Abstract
Purpose: To determine whether sociodemographic factors are associated with delays at specific steps in the diagnostic process of Duchenne and Becker muscular dystrophy.
Methods: We examined abstracted medical records for 540 males from population-based surveillance sites in Arizona, Colorado, Georgia, Iowa, and western New York. We used linear regressions to model the association of three sociodemographic characteristics with age at initial medical evaluation, first creatine kinase measurement, and earliest DNA analysis while controlling for changes in the diagnostic process over time. The analytical dataset included 375 males with information on family history of Duchenne and Becker muscular dystrophy, neighborhood poverty levels, and race/ethnicity.
Results: Black and Hispanic race/ethnicity predicted older ages at initial evaluation, creatine kinase measurement, and DNA testing (P < 0.05). A positive family history of Duchenne and Becker muscular dystrophy predicted younger ages at initial evaluation, creatine kinase measurement and DNA testing (P < 0.001). Higher neighborhood poverty was associated with earlier ages of evaluation (P < 0.05).
Conclusions: Racial and ethnic disparities in the diagnostic process for Duchenne and Becker muscular dystrophy are evident even after adjustment for family history of Duchenne and Becker muscular dystrophy and changes in the diagnostic process over time. Black and Hispanic children are initially evaluated at older ages than white children, and the gap widens at later steps in the diagnostic process.
Similar content being viewed by others
Main
Inequalities in healthcare access, treatment, and clinical outcomes are common between various segments of the US population.1 Health disparities originate in the variation among populations for race and ethnicity, social and economic status, insurance provider, geographic location, age, and language usage.2 Studies indicate that these factors act both alone and in concert to influence the severity of health disparities.3 Although some health disparities have been documented in the pediatric population,4,5 the literature is sparse,6 and no studies have examined disparities in the diagnostic process of relatively rare conditions such as Duchenne and Becker muscular dystrophy (DBMD).
DBMD are allelic, X-linked recessive, neuromuscular disorders with prevalence in males aged 5–24 years between 1.3 and 1.8 per 10,000.7 Spontaneous mutations in the dystrophin gene are reported in approximately 30% of cases.8 The clinical course of Duchenne muscular dystrophy is characterized by delayed development, progressive disability, and loss of ambulation by age 13 years.9 Becker muscular dystrophy is associated with a milder clinical course, but in practice, there is an overlap of clinical manifestations.10
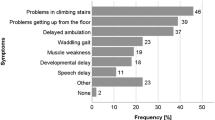
In the absence of a family history of the disease, symptoms of DBMD are usually recognized approximately 2.5 years before diagnosis and 1 year before the child is brought to a healthcare professional for evaluation of those symptoms.11 Clinic and population-based studies indicate a mean age of diagnosis of DBMD approximately 5 years, and this age has remained unchanged for 2 decades.11–13 Diagnostic testing for DBMD should be initiated, irrespective of family history, when characteristic physical signs and symptoms or abnormal laboratory findings are identified. Clinical symptoms include muscle weakness, delayed walking, and difficulty with running and climbing stairs, which should prompt creatine kinase (CK) measurement and subsequent referral to a neuromuscular specialist and genetic testing for definitive diagnosis.14
Clinical management of DBMD has improved over the past 20 years.14 Treatments that prolong ambulation, increase quality of life, and extend life expectancy are now available, but their efficacy may depend on a timely diagnosis.14 Studies of the diagnosis of other diseases in children and adults show disparities exist in at least two related diagnostic steps that are relevant to the experience of children as they undergo the DBMD diagnostic process, including (1) disparities at the family and physician level in noting symptoms and taking action as a result of symptoms15–18 and (2) disparities in access to and uptake of diagnostic and genetic testing.19–21 Disparities at each of these diagnostic steps result in a longer diagnostic timeline, which may have negative psychological effects on patients and their families21–23 and may unjustly exclude certain groups of patients from mutation-specific clinical trials and the highest standard of care.11 The purpose of this study, therefore, is to determine whether sociodemographic factors are associated with disparities at steps in the diagnostic process of DBMD. A better understanding of the factors that place children at risk for diagnostic delay can inform efforts to improve early diagnosis for all patients.
MATERIALS AND METHODS
Data collection
The Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet) is a group of five US sites (Arizona, Colorado, Georgia, Iowa, and western New York), which identifies and collects information on all cases of DBMD born or residing in those areas between 1982 and the present. The MD STARnet gathers information on cases from medical records, birth certificates, and interviews with caregivers and patients with DBMD. Using a computerized abstraction instrument, trained abstractors review and collect data from medical records for each case annually from multiple locations. Record sources include neuromuscular clinics, hospitals and hospital discharge databases, private physicians, service units for children with special healthcare needs, and birth defects surveillance systems. Following record abstraction, the MD STARnet Clinical Review Committee conducts a blind review of selected diagnostic variables and classifies each patient to one of the following case definitions: definite, probable, possible, asymptomatic, female, or not DBMD. A full characterization of the population-based MD STARnet surveillance methodology is published elsewhere.24
Data abstracted from medical records that we analyzed include family history of DBMD, date of first medical evaluation for symptoms associated with DBMD, initial CK measurement, and DNA analysis for dystrophin mutations. Information gathered from birth certificates that we analyzed included date of birth, education level of the mother, race/ethnicity of the child, and home address at birth.
Mother's education information was missing from birth certificates of 29 cases of the final dataset. For these cases, we used mother's education information collected as part of a telephone survey that targeted families of all cases known to the MD STARnet.
Sample
We compiled the analytic dataset by examining data from all 540 males included in the MD STARnet year 4 database who were born before January 1, 2001, and identified by the Clinical Review Committee as definite or probable cases before January 1, 2009. Both definite and probable cases have a clinical diagnosis of DBMD from a qualified professional, characteristic signs and symptoms of DBMD, and an elevated CK level. The only difference between the classifications is that probable cases do not have an identified dystrophin gene mutation or diagnostic muscle biopsy. Figure 1 presents our selection criteria for the final dataset containing 375 (69.4%) of those cases. We excluded 40 cases missing family history information, 85 cases missing race/ethnicity information, and 16 cases that were identified with race/ethnicity other than white, black, or Hispanic. Twenty-four cases missing poverty information were also excluded.
Study variables
We designated three time points in the diagnostic pathway as continuous outcome variables: (1) age at initial evaluation for symptoms associated with DBMD, (2) age at first CK measurement, and (3) age at first DNA test. We created three dichotomous individual level sociodemographic variables: maternal education, classified as “high school graduate or less” versus “some college or above”; race and ethnicity, limited to either non-Hispanic white or non-Hispanic Black and Hispanic; and family history, designated as negative if records indicated that a patient's caregivers had no knowledge of family members affected with DBMD before the diagnosis of the index child.
We used one area-based socioeconomic measure, census tract poverty level, to investigate the effect of neighborhood characteristics on disparities in diagnostic delay and testing. Methods to create area-based socioeconomic measures and their sensitivity to detect theoretically expected gradients in healthcare access and outcomes are documented by the Public Health Disparities Geocoding Project.25 Census tract poverty level is the percent of residents within a census tract living below the federally stated poverty line at the time of the census. We derived this variable for each case by first geocoding patient birth addresses to the census tract level using University of Southern California WebGIS Services.26 We then matched census tract identification numbers with poverty levels for that tract calculated by the Public Health Disparities Geocoding Project for the 1980, 1990, and 2000 census. We used poverty data from the census year proximal to the patient's birth year in the analysis, and this measure was modeled as a continuous variable. Among cases for which race and family history were known, 53 cases were missing poverty information. We found that increased poverty levels were inversely correlated with mother's education (Point Biserial Correlation, −0.032, P < 0.001). Twenty-nine of the 53 cases missing poverty data had data for mother's education level. For these cases, we imputed the average percent poverty level corresponding to the mother's education level. The remaining 24 cases missing poverty information were excluded from the analysis.
We theorized that society wide improvements in disease awareness, increases in perceived utility of early diagnosis, or better access to diagnostic laboratory technology likely reduced the time to diagnosis over the past 25 years. We controlled for these societal changes over time by creating a continuous variable that we defined as months between January 1, 1982, and the patient's birth date. We also restricted our analysis of age at DNA testing to individuals within the analytic dataset born after 1991 because expert opinion and a literature review indicated that mutation analysis was neither commonly recommended nor widely available before 1991.27,28
Statistical analysis
We used SPSS 17.0 software (SPSS Inc., Chicago, IL) to analyze the data. We evaluated the distribution of outcome variables (age at evaluation, CK measurement, and DNA testing) by inspecting histograms, normal probability plots, and by conducting Kolmogorov-Smirnov and Shapiro-Wilks tests. Each variable deviated from normality, but we judged the distribution to be sufficiently close to normal to proceed using common parametric statistical techniques and linear regression. We compared mean differences between dichotomous groups by conducting Student's t tests and searched for potential differences across MD STARnet regional sites using analysis of variance.
We used linear regressions to determine which sociodemographic variables affected outcome variables. Because we had no preconception as to which independent variables might best predict outcome variables, we entered all variables into the model simultaneously. Interaction terms were generated and entered into the model, but none showed association with the outcome variable and thus were removed from the final model. We reviewed regression residual plots to confirm that model assumptions were justified and evaluated the colinearity of independent variables by examining variance inflation factors and tolerances. The analytic dataset contains 27 sibling pairs, one triplet, and one quadruplet. We found no evidence of correlations between brothers on outcome variables and treated these cases as independent observations.
RESULTS
Participants
Table 1 illustrates the demographic composition of the analytical dataset and compares it with all males in the year 4 database born 1982–2000. The distribution of sociodemographic variables in the analytical dataset did not differ significantly from the year 4 database. Among included cases, approximately two thirds (65.9%) had no family history of DBMD. Approximately 70% of cases were white and 30% were black (21 patients) or Hispanic (95 patients). On average, 13.6% of people in each census tract lived below the poverty line.
Bivariate analysis
Figure 2 presents box plots of the relationship between age and diagnostic steps stratified by family history. Table 2 complements the box plots, reporting on mean case age at diagnostic steps stratified by family history. On average, cases with a family history were first evaluated at 30.8 months, 26 months earlier than cases with no family history. Cases with a family history had CK testing at 35.1 months, 28.9 months before cases without a family history. Age at DNA testing was not significantly different by family history.
Multivariate analysis
Table 3 provides results of linear regressions predicting age at evaluation, CK measurement, and DNA testing. Race/ethnicity and neighborhood poverty independently predicted age at first evaluation after adjusting for family history and birth date. Black and Hispanic children were evaluated 11.7 months after white children and children who lived in poorer neighborhoods were evaluated at younger ages; a 10% rise in neighborhood poverty reduced age at first evaluation by 3.8 months. Mother's education was not a significant predictor in our preliminary analysis and was removed from the final models.
In the regression analysis modeling age to CK measurement, adjusting for family history and birth date, we found white children had CK measurement 12.9 months earlier than black and Hispanic children. Similarly, our model of age to DNA testing indicates that white children were tested 24.5 months earlier than black and Hispanic children. Neighborhood poverty level was not a significant predictor of age at CK measurement or DNA testing.
DISCUSSION
This population-based study found that children without a family history of DBMD born between 1982 and 2000 were evaluated for symptoms later and received CK measurement at older ages than was found by Ciafaloni et al.11 using a prior dataset derived from the MD STARnet. This study also found a trend toward younger ages at all steps in the diagnostic process over time that was not found previously.12 There are important differences in the case composition of the prior and current analytical datasets. The prior study drew cases from 384 definite or probable cases identified by the MD STARnet at that time. That study excluded cases with a family history, those with no recorded symptoms before age 7 years, those who were recognized by newborn screening or because of concomitant conditions, and those with incomplete data on diagnostic steps. In contrast, this study excludes cases missing information on sociodemographic variables of interest. Also, more cases are now identified by the MD STARnet, including 92 cases from the Georgia MD STARnet site, which were not identified at the time of the previous study. However, we found that mean ages did not differ by site; it is, therefore, unlikely that additional cases from Georgia are responsible for observed differences.
As expected, boys with a family history of DBMD were evaluated and had CK levels measured at younger ages than boys without a family history. Although still a significant predictor in regression modeling, the effect of a positive family history on age at DNA testing was much reduced. Parents may perceive less utility in quickly obtaining DNA testing of a boy if a disease-causing mutation in another family member has been identified. Insurance providers may also be reluctant to authorize confirmational testing of boys born into families with known mutations.
Boys born in neighborhoods with higher poverty levels were evaluated at younger ages. Although the effect size was small, this trend is contrary to the influence that poverty gradients generally have on health outcomes.29 However, in a study examining family level factors associated with age at diagnosis for autism spectrum disorders, Mandell et al.30 found that poverty effects were not linear: children of near-poor families were diagnosed later than either poor or wealthy families. We did not, however, find evidence of nonlinear trends in bivariate plots of poverty data and any outcome variable.
Measures of neighborhood poverty levels are not merely a proxy for family level income information but rather capture a mix of individual and area-based socioeconomic effects.31 It may be that poorer neighborhoods are more likely to have a range of social services available that monitor child development closely and promote earlier recognition of symptoms, which in turn may lead to earlier evaluation. It is also possible that residents from poorer neighborhoods are more likely to be publicly insured. Families in this group may have better access to health system resources than near-poor families who do not qualify for public assistance.32–34
Racial and ethnic disparities in the diagnostic process might be linked to the actions parents and providers take after the initial evaluation. Although race/ethnicity differences were apparent in the ages of boys at initial evaluation, delays to CK measurement and DNA testing were progressively longer, even after adjustment for other sociodemographic characteristics. At the family level, blacks and Hispanics may be less likely to pursue CK and DNA testing due to a variety of access and knowledge barriers, as well as cultural factors among these groups. For example, Peters et al.35 found that blacks have a lower awareness of diagnostic genetic testing. Suther and Kiros36 found that blacks and Hispanics have less knowledge of genetic testing, less trust in a medical doctor, and greater concerns about the misuse of genetic testing than do whites; and Zimmerman et al.37 found that blacks were more likely to believe that genetic testing would lead to discrimination.
Barriers contributing to increased diagnostic delays for black and Hispanic children likely also reside at the physician and system levels. Previous investigations found that minority patients are more likely to seek care from lower performing or underresourced hospitals38; and Bach et al.39 found that doctors who treat blacks were less likely to be board certified and to report more access barriers when referring their patients to subspecialists or diagnostic imaging services. Parental survey data suggest that providers refer black and Hispanic children to specialists less often than white children.6 Access to medical insurance may also represent a barrier for minority populations. Hispanics and blacks are more likely to be uninsured than whites40 and, therefore, may be unable to pay for expensive DNA testing. For Spanish speaking Hispanics, language difficulties may also play a role. Non-English-primary-language households experience multiple disparities in medical health, access to care, and use of services.41
This study has limitations. First, the distribution of black and Hispanic cases in the MD STARnet varies significantly by site (χ2 = 23.13, P = 0.000). Arizona, Colorado, and Georgia contribute 79% of black and Hispanic cases. Racial and ethnic disparities evident in our regression models may, therefore, be confounded by regional variation in clinical standards of practice or insurance provider policies. However, the inclusion of site in our exploratory regression modeling did not increase the explanatory power of the models, and no individual site was a significant predictor of age at any step in the diagnostic process.
Second, our regression models account for a range of 20–24% of the variance in ages at specific steps in diagnosis, indicating that factors other than those considered herein also play a role in diagnostic delay. Socioeconomic characteristics, such as insurance status, urban rural contrasts, and family level income likely affect the diagnostic process. Because the MD STARnet database is compiled from retrospective chart reviews, patient information on these and other potentially important variables was unavailable or of poor quality and not suitable for inclusion.
CONCLUSION
A delay in the diagnosis of DBMD may create negative consequences for patients and caregivers, including prolonged anxiety about the cause of patients' disabilities; unnecessary, costly, and sometimes painful diagnostic testing, and lost opportunities for time-sensitive treatments such as corticosteroid therapy. These negative outcomes are likely to be particularly acute among black and Hispanic families because their children face relatively longer diagnostic delays than do whites. This population-based study suggests that racial and ethnic disparities arise before the first clinical encounter for symptoms related to DBMD and often continue throughout the diagnostic process. Improving diagnostic disparities for DBMD will require healthcare providers and healthcare systems to identify all children who exhibit DBMD symptoms, so they can receive timely CK measurement, early referral to neuromuscular specialists, and definitive diagnostic testing.
REFERENCES
Smedley BD, Stith AY, Nelson AR, editors. Unequal treatment: confronting racial and ethnic disparities in health care. Washington, DC: National Academies Press, 2003.
Shi L, Stevens GD . Vulnerable populations in the United States, 1st ed. San Francisco, CAL: Jossey-Bass, 2005.
Agency for Healthcare Research and Quality. 2007 National Healthcare Disparities Report. AHRQ Publication 08-0041. Rockville, MD: US Department of Health and Human Services, Agency for Healthcare Research and Quality. 2007.
Flores G, Tomany-Korman SC . Racial and ethnic disparities in medical and dental health, access to care, and use of services in US children. Pediatrics 2008; 121: e286–e298.
Mukherjee D, Kosztowski T, Zaidi HA, et al. Disparities in access to pediatric neurooncological surgery in the United States. Pediatrics 2009; 124: e688–e696.
Flores G, Olson L, Tomany-Korman SC . Racial and ethnic disparities in early childhood health and health care. Pediatrics 2005; 115: e183–e193.
Centers for Disease Control and Prevention (CDC). Prevalence of Duchenne/Becker muscular dystrophy among males aged 5–24 years—four states, 2007. Morb Mortal Wkly Rep 2009; 58: 1119–1122.
Emery AH, Muntoni F . Duchenne muscular dystrophy, 3rd ed. Oxford, NY: Oxford University Press, 2003.
Brook MH, Fenichel GM, Griggs RC, et al. Duchenne muscular dystrophy: patterns of clinical progression and effects of supportive therapy. Neurology 1989; 39: 475–481.
Nicholson LV, Johnson MA, Bushby KM . Integrated study of 100 patients with Xp21 linked muscular dystrophy using clinical, genetic, immunochemical, and histopathological data. Part 1. Trends across the clinical groups. J Med Genet 1993; 30: 728–736.
Ciafaloni E, Fox DJ, Pandya S, et al. Delayed diagnosis in Duchenne muscular dystrophy: data from the Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet). J Pediatr 2009; 155: 380–385.
Bushby K, Hill A, Steele J . Failure of early diagnosis in symptomatic Duchenne muscular dystrophy. The Lancet 1999; 353: 557–558.
Mohamed K, Appleton R, Nicolaides P . Delayed diagnosis of Duchenne muscular dystrophy. Eur J Paediatr Neurol 2000; 4: 219–223.
Bushby K, Finkel R, Birnkrant DJ, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol 2010; 9: 77–93.
Mandell DS, Ittenback RF, Levy SE, Pinto-Martin JA . Disparities in diagnoses received prior to a diagnosis of autism spectrum disorder. J Autism Dev Disord 2007; 37: 1795–1802.
Mandell DS, Listerud J, Levy SE, Pinto-Martin JA . Race differences in the age at diagnosis among medicaid-eligible children with autism. J Am Acad Child Adolesc Psychiatry 2002; 41: 1447–1453.
Hansen R, Olesen F, Sorensen H, Sokolowski I, Sondergaard J . Socioeconomic patient characteristics predict delay in cancer diagnosis: a Danish cohort study. BMC Health Serv Res 2008; 8: 49.
Schwartz KL, Crossley-May H, Vigneau FD, Brown K, Banerjee M . Race, socioeconomic status and stage at diagnosis for five common malignancies. Cancer Causes Control 2003; 14: 761–766.
Quintana JM, Goldmann D, Homer C . Social disparities in the use of diagnostic tests for children with gastroenteritis. Int J Qual Health Care 1997; 9: 419–425.
Satcher D, Pamies RJ, Woelfl NN, editors. Multicultural medicine and health disparities. New York: McGraw-Hill, 2006.
Hall MJ, Olopade OI . Disparities in genetic testing: thinking outside the BRCA box. J Clin Oncol 2006; 24: 2197–2203.
Green JM, Murton FE . Diagnosis of Duchenne muscular dystrophy: parents' experiences and satisfaction. Child Care Health Dev 1996; 22: 113–128.
Poysky J, Kinnett K . Facilitating family adjustment to a diagnosis of Duchenne muscular dystrophy: April 24–25, 2008, Miami, Florida. Neuromuscul Disord 2009; 19: 733–738.
Miller LA, Romitti PA, Cunniff C, et al. The Muscular Dystrophy Surveillance Tracking and Research Network (MD STARnet): surveillance methodology. Birth Defects Res A Clin Mol Teratol 2006; 76: 793–797.
Krieger N, Chen JT, Waterman PD, Soobader M, Subramanian SV, Carson R . Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer Incidence: does the choice of area-based measure and geographic level matter?: the Public Health Disparities Geocoding Project. Am J Epidemiol 2002; 156: 471–482.
Goldberg DW, Wilson JP . USC WebGIS services. Available at: https://webgis.usc.edu. Accessed December 31, 2009.
Beggs AH, Kunkel LM . Improved diagnosis of Duchenne/Becker muscular dystrophy. J Clin Invest 1990; 85: 613–619.
Beggs AH, Koenig M, Boyce FM, Kunkel LM . Detection of 98% of DMD/BMD gene deletions by polymerase chain reaction. Hum Genet 1990; 86: 45–48.
Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV . Painting a truer picture of US socioeconomic and racial/ethnic health inequalities: the Public Health Disparities Geocoding Project. Am J Public Health 2005; 95: 312–323.
Mandell DS, Novak MM, Zubritsky CD . Factors associated with age of diagnosis among children with autism spectrum disorders. Pediatrics 2005; 116: 1480–1486.
Krieger N, Zierler S, Hogan JW, et al. Geocoding and measurement of neighborhood socioeconomic position: a U.S. perspective. In: Kawachi I, Berkman LF, editors. Neighborhoods and health. New York: Oxford University Press, 2003; 146–177.
Moffitt RA, Slade EP . Health care coverage for children who are on and off welfare. Future Child 1997; 7: 87–98.
Starfield B, Shi L . The medical home, access to care, and insurance: a review of evidence. Pediatrics 2004; 113: 1493–1498.
Dubay L, Kenney GM . Health care access and use among low-income children: who fares best?. Health Aff 2001; 20: 112–121.
Peters N, Rose A, Armstrong K . The association between race and attitudes about predictive genetic testing. Cancer Epidemiol Biomarkers Prev 2004; 13: 361–365.
Suther S, Kiros G . Barriers to the use of genetic testing: a study of racial and ethnic disparities. Genet Med 2009; 11: 655–662.
Zimmerman RK, Tabbarah M, Nowalk MP, et al. Racial differences in beliefs about genetic screening among patients at inner-city neighborhood health centers. J Natl Med Assoc 2006; 98: 370–377.
Hasnain-Wynia R, Baker DW, Nerenz D, et al. Disparities in health care are driven by where minority patients seek care: examination of the Hospital Quality Alliance Measures. Arch Intern Med 2007; 167: 1233–1239.
Bach PB, Pham HH, Schrag D, Tate RC, Hargraves JL . Primary care physicians who treat blacks and whites. N Engl J Med 2004; 351: 575–584.
Flores G, Abreu M, Tomany-Korman SC . Why are Latinos the most uninsured racial/ethnic group of US children? A community-based study of risk factors for and consequences of being an uninsured Latino child. Pediatrics 2006; 118: e730–e740.
Flores G, Tomany-Korman SC . The language spoken at home and disparities in medical and dental health, access to care, and use of services in US children. Pediatrics 2008; 121: e1703–e1714.
Acknowledgements
This project was supported under a cooperative agreement from the Centers for Disease Control and Prevention through the Association of American Medical Colleges, Grant number U36/CCU319276, AAMC ID number MM-1064-09/09. This study was also funded by the Centers for Disease Control and Prevention under the Cooperative Agreement for Surveillance and Epidemiologic Research of Duchenne and Becker Muscular Dystrophy DD000187, DD000189, DD000190, DD000191.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure: The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Holtzer, C., Meaney, F., Andrews, J. et al. Disparities in the diagnostic process of Duchenne and Becker muscular dystrophy. Genet Med 13, 942–947 (2011). https://doi.org/10.1097/GIM.0b013e31822623f1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1097/GIM.0b013e31822623f1