Abstract
The cloaca is temporally formed and eventually divided by the urorectal septum (URS) during urogenital and anorectal organ development. Although congenital malformations, such as anorectal malformations (ARMs), are frequently observed during this process, the underlying pathogenic mechanisms remain unclear. β-Catenin is a critical component of canonical Wnt signaling and is essential for the regulation of cell differentiation and morphogenesis during embryogenesis. The expression of β-catenin is observed in endodermal epithelia, including URS epithelia. We modulated the β-catenin gene conditionally in endodermal epithelia by utilizing tamoxifen-inducible Cre driver line (ShhCreERT2). Both β-catenin loss- and gain-of-function (LOF and GOF) mutants displayed abnormal clefts in the perineal region and hypoplastic elongation of the URS. The mutants also displayed reduced cell proliferation in the URS mesenchyme. In addition, the β-catenin GOF mutants displayed reduced apoptosis and subsequently increased apoptosis in the URS epithelium. This instability possibly resulted in reduced expression levels of differentiation markers, such as keratin 1 and filaggrin, in the perineal epithelia. The expression of bone morphogenetic protein (Bmp) genes, such as Bmp4 and Bmp7, was also ectopically induced in the epithelia of the URS in the β-catenin GOF mutants. The expression of the Msx2 gene and phosphorylated-Smad1/5/8, possible readouts of Bmp signaling, was also increased in the mutants. Moreover, we introduced an additional mutation for a Bmp receptor gene: BmprIA. The ShhCreERT2/+; β-cateninflox(ex3)/+; BmprIAflox/− mutants displayed partial restoration of URS elongation compared with the β-catenin GOF mutants. These results indicate that some ARM phenotypes in the β-catenin GOF mutants were caused by abnormal Bmp signaling. The current analysis revealed the close relation of endodermal β-catenin signaling to the ARM phenotypes. These results are considered to shed light on the pathogenic mechanisms of human ARMs.
Similar content being viewed by others
Main
During embryonic development, the cloaca is a temporal structure that is subsequently divided into the urogenital sinus and rectum by the urorectal septum (hereafter referred to as the URS).1, 2, 3 The URS develops from the proximal umbilical mesenchyme (the rostral portion of the cloaca) around embryonic day 10.5 (E10.5) (Figure 1a, asterisk)3, 4, 5 and subsequently extends caudally along the cloaca reaching the cloacal membrane. Part of the cloacal membrane degrades with the approximation of the URS tip. As a result, the cloaca is divided into the urogenital sinus and rectum, and the tip of the URS (endodermal epithelia) contributes to forming the ectodermal epithelia of the perineum and external genitalia.6 The contribution of these cells and growth factors expressed in the endodermal epithelia are essential for the proper morphogenesis of the perineal region, based on the phenotypes of several mutants. For instance, Sonic hedgehog (Shh) is expressed in the endodermal epithelia and has an essential role in both URS formation and GT protrusion by affecting neighboring mesenchymal cells.7, 8, 9, 10, 11 Apoptotic cells are observed in the urogenital tract during URS formation, being distributed primarily in the epithelial layers of the URS, cloacal membrane and mesenchyme of the dorsal surface of the caudal hindgut at E11.5 and E11.75.5, 12 From E12.5, apoptotic cells are also observed in the URS mesenchyme and are thought to be involved in the transformation of the URS and disintegration of the cloacal membrane.5
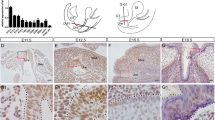
Temporally labeled Shh-expressing URS endodermal cells contribute to the ectodermal epithelia. The expression of the Shh gene in sagittal sections of wild-type embryos at E10.5, E11.5, E12.5 and E13.5 (a–d). The asterisk in (a) indicates future URS derived from the proximal umbilical mesenchyme. Ventral view of the genital tubercle (e–j). Tissue labeling experiments of Shh-expressing cells were performed at E15.5. ShhCreERT2/+; R26LacZ/+ embryos subsequent to TM administration at E8.5, E9.5, E10.5, E11.5, E12.5 or E13.5 (e–j). The red arrows indicate the LacZ-positive ectodermal cells. β-Catenin was expressed in the endoderm, including the URS epithelia, at E11.5 in the wild-type embryos (k). b, Bladder; c, cloaca; gt, genital tubercle; hl, hindlimb; r, rectum; t, tail; uc, umbilical cord; urs, urorectal septum
Several congenital anomalies are frequently observed during urogenital organ development. These abnormalities are usually accompanied by deficient excretory and copulatory functions, influencing the quality of life of the patient. In particular, the incidence of anorectal malformations (ARMs) is approximately 1 in 5000 human births;13 however, the underlying pathogenic mechanisms of this condition are currently unknown. ARM phenotypes are observed in several diseases, including Currarino syndrome, Townes Brocks syndrome and VACTERL complex.14, 15, 16, 17 Affected patients often display other malformations, such as anal fistulas, sacral malformations and renal malformations. Human and mouse genetic analyses have shed light on the possible genetic causes of some of these abnormalities.
Several mouse mutants for hedgehog signaling genes, ephrin-Eph signaling genes, fibroblast growth factor (Fgf) signaling genes and Wnt signaling genes are reported to display ARM phenotypes.18, 19, 20, 21, 22 Another causative factor for the development of ARM is all-trans retinoic acid (RA), a teratogen and active form of vitamin A. RA treatment in pregnant mice results in imperforate anus in embryos.23, 24 Although these reports have identified causative factors for ARM phenotypes, the pathogenic mechanisms underlying the development of ARM and URS remain elusive.
The Wnt signaling pathway is essential for embryonic development, and its dysregulation has been implicated in developmental disorders and human diseases. Wnt signaling is transmitted primarily via three divergent pathways: the canonical Wnt/β-catenin pathway, the planner cell polarity pathway and the Wnt/Ca2+ pathway.25, 26 β-Catenin is a key factor for the canonical Wnt pathway and also acts as a subunit of the cadherin protein complex, which controls cell–cell adhesion. Owing to the early lethality of β-catenin-deficient mice,27 analyses of the function of β-catenin in organogenesis have been performed using conditional mutants. Previous reports have revealed that β-catenin induces the differentiation of hair follicles during hair/skin development according to loss- and gain-of-function (LOF and GOF) approaches.28, 29 The ectopic activation of canonical Wnt signaling results in the ectopic induction of bone morphogenetic protein (Bmp) signaling, which is essential for hair follicle formation.30, 31 With respect to genital tubercle (primordia of external genitalia: GT) development, region-specific (ectodermal, endodermal and mesenchymal) modulation of the β-catenin gene has revealed essential functions, such as regulation of cell proliferation, epithelial integrity and protrusion/elongation of GT.8, 32, 33 However, the regulatory functions of the β-catenin gene during the development of the URS have not been investigated.
The current study aimed to investigate the function of the β-catenin gene in URS development. We modulated the β-catenin activity in the endodermal epithelia and studied the phenotypic consequences of dysregulated endodermal β-catenin signaling for urorectal development. Both β-catenin LOF and GOF mutations resulted in ARM phenotypes. The β-catenin GOF mutation led to the ectopic induction of Bmp signaling. Moreover, the ARM phenotypes in the β-catenin GOF mutants were restored by additionally introducing the BmprIA gene mutation. These results suggest that adequately controlled β-catenin signaling and its downstream growth factor signaling are essential for proper URS formation.
Results
Genetic tissue lineage analyses revealed the temporally specific contribution of URS epithelia to ectodermal epithelia
We first studied the kinetics of the contribution of URS epithelial cells. We utilized ShhCreERT2 mice, which are knock-in mice of the tamoxifen (TM)-inducible form of Cre recombinase to the Sonic hedgehog (Shh) locus.34 The Shh gene was expressed in the endodermal epithelia at E10.5–13.5 (Figures 1a–d). We crossed ICR female mice with ShhCreERT2/+; R26LacZ/LacZ male mice. The pregnant females were subsequently treated with TM at E8.5–13.5, and the embryos were analyzed at E15.5 (Figures 1e–j). There were few LacZ-positive cells in the perineal epithelia, urethra and rectum in the embryos upon TM treatment at E8.5 (Figure 1e). In contrast, numerous LacZ-positive cells were observed in the perineal epithelia, urethra and rectum in the embryos with E9.5 or E10.5 treatment (Figures 1f and g, red arrows). We observed a reduced number of LacZ-positive cells in the perineal ectodermal epithelia following later stage TM treatment, such as at E11.5 and E12.5 (Figures 1h and i). Following E13.5 TM treatment, the perineal epithelia were not positive for LacZ signals (Figure 1j). These results indicate that the Shh-positive endodermal epithelia labeled by E9.5–10.5 TM treatment can primarily contribute to the perineal epithelium.
ARMs were induced by abnormal β-catenin activity
We then asked whether β-catenin regulates URS formation. We first analyzed the expression of β-catenin using immunohistochemistry. The β-catenin expression was detected in the endoderm, including the URS epithelia, in the wild-type embryos at E11.5 (Figure 1k). We next used the ShhCreERT2 system to either constitutively activate or inhibit the β-catenin activity in the endodermal epithelia and studied the effects of dysregulated endodermal β-catenin signaling on URS development.
β-Catenin floxed mutant mice (β-cateninflox), which contain two loxP sites flanking exons 3–6,28 were crossed to ShhCreERT2, and conditional deletion of β-catenin in the URS epithelia was achieved with TM treatment. ShhCreERT2/+; β-cateninflox/− (β-catenin LOF) mutants were analyzed at E18.5 subsequent to TM treatment at E9.5, E11.5 or E13.5 (Figures 2b–d and b′–d′). The formation of the URS was perturbed by early-stage TM treatment. The mutants treated with TM at E9.5 displayed cleft-like phenotypes in the perineal region (Figures 2b and b′, red arrowheads). In addition, anal stenosis was observed in the E9.5-TM-treated mutants (Figure 2b′). TM treatment at E11.5 resulted in hypospadias-like phenotypes, although no perineal clefts were observed (Figures 2c and c′). The perineal region in the E13.5-TM-treated embryos appeared to be normal (Figures 2d and d′). We further analyzed the differentiation of the perineal epithelial cells in the E9.5-TM-treated mutants. The expression of keratin 1 (a marker of prickle cells) and filaggrin (a marker of granule cells) was analyzed (Figures 3a–e). The perineal epithelia of the wild-type embryos expressed both markers at E18.5 (Figures 3a and d, red arrowheads). The expression of these markers was also observed in the perineal epithelia of the E9.5-TM-treated β-catenin LOF mutants (Figures 3b and e, blue arrowheads), indicating that the differentiation of the URS epithelial cells in the β-catenin LOF mutants proceeded normally.
Both the LOF and GOF mutants for the β-catenin gene displayed ARM phenotypes. Ventral view of the genital tubercle region (a–g) and sagittal sections of the embryos at E18.5 (a′–g′). The β-catenin LOF embryos at E18.5 subsequent to TM administration at E9.5 (b and b′), E11.5 (c and c′) and E13.5 (d and d′). The β-catenin GOF embryos at E18.5 subsequent to TM administration at E9.5 (e and e′), E11.5 (f and f′) and E13.5 (g and g′). The red arrowheads indicate the cleft phenotype of the perineal region. gt, genital tubercle; r, rectum; t, tail; u, urethra
The analyses of the differentiation marker expression and cell proliferation in the URS/perineum of the β-catenin LOF and GOF mutants. Sagittal sections of the wild-type (a and d), β-catenin LOF (b and e) and β-catenin GOF (c and f) embryos. The expression of keratin 1 (a–c) and filaggrin (d–f) was assessed. Both markers were positive in perineal epithelia of wild-type embryos (red arrowheads) and in URS epithelia of β-catenin LOF embryos (blue arrowheads). The tissues surrounded by blue squares indicate the URS region (c and f). Cell proliferation was assessed using a BrdU incorporation assay (g–k). Sagittal sections of the wild-type (g), β-catenin LOF (h) and β-catenin GOF embryos (i) at E11.5. The red squares of the lower magnification pictures indicate magnified areas of (g–k). In the β-catenin LOF mutants, the cell proliferation of the URS mesenchyme was decreased at E11.5 (h). The cell proliferation in URS epithelia was increased in the β-catenin GOF mutants at E11.5 (i). Sagittal sections of the wild-type and β-catenin GOF mutants at E13.5 (j and k). The cell proliferation in the mesenchyme of the URS in the β-catenin GOF mutants was decreased (k). c, cloaca; gt, genital tubercle; r, rectum; u, urethra; urs, urorectal septum
Next, a constitutively active β-cateninflox(ex3) mutant allele was analyzed to determine whether the enhanced β-catenin activity affects the formation of the URS. The Cre activity derived from the ShhCreERT2 allele regulated by TM treatment at E9.5, E11.5 or E13.5 induced a constitutively active β-catenin lacking the N-terminal domain phosphorylation sites essential for its degradation (Figures 2e–g and e′–g′). An augmentation of β-catenin expression and its activity as a component of Wnt/β-catenin signaling in E9.5-TM-treated β-catenin GOF mutants were assessed by immunohistochemistry and transgenic reporter, respectively (Supplementary Figures S1a–f). Similar to that observed in the β-catenin LOF mutants, early TM treatment resulted in hypoplasia during URS/perineal formation in the β-catenin GOF mutants (Figures 2e, f, e′ and f′, red arrowheads). The URS in the E9.5-TM-treated mutants did not reach to the cloacal membrane, leading to incomplete cloacal division of the urogenital sinus and rectum (Figures 2e and e′). This phenotype was slightly milder in the E11.5-TM-treated mutants and was not observed in the E13.5-TM-treated mutants (Figures 2f, g, f′ and g′). The expression of keratin 1 and filaggrin was also examined in the β-catenin GOF mutants (Figures 3c and f). Expression of these markers was not detected in the perineal epithelial region in the E9.5-TM-treated mutants (Figures 3c and f, demarcated by blue squares). These results suggest that the GOF β-catenin mutation perturbs the cell differentiation of the URS epithelium and that the proper dose of β-catenin activity in the URS epithelium at early embryonic stages is essential for the formation of the URS.
Defective cell proliferation of URS mesenchymal cells may contribute to the abnormal URS formation of β-catenin mutants
To analyze cellular mechanisms inducing mutant phenotypes, we next performed cell proliferation and death analyses in the E9.5-TM-treated β-catenin LOF and GOF mutants. The β-catenin LOF mutants displayed hypoplastic URS and markedly reduced cell proliferation of the URS mesenchyme at E11.5 (Figure 3h). Decreased mesenchymal cell proliferation was also observed at E13.5 using phosphohistone H3 (pHH3) immunostaining (Supplementary Figure S2). However, the cell proliferation of the URS epithelia was comparable with that observed in the controls (Figure 3h). On the other hand, the β-catenin GOF mutants displayed hypertrophy and an increased number of cell proliferation in the URS epithelia at E11.5 (Figure 3i). This hypertrophic layer was confirmed to be derived from endodermal epithelia using labeling with the ShhCreERT2 system (Supplementary Figure S3). As for the mesenchymal cell proliferation in the URS, the β-catenin GOF mutants did not display marked alteration at E11.5 (Figure 3i); however, cell proliferation was decreased at E13.5 (Figure 3k). Moreover, we also analyzed the cell death status to judge whether the apoptotic cells were prominent enough to induce elongation defects of the URS in both mutants. The distribution of apoptotic cells in the URS mesenchyme was not increased in either the β-catenin LOF or GOF mutants at E11.5 and E13.5 (Figures 4f and h; Supplementary Figure S4). These results suggest that the reduced cell proliferation of the URS mesenchyme observed in both the β-catenin LOF and GOF mutants possibly contributes to defective URS elongation.
Time-course expression analysis of TUNEL and cleaved caspase-3 in the β-catenin GOF mutants. Sagittal sections of the embryos at E11.5, E12.5, E13.5, E14.5 and E15.5 subsequent to E9.5-TM treatment (a–t). The number of apoptotic cells in the URS epithelia of the β-catenin GOF mutants was less than that observed in the controls at E11.5 and E12.5 (a, b, f, g, k, l, p and q; red arrows and surrounded by red dotted line). The number of apoptotic cells was increased in the mutant URS epithelia at E13.5, and such cells were also observed in the mutant URS at E14.5 and E15.5 (c–e, h–j, m–o and r–t; blue arrows and surrounded by blue dotted line). urs, urorectal septum
Of note, the β-catenin GOF mutants displayed less apoptotic cells in the URS epithelia compared with the controls at E11.5 and E12.5 (Figures 4a, b, f, g, k, l, p and q, red arrows and surrounded by red dotted line). This reduced amount of cell death and increased level of cell proliferation possibly resulted in hypertrophy of the URS epithelia at E11.5 (Figure 3i). Subsequently, at E13.5 and later embryonic stages, augmented apoptotic cells were observed in the hypertrophic URS epithelia of the β-catenin GOF mutants (Figures 4c–e, h–j, m–o and r–t, blue arrows and surrounded by blue dotted line). Such elevated URS epithelial apoptosis may contribute to the lack of differentiation of perineal epithelial cells in the β-catenin GOF mutants (Figures 3c and f).
Ectopic induction of Bmp signaling may contribute to ARM phenotypes in β-catenin GOF mutants
Next, we investigated possible causes of the ARM phenotype in the β-catenin GOF mutants. Several previous reports have demonstrated the ectopic activation of Bmp signaling in ectodermal β-catenin GOF mutants.31, 32 We therefore analyzed the expression of several Bmp signaling-related molecules in the β-catenin GOF mutants. The expression of the Bmp4 gene was observed in the mesenchyme of the URS in the wild-type embryos at E13.5 (Figure 5a). The Bmp4 gene expression was ectopically observed in the URS epithelia, in addition to the URS mesenchyme, in the β-catenin GOF mutants (Figure 5e, red arrowheads). The ectopic expression of Bmp7 was also observed in the URS epithelia (Figures 5b and f, red arrowheads). We also analyzed the downstream molecules of Bmp signaling. One of the downstream genes of Bmp signaling, Msx2, was ectopically expressed in the URS epithelia (Figures 5c and g, red arrowheads). Moreover, the expression of phosphorylated Smad (pSmad)1/5/8 was increased in both the epithelia and mesenchyme of the mutant URS (Figures 5d and h, red arrowheads).
Ectopic Bmp signaling in the epithelia of the URS in the E9.5-TM-treated β-catenin GOF mutants. Sagittal sections of the embryos at E13.5 (a–h). The expression of Bmp4 and Bmp7 was increased in the mutant URS epithelium (e and f). Msx2, a downstream target of Bmp signaling, and pSmad1/5/8 were ectopically induced (g and h). The red arrowheads indicate ectopic induction of the gene and protein expression (e–h). Sagittal sections of the wild-type, ShhCreERT2/+; β-cateninflox(ex3)/+, ShhCreERT2/+; β-cateninflox(ex3)/+; BmprIAflox/− embryos at E18.5 subsequent to E9.5-TM treatment (i–k). Ventral view of a ShhCreERT2/+; β-cateninflox(ex3)/+; BmprIAflox/− embryo (k′). The URS abnormality was partly restored in the ShhCreERT2/+; β-cateninflox(ex3)/+; BmprIAflox/− mutants compared with that observed in the ShhCreERT2/+; β-cateninflox(ex3)/+ mutants (j and k). The blue arrowhead indicates the tip of the URS. gt, genital tubercle; r, rectum; t, tail; u, urethra; urs, urorectal septum
To further ascertain the contribution of ectopic Bmp signaling in the abnormal development of the URS in the β-catenin GOF mutants, we analyzed the URS development in compound mutants for the β-catenin GOF mutation and one of the Bmp receptors (BmprIA). Although the compound mutants (ShhCreERT2/+; β-cateninflox(ex3)/+; BmprIAflox/−) displayed the perineal cleft, URS elongation was substantially reversed in these mutants compared with that observed in the ShhCreERT2/+; β-cateninflox(ex3)/+ mutants under E9.5-TM administration (Figures 5i–k and k′, blue arrowhead). These results may suggest that the ectopically induced endodermal Bmp signaling by augmented β-catenin signaling partly contributes to the ARM phenotypes of β-catenin GOF mutants.
Discussion
ARM is one of the most frequently observed congenital diseases in humans. However, the pathogenic mechanisms of ARM, including the developmental mechanisms of the URS, a key structure for the formation of the urogenital sinus and rectum, have not been elucidated. The current analysis demonstrated perineal clefts and deficient URS elongation phenotypes in both β-catenin LOF and GOF mutants. In the case of human ARMs, there are several types of symptoms.35 Focusing on the location of the anal opening, the phenotype of β-catenin LOF mutants may be similar to perineal fistulas, a benign type of ARM. In contrast, the phenotype of β-catenin GOF mutants may be similar to a severe type of ARM, namely rectourethral bulbar fistulas, abnormal communications or fistulas, between the rectum and urinary tract. These classifications are usually thought to reflect the severity of symptoms, presumably because of the different pathogenic causes.
β-Catenin is expressed in the normal URS epithelia. β-Catenin LOF mutants displayed decreased cell proliferation in the URS mesenchyme. This is considered to affect directly to the size of the URS. β-Catenin is essential for inducing the expression of several growth factors in the gut endoderm, including the small intestine.36, 37 Likewise, the β-catenin expressed in the URS epithelium may affect downstream growth factor signals. The regulation of mesenchymal development is known to occur via the actions of endodermally derived growth factors, such as Shh. It has also been suggested that Shh positively regulates the cell proliferation of URS mesenchymal cells.7 The level of β-catenin in the endodermal epithelia is reduced in Shh-null mutants, and the Shh expression is reduced in β-catenin conditional mutants.8 These previous results suggest the possibility of the reduction of downstream growth factor signaling associated with reduced cell proliferation in β-catenin LOF mutants. The current results suggest that growth factor signaling, including β-catenin, can regulate URS elongation by affecting URS mesenchymal cell proliferation.
On the other hand, the β-catenin GOF mutants also displayed ARM phenotypes. In this study, we focused on the ectopic induction of Bmp signaling in the URS epithelia and mesenchyme and found that the ARM phenotypes of the β-catenin GOF mutants were partly restored by the additional mutation of Bmp signaling. In addition, previous reports have suggested that the ectopic induction of β-catenin signaling can induce the ectopic Fgf8 expression in the cloacal endoderm.8, 11, 32 These findings may indicate that the ectopic induction of several growth factors perturbs proper URS development. We also observed transiently reduced apoptosis and subsequently increased apoptosis in the URS epithelium in the β-catenin GOF mutants. Such continuous cell death may affect the maturation of the URS epithelia and contribute to phenotypic differences between β-catenin LOF and GOF mutants.
In support of these findings, null mutants of the Wnt inhibitory factor 1 (Wif1) gene, which is primarily expressed at the apical URS endoderm and cloacal membrane, displayed urorectal defects, and the exogenous addition of Wif1 proteins in an urorectal culture caused cloacal membrane disintegration (Ng et al.38). Our results demonstrate that spatiotemporally and properly controlled levels of URS epithelial β-catenin signaling are essential for cloacal septation and that dysregulation of endodermal β-catenin signaling leads to abnormal downstream growth factor activity. The current results are considered to contribute to the better understanding of the pathogenesis of ARM.
Materials and Methods
Mice
The mutant mice used in this study were ShhCreERT2, Rosa26R (R26LacZ), β-cateninflox, β-cateninflox(ex3), BmprIAflox, TopGAL and CAG-Cre.28, 34, 36, 39, 40, 41, 42 The genotypes of each strain were determined as reported previously. To obtain ShhCreERT2/+; R26LacZ/+ mice, ShhCreERT2/+; R26LacZ/LacZ male and Jcl:ICR (CLEA Japan, Osaka, Japan) female mice were crossed. The BmprIA-null allele (BmprIA+/−) was generated using BmprIAflox and CAG-Cre driver mice.41 The mouse embryos were processed for X-gal or immunohistochemical staining. Noon of the day on which the vaginal plug appeared was designated as E0.5. All experimental procedures and protocols were approved by the Committees on Animal Research at Wakayama Medical University and Kumamoto University.
Histological analyses
The mouse embryos were fixed overnight in 4% paraformaldehyde (Sigma-Aldrich, Tokyo, Japan) with PBS, dehydrated with methanol and embedded in paraffin. Then, 6-μm serial sections were prepared. Hematoxylin and eosin staining was performed using standard procedures. X-gal staining was carried out as described previously.43 The X-gal-stained samples were embedded in paraffin and sectioned. The immunohistochemical analysis was performed according to standard procedures using anti-β-catenin antibodies (1 : 100; BD Biosciences, Tokyo, Japan), anti-cleaved caspase-3 (Asp175) antibodies (1 : 1000; Cell Signaling Technology, Tokyo, Japan), anti-keratin 1 antibodies (1 : 200; Covance, Tokyo, Japan), anti-filaggrin antibodies (1 : 300; Covance), anti-pSmad1/5/8 antibodies (1 : 300; Cell Signaling Technology) and anti-pHH3 (Ser10) antibodies (1 : 1000; Merck Millipore, Tokyo, Japan). Signal amplification was performed using the appropriate VECTASTAIN ABC IgG Kit (Vector Laboratories, Burlingame, CA, USA), and the immunocomplexes were detected with DAB staining. For the cell proliferation assays, pregnant females were injected with 100 mg of 5-bromo-2'-deoxyuridine (BrdU; Sigma-Aldrich) per kg of body weight. One hour after injection, the embryos were collected and processed for immunohistochemistry with anti-BrdU antibodies (1:300; Roche, Mannheim, Germany). For the cell death analyses, a TUNEL assay was performed with the In situ Apoptosis Detection Kit (Takara, Ohtsu, Japan).
RNA in situ hybridization
Section in situ hybridization for the gene expression analyses was performed as described previously.44 The antisense riboprobe templates have been described previously: Shh (kindly provided by Dr. C Shukunami, Hiroshima, Japan), Bmp4,45 Bmp7 (kindly provided by Dr. M Yoshida, Kobe, Japan) and Msx2 (kindly provided by Dr. Hill RE, Edinburgh, UK and Dr. I Satokata, Niigata, Japan).
TM administration for conditional gene recombination
The TM-inducible Cre recombinase system removes the floxed sequence from the target genome.46, 47, 48 A 10 mg/ml stock solution of TM (T-5648; Sigma-Aldrich) was prepared in sesame oil.8 The pregnant females received 0.1 mg of TM per g of maternal body weight intraperitoneally at the indicated time points. No overt teratological effects were observed under these conditions.8, 43, 49
Abbreviations
- URS:
-
urorectal septum
- ARM:
-
anorectal malformation
- LOF:
-
loss-of-function
- GOF:
-
gain-of-function
- Shh:
-
sonic hedgehog
- Bmp:
-
bone morphogenetic protein
- GT:
-
genital tubercle
- TM:
-
tamoxifen
References
de Santa Barbara P, Roberts D . Tail gut endoderm and gut/genitourinary/tail development: a new tissue-specific role for Hoxa13. Development 2002; 129: 551–561.
Kimmel S, Mo R, Hui C, Kim P . New mouse models of congenital anorectal malformations. J Pediatr Surg 2000; 35: 227–230 discussion 230-1.
Hynes PJ, Fraher JP . The development of the male genitourinary system. I. The origin of the urorectal septum and the formation of the perineum. Br J Plast Surg 2004; 57: 27–36.
Forsberg JG . On the development of the cloaca and the perineum and the formation of the urethral plate in female rat embryos. J Anat 1961; 95: 423–436.
Sasaki C, Yamaguchi K, Akita K . Spatiotemporal distribution of apoptosis during normal cloacal development in mice. Anat Rec A 2004; 279: 761–767.
Seifert AW, Harfe BD, Cohn MJ . Cell lineage analysis demonstrates an endodermal origin of the distal urethra and perineum. Dev Biol 2008; 318: 143–152.
Haraguchi R, Mo R, Hui C, Motoyama J, Makino S, Shiroishi T et al. Unique functions of Sonic hedgehog signaling during external genitalia development. Development 2001; 128: 4241–4250.
Miyagawa S, Moon A, Haraguchi R, Inoue C, Harada M, Nakahara C et al. Dosage-dependent hedgehog signals integrated with Wnt/beta-catenin signaling regulate external genitalia formation as an appendicular program. Development 2009; 136: 3969–3978.
Seifert AW, Zheng Z, Ormerod BK, Cohn MJ . Sonic hedgehog controls growth of external genitalia by regulating cell cycle kinetics. Nat Commun 2010; 1: 23.
Seifert AW, Bouldin CM, Choi KS, Harfe BD, Cohn MJ . Multiphasic and tissue-specific roles of sonic hedgehog in cloacal septation and external genitalia development. Development 2009; 136: 3949–3957.
Lin C, Yin Y, Veith GM, Fisher AV, Long F, Ma L . Temporal and spatial dissection of Shh signaling in genital tubercle development. Development 2009; 136: 3959–3967.
Qi BQ, Williams A, Beasley S, Frizelle F . Clarification of the process of separation of the cloaca into rectum and urogenital sinus in the rat embryo. J Pediatr Surg 2000; 35: 1810–1816.
van der Putte SC . Normal and abnormal development of the anorectum. J Pediatr Surg 1986; 21: 434–440.
Belloni E, Martucciello G, Verderio D, Ponti E, Seri M, Jasonni V et al. Involvement of the HLXB9 homeobox gene in Currarino syndrome. Am J Hum Genet 2000; 66: 312–319.
Kohlhase J, Wischermann A, Reichenbach H, Froster U, Engel W . Mutations in the SALL1 putative transcription factor gene cause Townes–Brocks syndrome. Nat Genet 1998; 18: 81–83.
Kohlhase J, Taschner PE, Burfeind P, Pasche B, Newman B, Blanck C et al. Molecular analysis of SALL1 mutations in Townes–Brocks syndrome. Am J Hum Genet 1999; 64: 435–445.
Martínez-Frias ML, Bermejo E, Frias JL . The VACTERL association: lessons from the Sonic hedgehog pathway. Clin Genet 2001; 60: 397–398.
Kim P, Mo R, Hui CcC . Murine models of VACTERL syndrome: role of sonic hedgehog signaling pathway. J Pediatr Surg 2001; 36: 381–384.
Mo R, Kim JH, Zhang J, Chiang C, Hui CC, Kim PC . Anorectal malformations caused by defects in sonic hedgehog signaling. Am J Pathol 2001; 159: 765–774.
Dravis C, Yokoyama N, Chumley MJ, Cowan CA, Silvany RE, Shay J et al. Bidirectional signaling mediated by ephrin-B2 and EphB2 controls urorectal development. Dev Biol 2004; 271: 272–290.
Tai C, Sala F, Ford H, Wang K, Li C, Minoo P et al. Wnt5a knock-out mouse as a new model of anorectal malformation. J Surg Res 2009; 156: 278–282.
Garrido-Allepuz C, González-Lamuño D, Ros MA . Sirenomelia phenotype in bmp7;shh compound mutants: a novel experimental model for studies of caudal body malformations. PLoS One 2012; 7: e44962.
Nakata M, Takada Y, Hishiki T, Saito T, Terui K, Sato Y et al. Induction of Wnt5a-expressing mesenchymal cells adjacent to the cloacal plate is an essential process for its proximodistal elongation and subsequent anorectal development. Pediatr Res 2009; 66: 149–154.
Padmanabhan R . Retinoic acid-induced caudal regression syndrome in the mouse fetus. Reprod Toxicol 1998; 12: 139–151.
Niehrs C . The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol 2012; 13: 767–779.
Wansleeben C, Meijlink F . The planar cell polarity pathway in vertebrate development. Dev Dyn 2011; 240: 616–626.
Haegel H, Larue L, Ohsugi M, Fedorov L, Herrenknecht K, Kemler R . Lack of beta-catenin affects mouse development at gastrulation. Development 1995; 121: 3529–3537.
Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W . Beta-catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 2001; 105: 533–545.
Gat U, DasGupta R, Degenstein L, Fuchs E . De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell 1998; 95: 605–614.
Lo Celso C, Prowse DM, Watt FM . Transient activation of beta-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development 2004; 131: 1787–1799.
Suzuki K, Yamaguchi Y, Villacorte M, Mihara K, Akiyama M, Shimizu H et al. Embryonic hair follicle fate change by augmented beta-catenin through Shh and Bmp signaling. Development 2009; 136: 367–372.
Lin C, Yin Y, Long F, Ma L . Tissue-specific requirements of beta-catenin in external genitalia development. Development 2008; 135: 2815–2825.
Mazahery AR, Suzuki K, Nagafuchi A, Miyajima M, Nakagata N, Orvis GD et al. Functional analysis of ectodermal β-catenin during external genitalia formation. Congenit Anom (Kyoto) 2013; 53: 34–41.
Harfe B, Scherz P, Nissim S, Tian H, McMahon A, Tabin C . Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell 2004; 118: 517–528.
Tobias N, Mason D, Lutkenhoff M, Stoops M, Ferguson D . Management principles of organic causes of childhood constipation. J Pediatr Health Care 2008; 22: 12–23.
Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J 1999; 18: 5931–5942.
Kim BM, Mao J, Taketo MM, Shivdasani RA . Phases of canonical Wnt signaling during the development of mouse intestinal epithelium. Gastroenterology 2007; 133: 529–538.
Ng RC-L, Matsumaru D, Ho AS-H, Garcia-Barceló M-M, Yuan Z-W, Smith D et al. Dysregulation of Wnt inhibitory factor 1 (Wif1) expression resulted in aberrant Wnt-β-catenin signaling and cell death of the cloaca endoderm, and anorectal malformations. Cell Death Differ 2014 e-pub ahead of print 14 March 2014; doi:10.1038/cdd.2014.20.
Soriano P . Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 1999; 21: 70–71.
Mishina Y, Hanks MC, Miura S, Tallquist MD, Behringer RR . Generation of Bmpr/Alk3 conditional knockout mice. Genesis 2002; 32: 69–72.
Araki K, Imaizumi T, Okuyama K, Oike Y, Yamamura K . Efficiency of recombination by Cre transient expression in embryonic stem cells: comparison of various promoters. J Biochem 1997; 122: 977–982.
DasGupta R, Fuchs E . Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 1999; 126: 4557–4568.
Haraguchi R, Motoyama J, Sasaki H, Satoh Y, Miyagawa S, Nakagata N et al. Molecular analysis of coordinated bladder and urogenital organ formation by Hedgehog signaling. Development 2007; 134: 525–533.
Miyagawa S, Satoh Y, Haraguchi R, Suzuki K, Iguchi T, Taketo MM et al. Genetic interactions of the androgen and Wnt/beta-catenin pathways for the masculinization of external genitalia. Mol Endocrinol 2009; 23: 871–880.
Jones CM, Lyons KM, Hogan BL . Involvement of bone morphogenetic protein-4 (BMP-4) and Vgr-1 in morphogenesis and neurogenesis in the mouse. Development 1991; 111: 531–542.
Feil R, Brocard J, Mascrez B, LeMeur M, Metzger D, Chambon P . Ligand-activated site-specific recombination in mice. Proc Natl Acad Sci USA 1996; 93: 10887–10890.
Feil R, Wagner J, Metzger D, Chambon P . Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun 1997; 237: 752–757.
Indra A, Warot X, Brocard J, Bornert J, Xiao J, Chambon P et al. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res 1999; 27: 4324–4327.
Matsumaru D, Haraguchi R, Miyagawa S, Motoyama J, Nakagata N, Meijlink F et al. Genetic analysis of Hedgehog signaling in ventral body wall development and the onset of omphalocele formation. PLoS One 2011; 6: e16260.
Acknowledgements
We thank Drs. Pierre Chambon, Andrew P McMahon, Brian D Harfe, Walter Birchmeier, Terry P Yamaguchi, Taisen Iguchi, Makoto M Taketo, Yuji Mishina and Richard R Behringer for their invaluable supports. We would also thank Drs. Ken-ichi Yamamura, Kimi Araki, Philippe Soriano, Laurence S Baskin, Chisa Shukunami, Michio Yoshida, Yi Liu, Robert E Hill, Ichiro Satokata, Chi-Chung Hui, Jun Motoyama, Chin Chiang and Mark Lewandoski for encouragement and suggestions. We would also like to express our appreciation to Tomiko I Iba for her valuable assistance. This work is supported by Grant-in-Aid for Young Scientists B (23701070, 23790229 and 24790292), for Scientific Research C (23590216) and for Scientific Research on Innovative Areas: molecular mechanisms for establishment of sex differences (22132006) from the Ministry of Education, Culture, Sports, Science and Technology, Japan. This work is also supported by National Institutes of Health Grant No. R01ES016597.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by M Piacentini
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Miyagawa, S., Harada, M., Matsumaru, D. et al. Disruption of the temporally regulated cloaca endodermal β-catenin signaling causes anorectal malformations. Cell Death Differ 21, 990–997 (2014). https://doi.org/10.1038/cdd.2014.21
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cdd.2014.21
This article is cited by
-
Tgfbr1 controls developmental plasticity between the hindlimb and external genitalia by remodeling their regulatory landscape
Nature Communications (2024)
-
CircJag1 promotes apoptosis of ethylene thiourea–exposed anorectal malformations through sponging miR-137-3p by regulating Sox9 and suppressing Wnt/β-catenin pathway during the hindgut development of rat embryos
Cell Biology and Toxicology (2023)
-
LIM homeodomain transcription factor Isl1 affects urethral epithelium differentiation and apoptosis via Shh
Cell Death & Disease (2019)
-
Regulation of masculinization: androgen signalling for external genitalia development
Nature Reviews Urology (2018)
-
Epithelial–mesenchymal transformation and apoptosis in rat urethra development
Pediatric Research (2017)