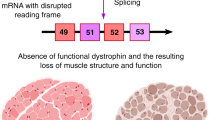
Abstract
Patients with Duchenne muscular dystrophy (DMD) show clinically relevant phenotypic variability, despite sharing the same primary biochemical defect (dystrophin deficiency). Factors contributing to this clinical variability include allelic heterogeneity (specific DMD mutations), genetic modifiers (trans-acting genetic polymorphisms) and variations in clinical care. Recently, a series of genetic modifiers have been identified, mostly involving genes and/or proteins that regulate inflammation and fibrosis — processes increasingly recognized as being causally linked with physical disability. This article reviews genetic modifier studies in DMD to date and discusses the effect of genetic modifiers on predicting disease trajectories (prognosis), clinical trial design and interpretation (inclusion of genotype-stratified subgroup analyses) and therapeutic approaches. The genetic modifiers identified to date underscore the importance of progressive fibrosis, downstream of dystrophin deficiency, in driving the disease process. As such, genetic modifiers have shown the importance of therapies aimed at slowing this fibrotic process and might point to key drug targets.
Key points
-
Clinically relevant variability in disease severity and progression is observed in Duchenne muscular dystrophy (DMD) and can be explained by allelic heterogeneity within the DMD locus (‘cis’ modifiers) and polymorphisms in different loci (‘trans’ modifiers).
-
Understanding the bases of clinical variability is important for patient and family counselling and prognosis, clinical trial design and the identification of therapeutic targets.
-
The main identified modifiers influence inflammation and fibrotic replacement, highlighting the central role of these processes in DMD and the importance of addressing them pharmacologically.
-
Modifier genes and proteins might become targets for therapeutic modulation themselves, predict responsiveness to treatments or enable precise subgroup analyses in clinical trials.
-
Future developments in the field include the identification of novel modifiers by genome mapping in large, deeply phenotyped cohorts and the development of multilocus interaction models.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Crisafulli, S. et al. Global epidemiology of Duchenne muscular dystrophy: an updated systematic review and meta-analysis. Orphanet J. Rare Dis. 15, 141 (2020).
Hoffman, E. P., Brown, R. H. & Kunkel, L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51, 919–928 (1987).
Bello, L., Hoffman, E. P. & Pegoraro, E. Dystrophinopathies. in Muscular Dystrophy: Causes and Management 67–96 (NOVA Publishers, 2013).
Darras, B.T., Urion, D.K., & Ghosh, P.S. Dystrophinopathies. in GeneReviews® [Internet] (eds Adam, M. P. et al) (University of Washington, 2022).
Monaco, A. P., Bertelson, C. J., Liechti-Gallati, S., Moser, H. & Kunkel, L. M. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics 2, 90–95 (1988).
McDonald, C. M. et al. The Cooperative International Neuromuscular Research Group Duchenne natural history study — a longitudinal investigation in the era of glucocorticoid therapy: design of protocol and the methods used. Muscle Nerve 48, 32–54 (2013).
Birnkrant, D. J. et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 17, 251–267 (2018).
Birnkrant, D. J. et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 17, 347–361 (2018).
Birnkrant, D. J. et al. Diagnosis and management of Duchenne muscular dystrophy, part 3: primary care, emergency management, psychosocial care, and transitions of care across the lifespan. Lancet Neurol. 17, 445–455 (2018).
Humbertclaude, V. et al. Motor and respiratory heterogeneity in Duchenne patients: implication for clinical trials. Eur. J. Paediatr. Neurol. 16, 149–160 (2012).
Winnard, A. V., Mendell, J. R., Prior, T. W., Florence, J. & Burghes, A. H. Frameshift deletions of exons 3–7 and revertant fibers in Duchenne muscular dystrophy: mechanisms of dystrophin production. Am. J. Hum. Genet. 56, 158–166 (1995).
van den Bergen, J. C., Ginjaar, H. B., Niks, E. H., Aartsma-Rus, A. & Verschuuren, J. J. G. M. Prolonged ambulation in Duchenne patients with a mutation amenable to exon 44 skipping. J. Neuromuscul. Dis. 1, 91–94 (2014).
Hufton, M. & Roper, H. Variations in Duchenne muscular dystrophy course in a multi-ethnic UK population: potential influence of socio-economic factors. Dev. Med. Child Neurol. 59, 837–842 (2017).
Emery, A. E. H. The muscular dystrophies. Lancet 359, 687–695 (2002).
Hoffman, E. P. Causes of clinical variability in Duchenne and Becker muscular dystrophies and implications for exon skipping therapies. Acta Myol. 39, 179–186 (2020).
Ervasti, J. M. Costameres: the Achilles’ heel of Herculean muscle. J. Biol. Chem. 278, 13591–13594 (2003).
Chen, Y.-W. et al. Early onset of inflammation and later involvement of TGF in Duchenne muscular dystrophy. Neurology 65, 826–834 (2005).
Dumont, N. A. et al. Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division. Nat. Med. 21, 1455–1463 (2015).
Brinkmeier, H. TRP channels in skeletal muscle: gene expression, function and implications for disease. Adv. Exp. Med. Biol. 704, 749–758 (2011).
Rando, T. A. Role of nitric oxide in the pathogenesis of muscular dystrophies: a ‘two hit’ hypothesis of the cause of muscle necrosis. Microsc. Res. Tech. 55, 223–235 (2001).
Consalvi, S. et al. Histone deacetylase inhibitors in the treatment of muscular dystrophies: epigenetic drugs for genetic diseases. Mol. Med. 17, 457–465 (2011).
Muntoni, F., Torelli, S. & Ferlini, A. Dystrophin and mutations: one gene, several proteins, multiple phenotypes. Lancet Neurol. 2, 731–740 (2003).
Henricson, E. K. et al. The cooperative international neuromuscular research group Duchenne natural history study: glucocorticoid treatment preserves clinically meaningful functional milestones and reduces rate of disease progression as measured by manual muscle testing and other commonly used clinical trial outcome measures: CINRG DMD Natural History Study. Muscle Nerve 48, 55–67 (2013).
Mazzone, E. S. et al. Timed rise from floor as a predictor of disease progression in Duchenne muscular dystrophy: an observational study. PLoS ONE 11, e0151445 (2016).
McDonald, C. M. et al. Long-term effects of glucocorticoids on function, quality of life, and survival in patients with Duchenne muscular dystrophy: a prospective cohort study. Lancet 391, 451–461 (2018).
Scott, E. et al. Development of a functional assessment scale for ambulatory boys with Duchenne muscular dystrophy: development of a scale for Duchenne MD. Physiother. Res. Int. 17, 101–109 (2012).
Mazzone, E. et al. North Star Ambulatory assessment, 6-minute walk test and timed items in ambulant boys with Duchenne muscular dystrophy. Neuromuscul. Disord. 20, 712–716 (2010).
McDonald, C. M. et al. The 6-minute walk test as a new outcome measure in Duchenne muscular dystrophy. Muscle Nerve 41, 500–510 (2010).
Mcdonald, C. M. et al. THE 6-minute walk test and other endpoints in Duchenne muscular dystrophy: longitudinal natural history observations over 48 weeks from a multicenter study: 6MWT and endpoints in DMD. Muscle Nerve 48, 343–356 (2013).
Barnard, A. M. et al. MR biomarkers predict clinical function in Duchenne muscular dystrophy. Neurology 94, e897–e909 (2020).
Bello, L. et al. Importance of SPP1 genotype as a covariate in clinical trials in Duchenne muscular dystrophy. Neurology 79, 159–162 (2012).
Forbes, S. C., Willcocks, R. J., Rooney, W. D., Walter, G. A. & Vandenborne, K. MRI quantifies neuromuscular disease progression. Lancet Neurol. 15, 26–28 (2016).
Rooney, W. D. et al. Modeling disease trajectory in Duchenne muscular dystrophy. Neurology 94, e1622–e1633 (2020).
Barnard, A. M. et al. Evaluating genetic modifiers of Duchenne muscular dystrophy disease progression using modeling and MRI. Neurology 99, e2406–e2416 (2022).
Pane, M. et al. Reliability of the performance of upper limb assessment in Duchenne muscular dystrophy. Neuromuscul. Disord. 24, 201–206 (2014).
Ricotti, V. et al. Respiratory and upper limb function as outcome measures in ambulant and non-ambulant subjects with Duchenne muscular dystrophy: a prospective multicentre study. Neuromuscul. Disord. https://doi.org/10.1016/j.nmd.2019.02.002 (2019).
Mayhew, A. et al. Development of the performance of the upper limb module for Duchenne muscular dystrophy. Dev. Med. Child Neurol. 55, 1038–1045 (2013).
Brooke, M. H. et al. Clinical trial in Duchenne dystrophy. I. The design of the protocol. Muscle Nerve 4, 186–197 (1981).
McDonald, C. M. et al. Longitudinal pulmonary function testing outcome measures in Duchenne muscular dystrophy: long-term natural history with and without glucocorticoids. Neuromuscul. Disord. 28, 897–909 (2018).
LoMauro, A. et al. Evolution of respiratory function in Duchenne muscular dystrophy from childhood to adulthood. Eur. Respir. J. 51, 1701418 (2018).
Bello, L. et al. Genetic modifiers of respiratory function in Duchenne muscular dystrophy. Ann. Clin. Transl. Neurol. 7, 786–798 (2020).
Vianello, A., Matarese, A. & Paladini, L. Ventilatory assistance. in Muscular Dystrophy: Causes and Management 381–391 (NOVA Publishers, 2013).
Eagle, M. et al. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscul. Disord. 12, 926–929 (2002).
Nigro, G., Comi, L. I., Politano, L. & Bain, R. J. The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. Int. J. Cardiol. 26, 271–277 (1990).
Puchalski, M. D. et al. Late gadolinium enhancement: precursor to cardiomyopathy in Duchenne muscular dystrophy? Int. J. Cardiovasc. Imaging 25, 57–63 (2009).
Jin, J. B., Carter, J. C., Sheehan, D. W. & Birnkrant, D. J. Cardiopulmonary phenotypic discordance is common in Duchenne muscular dystrophy. Pediatr. Pulmonol. 54, 186–193 (2019).
Melacini, P. et al. Cardiac and respiratory involvement in advanced stage Duchenne muscular dystrophy. Neuromuscul. Disord. 6, 367–376 (1996).
Duboc, D. et al. Effect of perindopril on the onset and progression of left ventricular dysfunction in Duchenne muscular dystrophy. J. Am. Coll. Cardiol. 45, 855–857 (2005).
Raman, S. V. et al. Eplerenone for early cardiomyopathy in Duchenne muscular dystrophy: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 14, 153–161 (2015).
Cotton, S., Voudouris, N. J. & Greenwood, K. M. Intelligence and Duchenne muscular dystrophy: full-scale, verbal, and performance intelligence quotients. Dev. Med. Child Neurol. 43, 497–501 (2001).
Doorenweerd, N. et al. Reduced cerebral gray matter and altered white matter in boys with Duchenne muscular dystrophy. Ann. Neurol. 76, 403–411 (2014).
Doorenweerd, N. et al. Resting-state functional MRI shows altered default-mode network functional connectivity in Duchenne muscular dystrophy patients. Brain Imaging Behav. https://doi.org/10.1007/s11682-020-00422-3 (2021).
Ricotti, V. et al. Neurodevelopmental, emotional, and behavioural problems in Duchenne muscular dystrophy in relation to underlying dystrophin gene mutations. Dev. Med. Child Neurol. 58, 77–84 (2016).
Doorenweerd, N. et al. Timing and localization of human dystrophin isoform expression provide insights into the cognitive phenotype of Duchenne muscular dystrophy. Sci. Rep. 7, 12575 (2017).
Bello, L., Pegoraro, E. & Hoffman, E. P. Genome-wide association studies in muscle physiology and disease. in Omics Approaches to Understanding Muscle Biology (eds Burniston, J. G. & Chen, Y.-W.) 9–30 (Springer, 2019).
Pegoraro, E. et al. SPP1 genotype is a determinant of disease severity in Duchenne muscular dystrophy. Neurology 76, 219–226 (2011).
Hogarth, M. W. et al. Evidence for ACTN3 as a genetic modifier of Duchenne muscular dystrophy. Nat. Commun. 8, 14143 (2017).
Heydemann, A. et al. Latent TGF-beta-binding protein 4 modifies muscular dystrophy in mice. J. Clin. Invest. 119, 3703–3712 (2009).
Vetrone, S. A. et al. Osteopontin promotes fibrosis in dystrophic mouse muscle by modulating immune cell subsets and intramuscular TGF-β. J. Clin. Investig. 119, 1583–1594 (2009).
Capote, J. et al. Osteopontin ablation ameliorates muscular dystrophy by shifting macrophages to a pro-regenerative phenotype. J. Cell Biol. 213, 275–288 (2016).
Giacopelli, F. et al. Polymorphisms in the osteopontin promoter affect its transcriptional activity. Physiol. Genomics 20, 87–96 (2004).
Jacobson, E. M., Concepcion, E., Oashi, T. & Tomer, Y. A Graves’ disease-associated Kozak sequence single-nucleotide polymorphism enhances the efficiency of CD40 gene translation: a case for translational pathophysiology. Endocrinology 146, 2684–2691 (2005).
Flanigan, K. M. et al. LTBP4 genotype predicts age of ambulatory loss in Duchenne muscular dystrophy. Ann. Neurol. 73, 481–488 (2013).
Bello, L. et al. DMD genotypes and loss of ambulation in the CINRG Duchenne natural history study. Neurology 87, 401–409 (2016).
Wang, R. T. et al. DMD genotype correlations from the Duchenne Registry: endogenous exon skipping is a factor in prolonged ambulation for individuals with a defined mutation subtype. Hum. Mutat. 39, 1193–1202 (2018).
Bello, L. et al. Genetic modifiers of ambulation in the cooperative international neuromuscular research group Duchenne natural history study: ambulation in CINRG-DNHS. Ann. Neurol. 77, 684–696 (2015).
Chen, M. et al. Genetic modifiers of Duchenne muscular dystrophy in Chinese patients. Front. Neurol. 11, 721 (2020).
Kosac, A. et al. LTBP4, SPP1, and CD40 variants: genetic modifiers of Duchenne muscular dystrophy analyzed in Serbian patients. Genes 13, 1385 (2022).
Nelson, S. F. & Griggs, R. C. Predicting the severity of Duchenne muscular dystrophy: implications for treatment. Neurology 76, 208–209 (2011).
Pascual-Morena, C. et al. Genetic modifiers and phenotype of Duchenne muscular dystrophy: a systematic review and meta-analysis. Pharmaceuticals 14, 798 (2021).
Visscher, P. M. et al. 10 Years of GWAS discovery: biology, function, and translation. Am. J. Hum. Genet. 101, 5–22 (2017).
Bello, L. et al. Association study of exon variants in the NF-κB and TGFβ pathways identifies CD40 as a modifier of Duchenne muscular dystrophy. Am. J. Hum. Genet. https://doi.org/10.1016/j.ajhg.2016.08.023 (2016).
Weiss, R. B. et al. Long-range genomic regulators of THBS1 and LTBP4 modify disease severity in duchenne muscular dystrophy. Ann. Neurol. 84, 234–245 (2018).
Flanigan, K. M. et al. A genome-wide association analysis of loss of ambulation in dystrophinopathy patients suggests multiple candidate modifiers of disease severity. Eur. J. Hum. Genet. https://doi.org/10.1038/s41431-023-01329-5 (2023).
Vieland, V. J., Seok, S.-C. & Stewart, W. C. L. A new linear regression-like residual for survival analysis, with application to genome wide association studies of time-to-event data. PLoS ONE 15, e0232300 (2020).
Vieland, V. J. & Seok, S.-C. The PPLD has advantages over conventional regression methods in application to moderately sized genome-wide association studies. PLoS ONE 16, e0257164 (2021).
Aartsma-Rus, A., Van Deutekom, J. C. T., Fokkema, I. F., Van Ommen, G.-J. B. & Den Dunnen, J. T. Entries in the Leiden Duchenne muscular dystrophy mutation database: an overview of mutation types and paradoxical cases that confirm the reading-frame rule. Muscle Nerve 34, 135–144 (2006).
Kesari, A. et al. Integrated DNA, cDNA, and protein studies in Becker muscular dystrophy show high exception to the reading frame rule. Hum. Mutat. 29, 728–737 (2008).
Bello, L. & Pegoraro, E. The ‘usual suspects’: genes for inflammation, fibrosis, regeneration, and muscle strength modify Duchenne muscular dystrophy. J. Clin. Med. 8, 649 (2019).
Mazzone, E. et al. Functional changes in Duchenne muscular dystrophy: a 12-month longitudinal cohort study. Neurology 77, 250–256 (2011).
Matthews, E., Brassington, R., Kuntzer, T., Jichi, F. & Manzur, A. Y. Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD003725.pub4 (2016).
Sarepta Therapeutics, Inc. A Phase 3 Multinational, Randomized, Double-Blind, Placebo-Controlled Systemic Gene Delivery Study to Evaluate the Safety and Efficacy of SRP-9001 in Patients with Duchenne Muscular Dystrophy (EMBARK). https://clinicaltrials.gov/ct2/show/NCT05096221 (2022).
Pane, M. et al. 6 Minute walk test in Duchenne MD patients with different mutations: 12 month changes. PLoS ONE 9, e83400 (2014).
Dwianingsih, E. K. et al. A novel splicing silencer generated by DMD exon 45 deletion junction could explain upstream exon 44 skipping that modifies dystrophinopathy. J. Hum. Genet. 59, 423–429 (2014).
Muntoni, F. et al. Deletions in the 5’ region of dystrophin and resulting phenotypes. J. Med. Genet. 31, 843–847 (1994).
Gualandi, F. et al. Intronic breakpoint definition and transcription analysis in DMD/BMD patients with deletion/duplication at the 5’ mutation hot spot of the dystrophin gene. Gene 370, 26–33 (2006).
Zambon, A. A. et al. Phenotypic spectrum of dystrophinopathy due to Duchenne muscular dystrophy exon 2 duplications. Neurology 98, e730–e738 (2022).
Nigro, V. et al. Detection of a nonsense mutation in the dystrophin gene by multiple SSCP. Hum. Mol. Genet. 1, 517–520 (1992).
Koeks, Z. et al. Clinical outcomes in Duchenne muscular dystrophy: a study of 5345 patients from the TREAT-NMD DMD global database. J. Neuromuscul. Dis. 4, 293–306 (2017).
Flanigan, K. M. et al. Nonsense mutation-associated Becker muscular dystrophy: interplay between exon definition and splicing regulatory elements within the DMD gene. Hum. Mutat. 32, 299–308 (2011).
Zatz, M. et al. Milder course in Duchenne patients with nonsense mutations and no muscle dystrophin. Neuromuscul. Disord. 24, 986–989 (2014).
Torella, A. et al. The position of nonsense mutations can predict the phenotype severity: a survey on the DMD gene. PLoS ONE 15, e0237803 (2020).
Flanigan, K. M. et al. DMD Trp3X nonsense mutation associated with a founder effect in North American families with mild Becker muscular dystrophy. Neuromuscul. Disord. 19, 743–748 (2009).
Pagel, C. N., Wasgewatte Wijesinghe, D. K., Taghavi Esfandouni, N. & Mackie, E. J. Osteopontin, inflammation and myogenesis: influencing regeneration, fibrosis and size of skeletal muscle. J. Cell Commun. Signal. 8, 95–103 (2014).
Dadgar, S. et al. Asynchronous remodeling is a driver of failed regeneration in Duchenne muscular dystrophy. J. Cell Biol. 207, 139–158 (2014).
Uaesoontrachoon, K. et al. Osteopontin and skeletal muscle myoblasts: association with muscle regeneration and regulation of myoblast function in vitro. Int. J. Biochem. Cell Biol. 40, 2303–2314 (2008).
Uaesoontrachoon, K., Wasgewatte Wijesinghe, D. K., Mackie, E. J. & Pagel, C. N. Osteopontin deficiency delays inflammatory infiltration and the onset of muscle regeneration in a mouse model of muscle injury. Dis. Model. Mech. 6, 197–205 (2013).
van den Bergen, J. C. et al. Validation of genetic modifiers for Duchenne muscular dystrophy: a multicentre study assessing SPP1 and LTBP4 variants. J. Neurol. Neurosurg. Psychiatr. 86, 1060–1065 (2015).
Hoffman, E. P. et al. Alterations in osteopontin modify muscle size in females in both humans and mice. Med. Sci. Sports Exerc. 45, 1060–1068 (2013).
Barfield, W. L. et al. Eccentric muscle challenge shows osteopontin polymorphism modulation of muscle damage. Hum. Mol. Genet. 23, 4043–4050 (2014).
Vianello, S. et al. SPP1 genotype and glucocorticoid treatment modify osteopontin expression in Duchenne muscular dystrophy cells. Hum. Mol. Genet. 26, 3342–3351 (2017).
Barp, A. et al. Genetic modifiers of Duchenne muscular dystrophy and dilated cardiomyopathy. PLoS ONE 10, e0141240 (2015).
Sabbatini, D. et al. Genetic modifiers of upper limb function in Duchenne muscular dystrophy. J. Neurol. 269, 4884–4894 (2022).
Rosenberg, A. S. et al. Immune-mediated pathology in Duchenne muscular dystrophy. Sci. Transl. Med. 7, 299rv4 (2015).
Dobaczewski, M., Chen, W. & Frangogiannis, N. G. Transforming growth factor (TGF)-β signaling in cardiac remodeling. J. Mol. Cell. Cardiol. 51, 600–606 (2011).
North, K. N. et al. A common nonsense mutation results in alpha-actinin-3 deficiency in the general population. Nat. Genet. 21, 353–354 (1999).
Schiaffino, S. Knockout of human muscle genes revealed by large scale whole-exome studies. Mol. Genet. Metab. 123, 411–415 (2018).
Clarkson, P. M. et al. ACTN3 genotype is associated with increases in muscle strength in response to resistance training in women. J. Appl. Physiol. 99, 154–163 (2005).
Yang, N. et al. ACTN3 genotype is associated with human elite athletic performance. Am. J. Hum. Genet. 73, 627–631 (2003).
Moran, C. N. et al. Association analysis of the ACTN3 R577X polymorphism and complex quantitative body composition and performance phenotypes in adolescent Greeks. Eur. J. Hum. Genet. 15, 88–93 (2007).
Eynon, N. et al. ACTN3 R577X polymorphism and Israeli top-level athletes. Int. J. Sports Med. 30, 695–698 (2009).
MacArthur, D. G. et al. An Actn3 knockout mouse provides mechanistic insights into the association between alpha-actinin-3 deficiency and human athletic performance. Hum. Mol. Genet. 17, 1076–1086 (2008).
Seto, J. T. et al. ACTN3 genotype influences muscle performance through the regulation of calcineurin signaling. J. Clin. Invest. 123, 4255–4263 (2013).
St-Pierre, S. J. G. et al. Glucocorticoid treatment alleviates dystrophic myofiber pathology by activation of the calcineurin/NF-AT pathway. FASEB J. 18, 1937–1939 (2004).
Nagai, M. et al. The ACTN3 577XX null genotype is associated with low left ventricular dilation-free survival rate in patients with Duchenne muscular dystrophy. J. Card. Fail. 26, 841–848 (2020).
Uhlén, M. et al. Proteomics. Tissue-based map of the human proteome. Science 347, 1260419 (2015).
Spitali, P. et al. TCTEX1D1 is a genetic modifier of disease progression in Duchenne muscular dystrophy. Eur. J. Hum. Genet. 28, 815–825 (2020).
Rolland, T. et al. A proteome-scale map of the human interactome network. Cell 159, 1212–1226 (2014).
Kotlyar, M., Pastrello, C., Sheahan, N. & Jurisica, I. Integrated interactions database: tissue-specific view of the human and model organism interactomes. Nucleic Acids Res. 44, D536–D541 (2016).
Farmer, P. & Pugin, J. Beta-adrenergic agonists exert their ‘anti-inflammatory’ effects in monocytic cells through the IkappaB/NF-kappaB pathway. Am. J. Physiol. Lung Cell Mol. Physiol. 279, L675–L682 (2000).
Tang, W. et al. The Arg16Gly polymorphism of the beta2-adrenergic receptor and left ventricular systolic function. Am. J. Hypertens. 16, 945–951 (2003).
Zhang, G. et al. Association of haplotypes of beta2-adrenoceptor polymorphisms with lung function and airway responsiveness in a pediatric cohort. Pediatr. Pulmonol. 41, 1233–1241 (2006).
Groenning, B. A. et al. Antiremodeling effects on the left ventricle during beta-blockade with metoprolol in the treatment of chronic heart failure. J. Am. Coll. Cardiol. 36, 2072–2080 (2000).
Kelley, E. F. et al. Influence of β2 adrenergic receptor genotype on risk of nocturnal ventilation in patients with Duchenne muscular dystrophy. Respir. Res. 20, 221 (2019).
Kelley, E. F., Cross, T. J., McDonald, C. M., Hoffman, E. P. & Bello, L. Influence of β2 adrenergic receptor genotype on longitudinal measures of forced vital capacity in patients with Duchenne muscular dystrophy. Neuromuscul. Disord. 32, 150–158 (2022).
Hagg, A. et al. Using AAV vectors expressing the β2-adrenoceptor or associated Gα proteins to modulate skeletal muscle mass and muscle fibre size. Sci. Rep. 6, 23042 (2016).
Kim, M. O., Na, S. I., Lee, M. Y., Heo, J. S. & Han, H. J. Epinephrine increases DNA synthesis via ERK1/2s through cAMP, Ca(2+)/PKC, and PI3K/Akt signaling pathways in mouse embryonic stem cells. J. Cell Biochem. 104, 1407–1420 (2008).
Kelley, E. F. et al. Influence of β1 adrenergic receptor genotype on longitudinal measures of left ventricular ejection fraction and responsiveness to ß-blocker therapy in patients with Duchenne muscular dystrophy. Clin. Med. Insights Cardiol. 16, 11795468221116838 (2022).
Johnson, J. A. et al. Beta 1-adrenergic receptor polymorphisms and antihypertensive response to metoprolol. Clin. Pharmacol. Ther. 74, 44–52 (2003).
Liggett, S. B. et al. A polymorphism within a conserved beta(1)-adrenergic receptor motif alters cardiac function and beta-blocker response in human heart failure. Proc. Natl Acad. Sci. USA 103, 11288–11293 (2006).
Bello, L. et al. Prednisone/prednisolone and deflazacort regimens in the CINRG Duchenne natural history study. Neurology 85, 1048–1055 (2015).
Research Center for Drug Evaluations. Duchenne muscular dystrophy and related dystrophinopathies: developing drugs for treatment guidance for industry. US Food and Drug Administration. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/duchenne-muscular-dystrophy-and-related-dystrophinopathies-developing-drugs-treatment-guidance (2020).
Treat-NMD Neuromuscular Network. Immune response to micro-dystrophin. Gene Therapy. https://treat-nmd.org/resources-support/research-overview/dmd-research-overview/gene-therapy/.
Muntoni, F. et al. DMD genotypes and motor function in Duchenne muscular dystrophy: a multi-institution meta-analysis with implications for clinical trials. Neurology https://doi.org/10.1212/WNL.0000000000201626 (2023).
Demonbreun, A. R. et al. Anti-latent TGFβ binding protein 4 antibody improves muscle function and reduces muscle fibrosis in muscular dystrophy. Sci. Transl. Med. 13, eabf0376 (2021).
Acknowledgements
E.P. and L.B. are part of the European Reference Network (ERN) for neuromuscular diseases and wish to acknowledge funding from the Cariparo foundation (Progetti di Eccellenza 2017).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
L.B. reports honoraria (speaker, advisory boards) from PTC Therapeutics, Sarepta Therapeutics, Edgewise Therapeutics, Epirium Bio and research funding from PTC Therapeutics. E.P.H. reports salary support from Reveragen and AGADA Biosciences. E.P. reports personal fees from Sarepta, grants and non-financial support from Santhera, personal fees and non-financial support from PTC Pharmaceuticals, non-financial support from Genzyme and personal fees from Roche, outside the submitted work.
Peer review
Peer review information
Nature Reviews Neurology thanks Marianela Schiava, who co-reviewed with Michela Guglieri, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Review criteria
L.B., E.P.H. and E.P. carried out independent literature searches using PubMed for published articles on genetic modifiers in Duchenne muscular dystrophy, with the following keywords: ‘Duchenne AND genetic modifiers’, ‘Duchenne AND genotype’. All results describing findings from human patients were considered for inclusion in the article. Abstracts were not considered. No time period restrictions or language restrictions utilized in the search criteria.
Related links
BGee database: https://bgee.org/
GTEx database: https://gtexportal.org/home/
Glossary
- Allelic heterogeneity
-
When patients with a single gene disorder show multiple different types of mutations in the specific gene involved in the disorder.
- Cis-acting genetic modifiers
-
Mutations in disease-causing genes that are expected to give rise to a ‘typical’ phenotype for the disease of interest (for example, out-of-frame mutations in the DMD gene expected to give rise to a severe DMD phenotype), but that, in some cases, is associated with milder or more severe phenotypes, because of complex molecular mechanisms (for example, out-of-frame deletion of exon 45 of the DMD gene allowing low-level expression of internally deleted dystrophin owing to endogenous skipping of exon 44).
- GWASs
-
Genome-wide association studies, in which millions of genetic polymorphisms throughout the human genome are studied for association with a human trait (such as clinical severity in DMD).
- Non-null (leaky)
-
The protein product of the mutated gene is detectable, often at low levels, and with only partly retained biochemical function.
- Null
-
The protein product of the mutated gene is not detectable.
- Trans-acting genetic modifiers
-
Genetic variations in genes remote from a disease-causing gene, which typically show a high degree of polymorphism in the general population and significantly alter the phenotypic manifestations of the disease, by affecting molecular pathways in the disease pathophysiology.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bello, L., Hoffman, E.P. & Pegoraro, E. Is it time for genetic modifiers to predict prognosis in Duchenne muscular dystrophy?. Nat Rev Neurol 19, 410–423 (2023). https://doi.org/10.1038/s41582-023-00823-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-023-00823-0