Circulation 1998; 97: 2017–2024.
Laufs U, Liao JK . Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem 1998; 273: 24266–24271.
Sawada N, Itoh H, Ueyama K, Yamashita J, Doi K, Chun TH, Inoue M, Masatsugu K, Saito T, Fukunaga Y, Sakaguchi S, Arai H, Ohno N, Komeda M, Nakao K . Inhibition of rho-associated kinase results in suppression of neointimal formation of balloon-injured arteries. Circulation 2000; 101: 2030–2033.
Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S . Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 1997; 389: 990–994.
Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K . Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science 1997; 275: 1308–1311.
Hall A . Rho GTPases and the actin cytoskeleton. Science 1998; 279: 509–514.
Masumoto A, Mohri M, Shimokawa H, Urakami L, Usui M, Takeshita A . Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation 2002; 105: 1545–1547.
Masumoto A, Hirooka Y, Shimokawa H, Hironaga K, Setoguchi S, Takeshita A . Possible involvement of Rho-kinase in the pathogenesis of hypertension in humans. Hypertension 2001; 38: 1307–1310.
Shimokawa H, Hiramori K, Iinuma H, Hosoda S, Kishida H, Osada H, Katagiri T, Yamauchi K, Yui Y, Minamino T, Nakashima M, Kato K . Anti-anginal effect of fasudil, a Rho-kinase inhibitor, in patients with stable effort angina: a multicenter study. J Cardiovasc Pharmacol 2002; 40: 751–761.
Noma K, Higashi Y, Jitsuiki D, Hara K, Kimura M, Nakagawa K, Goto C, Oshima T, Yoshizumi M, Chayama K . Smoking activates rho-kinase in smooth muscle cells of forearm vasculature in humans. Hypertension 2003; 41: 1102–1105.
Kikuchi Y, Yasuda S, Aizawa K, Tsuburaya R, Ito Y, Takeda M, Nakayama M, Ito K, Takahashi J, Shimokawa H . Enhanced Rho-kinase activity in circulating neutrophils of patients with vasospastic angina: a possible biomarker for diagnosis and disease activity assessment. J Am Coll Cardiol 2011; 58: 1231–1237.
Hung MJ, Cherng WJ, Hung MY, Kuo LT, Cheng CW, Wang CH, Yang NI, Liao JK . Increased leukocyte Rho-associated coiled-coil containing protein kinase activity predicts the presence and severity of coronary vasospastic angina. Atherosclerosis 2012; 221: 521–526.
Noma K, Goto C, Nishioka K, Hara K, Kimura M, Umemura T, Jitsuiki D, Nakagawa K, Oshima T, Chayama K, Yoshizumi M, Higashi Y . Smoking, endothelial function, and Rho-kinase in humans. Arterioscler Thromb Vasc Biol 2005; 25: 2630–2635.
Noma K, Goto C, Nishioka K, Jitsuiki D, Umemura T, Ueda K, Kimura M, Nakagawa K, Oshima T, Chayama K, Yoshizumi M, Liao JK, Higashi Y . Roles of rho-associated kinase and oxidative stress in the pathogenesis of aortic stiffness. J Am Coll Cardiol 2007; 49: 698–705.
Wolfrum S, Dendorfer A, Rikitake Y, Stalker TJ, Gong Y, Scalia R, Dominiak P, Liao JK . Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Arterioscler Thromb Vasc Biol 2004; 24: 1842–1847.
Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Smolenski A, Lohmann SM, Bertoglio J, Chardin P, Pacaud P, Loirand G . Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J Biol Chem 2000; 275: 21722–21729.
Liu PY, Chen JH, Lin LJ, Liao JK . Increased Rho kinase activity in a Taiwanese population with metabolic syndrome. J Am Coll Cardiol 2007; 49: 1619–1624.
Noma K, Oyama N, Liao JK . Physiological role of ROCKs in the cardiovascular system. Am J Physiol Cell Physiol 2006; 290: C661–C668.
Mallat Z, Gojova A, Sauzeau V, Brun V, Silvestre JS, Esposito B, Merval R, Groux H, Loirand G, Tedgui A . Rho-associated protein kinase contributes to early atherosclerotic lesion formation in mice. Circ Res 2003; 93: 884–888.
Sato M, Tani E, Fujikawa H, Kaibuchi K . Involvement of Rho-kinase-mediated phosphorylation of myosin light chain in enhancement of cerebral vasospasm. Circ Res 2000; 87: 195–200.
Higashi M, Shimokawa H, Hattori T, Hiroki J, Mukai Y, Morikawa K, Ichiki T, Takahashi S, Takeshita A . Long-term inhibition of Rho-kinase suppresses angiotensin II-induced cardiovascular hypertrophy in rats in vivo: effect on endothelial NAD(P)H oxidase system. Circ Res 2003; 93: 767–775.
Liu PY, Liu YW, Lin LJ, Chen JH, Liao JK . Evidence for statin pleiotropy in humans: differential effects of statins and ezetimibe on rho-associated coiled-coil containing protein kinase activity, endothelial function, and inflammation. Circulation 2009; 119: 131–138.
Hata T, Soga J, Hidaka T, Idei N, Fujii Y, Fujimura N, Mikami S, Maruhashi T, Kihara Y, Chayama K, Kato H, Noma K, Liao JK, Higashi Y . Calcium channel blocker and Rho-associated kinase activity in patients with hypertension. J Hypertens 2011; 29: 373–379.
Nishioka S, Yoshioka T, Nomura A, Kato R, Miyamura M, Okada Y, Ishizaka N, Matsumura Y, Hayashi T . Celiprolol reduces oxidative stress and attenuates left ventricular remodeling induced by hypoxic stress in mice. Hypertens Res 2013; 36: 934–939.
Paulo M, Araujo AV, Bendhack LM . Sodium nitroprusside activates potassium channels in the vena cava in normotensive but not in hypertensive rats. Hypertens Res 2013; 36: 765–769.
Gori T, Parker JD . Nitrate-induced toxicity and preconditioning: a rationale for reconsidering the use of these drugs. J Am Coll Cardiol 2008; 52: 251–254.
Packer M, Lee WH, Kessler PD, Gottlieb SS, Medina N, Yushak M . Prevention and reversal of nitrate tolerance in patients with congestive heart failure. N Engl J Med 1987; 317: 799–804.
Hata T, Goto C, Soga J, Hidaka T, Fujii Y, Idei N, Fujimura N, Maruhashi T, Mikami S, Kihara Y, Chayama K, Noma K, Liao JK, Higashi Y . Measurement of Rho-associated kinase (ROCK) activity in humans: validity of leukocyte p-MBS/t-MBS in comparison with vascular response to fasudil. Atherosclerosis 2011; 214: 117–121.
Soga J, Noma K, Hata T, Hidaka T, Fujii Y, Idei N, Fujimura N, Mikami S, Maruhashi T, Kihara Y, Chayama K, Kato H, Liao JK, Higashi Y, Group RS . Rho-associated kinase activity, endothelial function, and cardiovascular risk factors. Arterioscler Thromb Vasc Biol 2011; 31: 2353–2359.
Dong M, Liao JK, Fang F, Lee AP, Yan BP, Liu M, Yu CM . Increased Rho kinase activity in congestive heart failure. Eur J Heart Fail 2012; 14: 965–973.
Nohria A, Grunert ME, Rikitake Y, Noma K, Prsic A, Ganz P, Liao JK, Creager MA . Rho kinase inhibition improves endothelial function in human subjects with coronary artery disease. Circ Res 2006; 99: 1426–1432.
Acknowledgements
We thank Naomi Idei, Shinsuke Mikami, Yumiko Iwamoto, Akimichi Iwamoto, Nozomu Oda, Yoshiki Aibara, Chikara Goto, Kazuaki Chayama, Megumi Wakisaka, Miki Kumiji, Ki-ichiro Kawano and Satoko Michiyama for their excellent experimental and secretarial assistance. This work was carried out at the Analysis Center of Life Science, Hiroshima University. Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (23591043), Japan Heart Foundation/Novartis Grant and Tsuchiya Foundation Grant, Japan (to KN) and a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (1859081500 and 21590898 to YH) and a Grant-in-Aid of Japanese Arteriosclerosis Prevention Fund (to YH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Maruhashi, T., Noma, K., Fujimura, N. et al. Exogenous nitric oxide inhibits Rho-associated kinase activity in patients with angina pectoris: a randomized controlled trial. Hypertens Res 38, 485–490 (2015). https://doi.org/10.1038/hr.2015.24
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2015.24
Keywords
This article is cited by
-
A novel cardioprotective mechanism of exogenous nitric oxide: inhibition of Rho-associated kinase activity
Hypertension Research (2015)