Abstract
The hepatocyte nuclear factor 1β gene (HNF1B) is responsible for maturity-onset diabetes of the young type 5 (MODY5), which is characterized by early-onset diabetes mellitus and urogenital malformations. HNF1B is expressed during visceral endoderm formation. We identified a disruption of the great pancreatic artery in a patient with MODY5 with no pancreatic body or tail. Our finding supports the significance of HNF1B in the development of the pancreas.
Similar content being viewed by others
Maturity-onset diabetes of the young (MODY; MIM#606391) was first reported by Fajans in 1960, as an early-onset form of mild diabetes with autosomal dominant inheritance.1 The minimum prevalence of MODY is estimated to be 108/1,000,000 in the general population in the UK.2 In Germany, MODY affects ~1% of patients with diabetes mellitus diagnosed before 20 years of age, indicating that the prevalence of MODY is not rare among patients with diabetes mellitus.3 Pathogenic mutations have been detected in >16 genes, the majority of which code transcription factors.4 The most common causes of MODY are mutations in HNF1A (hepatocyte nuclear factor-1α; MODY 3, MIM#600496), which encodes a transcription factor, and the second most commonly mutated gene is GCK (glucokinase; MODY2, MIM#125851), which encodes a glycolytic enzyme. Additional mutations are found in HNF4A (hepatocyte nuclear factor-4α; MODY1, MIM#600496) and HNF1B (MODY5, #MIM189907). MODY5 is frequently associated with renal cysts and/or other urogenital manifestations.5 Moreover, some cases with MODY5 develop associated with ketoacidosis, like type 1 diabetes mellitus.5 The prevalence of MODY5 among all clinically diagnosed MODY is reported to be 2–6%.6,7 More than 50 types of mutations are reported, comprising base substitutions, small insertion–deletions and whole-gene deletions. The majority of mutations occur in first four exons of the gene. Exons 2, 4 and the intron 2 splice site are mutation site hotspots.8
We identified a 24-year-old female patient with a 17q12 hemizygous deletion including HNF1B. She had a sudden onset of diabetes mellitus. On admission, the patient had a normal level of consciousness, with temperature 36.8 °C, regular heart rate at 102 per minute, and blood pressure of 126/66 mm Hg. Anthropometric data were height 148 cm, body weight 43 kg and body mass index 19.6. No diabetic retinopathy was observed in either eye. Plasma glucose was 385 mg/dl, HbA1c was 16.7%. Antibodies to glutamic acid decarboxylase (GAD-Ab), insulin (IAA), and IA-2 were negative. Liver dysfunction was observed (aspartate aminotransferase 30 IU/l, alanine aminotransferase 39 IU/l, lactose dehydrogenase 155 IU/l, alkaline phosphatase 283 IU/l and γ-GTP 252 IU/l), but no renal dysfunction was detected (blood urea nitrogen 8.0 mg/dl, creatinine 0.36 mg/dl, negative for urinary protein). Markers for β-cell function (immunoreactive insulin 3.3 μU/ml (5–30 μU/ml), urinary-C-peptide immunoreactivity 12 μg/day (16–120 μg per day)) indicated severely decreased insulin secreting capacity. Although the initial presentation was acute onset, the diagnosis of type 1 diabetes mellitus was not likely. Younger age of onset without obesity, and a family history of diabetes mellitus through three generations were consistent with a clinical diagnosis of MODY (Figure 1a). She had renal cysts and lacked a pancreatic body and tail (Figure 2a). These manifestations strongly suggested a diagnosis of MODY5 and genetic testing was performed.
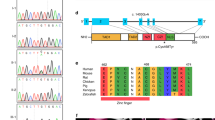
Pedigrees and the results of microarray-based comparative hybridization analysis. (a) The proband was diagnosed with diabetes at 24 years of age. Her mother and maternal grandmother were also diagnosed with diabetes mellitus, but their ages at the time of the diagnosis are not known. (b) A chromosomal aberration at 17q12 is detected by Chromosome View (left) using Agilent Genomic Workbench v6.5 (Agilent Technologies). The identified aberration with a 1.3 Mb in size is expanded in Gene View (right), in which HNF1B is included (highlighted by a red circle). There are segmental duplications at both ends of the aberration (there are no probes owing to repeated sequences). AMI, acute myocardial infarction; GB Ca, gall bladder cancer.
Radiologic imaging. (a) Abdominal CT with enhancement. The pancreatic head is shown, but the body and tail of the pancreas are not observed in this slice. A small cortical renal cyst can be seen in the right kidney. (b) MRCP. The main pancreatic duct was not contrasted (normal appearance of a main pancreatic duct is indicated by the red dotted line). (c) Angiography of celiac artery. The catheter reaches the contrasted celiac artery, which bifurcates into the gastroduodenal artery and splenic artery. The great pancreatic artery, which bifurcates from the splenic artery, ends abruptly and the transverse pancreatic artery, which is a peripheral branch of the great pancreatic artery, is nonexistent (indicated by the red dotted lines). The silhouette of the pancreas is indicated by the yellow dotted line. CT, computed tomography; MRCP, magnetic resonance cholangiopancreatography.
The study was approved by the Ethics Committee of Tokyo Women’s Medical University and performed in accordance with the principles of the Declaration of Helsinki. Informed consent was obtained from the subject before participation.
Genomic DNA was derived from peripheral blood monocytes and used for routine PCR-based direct sequencing for HNF1B, using ABI 3100 (Applied Biosystems by Life Technologies, Carlsbad, CA, USA). The nine exons, flanking introns and minimal promoter region of HNF1B were screened in both directions. None of the part of fluorescence electrophoresis was heterozygous, implying that she had only one allele in the entire HNF1B. Then genomic copy-number aberrations were examined by microarray-based comparative hybridization analysis using Human Genome CGH 244A 243504 probes, 6.4-kb interval (Agilent Technologies, Santa Clara, CA, USA) as described.9 Subsequently, a hemizygous deletion at chromosome 17q12 spanning 1.3 Mb, arr 17q12(34,815,184–36,241,526)×1 (bild19), the region including entire HNF1B (Figure 1b) was found. There is a family history of diabetes in an autosomal dominant trait, and her mother (II-7) and grandmother (I-4) were suspected as having the same deletion (Figure 1a). However, the mother and grandmother declined genotyping despite careful genetic counseling.
The first case of MODY5 was reported in 1997.10 Three affected family members who carried the mutation on exon 2 (R177X) in HNF1B had early onset and an insulin-deficient type of diabetes mellitus. These three cases also had renal cysts and various degrees of renal dysfunction. Later, it was clearly demonstrated, based on a Xenopus embryo model, that renal malformation, including renal cysts, are caused by gain-of-function or loss-of-function mutations in HNF1B in humans.11 Additional urogenital manifestations, such as dysplastic kidney, including horse-shoe kidney, duplex kidneys and single kidney, bicornuate uterus, and agenesis of the vas deferens have been reported.12,13 HNF1B is also involved in the development of the ureters, which may explain stenosis of the ureter responsible for hydronephrosis.13,14 Moreover, HNF1B is also responsible for renal cysts and diabetes syndrome (MIM#137920), which does not require diabetes mellitus as a diagnostic criterion. This region is also responsible for 17q12 deletion syndrome (MIM#614527) and 17q12 duplication syndrome (MIM# 614526). These syndromes are characterized by autism spectrum disorder and schizophrenia, and are independent from diabetes mellitus. Thus, HNF1B-related phenotypes are heterogeneous, and are considered a multi-system disorder.13 Moreover, some phenotypes are similar between cases with mutations in HNF1B and copy-number variations in 17q12 spanning 1.3 Mb, including HNF1B, suggesting a gene dosage effect as a molecular mechanism in combination with other genes in this region. The precise signaling pathways, however, are not fully elucidated.13 Clinical manifestations related to mutations in HNF1B have been intensively reviewed, especially urogenital tract malformations, but there are few reports of pancreatic manifestations.
The proband underwent magnetic resonance cholangiopancreatography (MRCP), which showed disruption of the main pancreatic duct. Interestingly, celiac artery angiography demonstrated aplasia of the great pancreatic artery and the transverse pancreatic artery (Figure 2b,c).
HNF1B and HNF1A are homeobox transcription factor family members with a highly conserved N-terminal DNA-binding domain and a more divergent C-terminal transactivation domain. Those two genes developed by duplication of an ancestral gene and HNF1B functions as a homodimer or a heterodimer with HNF1A.15 In mice, vHnf1 (mice HNF1B) expression is uniquely detected in the primitive and visceral endoderm from embryonic days 4.5 to 7.5, and in the liver, pancreas and ureter buds at embryonic days 9.5–11. Expression of vHnf1 is also detected during neural tube formation, and in the developing lungs and genital tract. A vHnf1 deficiency in mouse embryos is lethal due to defects in visceral endoderm formation.15 Haumaitre et al.16 reported that a lack of vHnf1 results in pancreas agenesis by embryonic day 13.5. In earlier stages, the dorsal bud rudiment forms only transiently and lacks key transcription factors for proper development of the pancreas. In another study, Haumaitre et al. proposed that the absence of vHnf1 results in decreased Ptf1a expression, which leads to defects in the development of the ventral pancreas and a reduced dorsal pancreas.17 Partial loss of hnf1ba function in zebrafish also causes pancreas defects, and normal hnf1ab function is distinctly required for pancreas specification. Haumaitre et al. also suggested a common genetic program in relation with hnf1ba, with a narrow developmental time window, that specifies progenitors of the liver, ventral pancreas, gall bladder and associated ducts.17 The pancreatic head develops by fusion of the dorsal and ventral bud, and the pancreatic body and tail develop only from dorsal bud. Atrophy of the pancreas was detected by abdominal computed tomography in five of six cases with MODY5, but it was not clear whether these cases were due to atrophy or agenesis.5 To our knowledge, celiac angiography and MRCP of a patient with MODY 5 associated with pancreatic abnormality has not been reported previously. This observation implies that the absence of a pancreatic body and tail are due to a developmental defect rather than to atrophy after development of the pancreas. The liver dysfunction observed in this patient is a typical feature of MODY5.5,13,18
In conclusion, systematic evaluation of the pancreas including MRCP and celiac artery angiography in a patient with MODY5 associated with 17q12 hemizygous deletion provided important clues to the molecular mechanism of HNF1B in development of the pancreas in humans.
References
References
Fajans SS, Conn JW . Tolbutamide-induced improvement in carbohydrate tolerance of young people with mild diabetes mellitus. Diabetes 1960; 9: 83–88.
Shields B, Hicks S, Shepherd M, Colclough K, Hattersley A, Ellard S . Maturity-onset diabetes of the young (MODY): how many cases are we missing? Diabetologia 2010; 53: 2504–2508.
Schober E, Rami B, Grabert M, Thon A, Kapellen T, Reinehr T et al. Phenotypical aspects of maturity-onset diabetes of the young (MODY diabetes) in comparison with type 2 diabetes mellitus (t2DM) in children and adrescents: experience from a large multicenter database. Diab Med 2009; 26: 466–473.
Bonnefond A, Froguel P . Rare and common genetic events in type 2 diabetes: what should biologist know? Cell Metab 2015; 21: 357–368.
Bellanne-Chantelot C, Chaubeau D, Gautier JF, Dubis-Laforgue D, Clauin S, Beaufils S et al. Clinical spectrum associated with hepatocyte nuclear factor-1β mutations. Ann Int Med 2004; 140: 510–517.
Fajans SS, Bell GI . MODY: History, genetics, pathophysiology, and clinical decision making. Diabetes Care 2011; 34: 1878–1884.
Kavvoura FK, Owen KR . Monogenic diabetes. Medicine 2014; 12: 692–697.
Chen YZ, Gao Q, Zhao XZ, Chen YA, Bennet CL, Xiong XS et al. Systematic review of TCF2 anomalies in renal cysts and diabetes syndrome/maturity onset diabetes of the young type 5. Chin Med J 2010; 123: 3326–3333.
Yamamoto T, Shimojima K, Shimada K, Yokochi K, Yoshitomi S, Yanagihara K et al. Clinical impacts of genomic copy number gains at Xq28. Hum Genome Variat 2014; 1: 14001.
Horikawa Y, Iwasaki N, Hara M, Furuta H, Hinokio Y, Cockburn B et al. Mutation in hepatocyte nuclear factor-1b gene (TCF2) associated with MODY. Nat Genet 1997; 17: 384–385.
Wild W, Pogge von Strandmann P, Nastos A, Sankel S, Lingott-Freig A, Bulman M et al. The mutated human gene encoding hepatocyte nuclear factor 1beta inhibits kidney formation in developing Xenopus embryos. Proc Natl Acad Sci USA 2000; 97: 4695–4700.
Iwasaki N, Okabe I, Momoi MY, Ohashi H, Ogata M, Iwamoto Y . Splice site mutation in the hepatocyte nuclear factor -1b gene, IVS2nt+1G>A, associated with maturity onset diabetes of the young, renal dysplasia and bicornuate uterus. Diabetologia 2001; 44: 387–388.
Clissold RL, Hamilton AJ, Hattersley AT, Ellard S, Bingham C . HNF1B-associated renal and extra renal disease- an expanding clinical spectrum. Nat Rev Nephrol 2015; 11: 102–112.
Lokmane L, Heliot C, Garcia-Villava P, Fabre M, Cereghini S . vHNF1 functions in distinct regulatory circuits to control ureteric bud branching and early nephrogenesis. Development 2010; 137: 347–357.
Haumaitre C, Reber M, Cereghini S . Functions of HNF1 family members in differentiation of the visceral endoderm cell lineage. J Biol Chem 2003; 278: 40933–40942.
Haumaitre C, Barbacci E, Jenny M, Ott MO, Gradwohl G, Cereghini S . Lack of TCF2/vNHF1 in mice leads to pancreas agenesis. Proc Natl Acad Sci USA 2005; 102: 1490–1495.
Lancman JJ, Zvenigorodsky N, Gates KP, Zhang D, Solomon K, Humphrey RK et al. Specification of hepatopancreas progenitors in zebrafish by hnf1ba and wnt2bb. Development 2013; 140: 2669–2679.
Iwasaki N, Ogata M, Tomonaga O, Kuroki H, Kasahara T, Yano N et al. Liver and kidney function in Japanese patients with maturity-onset diabetes of the young. Diabetes Care 1998; 21: 2144–2148.
Data Citations
Naoko, Iwasaki HGV Database (2016) http://dx.doi.org/10.6084/m9.figshare.hgv.842
Acknowledgements
We thank the patient and are grateful for the technical skills of Mitsue Tomioka and Yumiko Sagisaka. This study was supported by a Grant from the National Center for Global Health and Medicine (21A114) from the Ministry of Health, Labor, and Welfare of Japan (MHLW), and a Grant-in-Aid (22510214) for Scientific Research from the MEXT to N.I.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Iwasaki, N., Tsurumi, M., Asai, K. et al. Pancreatic developmental defect evaluated by celiac artery angiography in a patient with MODY5. Hum Genome Var 3, 16022 (2016). https://doi.org/10.1038/hgv.2016.22
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/hgv.2016.22
This article is cited by
-
The HNF1B mutations and deletion associated with diabetes and their resulting diabetic phenotypes: a systematic review
International Journal of Diabetes in Developing Countries (2024)
-
A rare combination of MODY5 and duodenal atresia in a patient: a case report
BMC Medical Genetics (2020)