Abstract
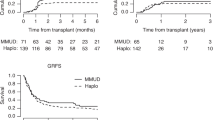
We designed a novel haploidentical hematopoietic stem cell transplantation (haplo-HSCT) system using idarubicin (IDA) intensified conditioning regimens and combination of antithymocyte globulin and basiliximab for GvHD prophylaxis. The outcomes of 110 high-risk acute leukemia patients undergoing haplo-HSCT were compared with 69 contemporaneous high-risk patients receiving HLA-matched sibling transplantation using uniform IDA-intensified regimens. The relapse incidence of haplo-HSCT was 23.4%, and 3-year overall survival (OS) and disease-free survival (DFS) achieved 62.9%, 59.1%, respectively. The cumulative incidences of II–IV and III–IV aGvHD were 28.6 and 14.3%, while limited and extensive cGvHD were 19.4, 13.8%. All these results were equivalent to those of concurrent identical sibling transplantation. Three-year OS and DFS for patients in advance stage reached 48.5, 47.3%. Furthermore, the relapse, 3-year OS of positive minimal residual disease (MRD) patients did not differ from negative MRD patients (18.9% vs 11.5%, 63.6% vs 69.6%), indicating our intensified haplo-HSCT technique could circumvent the dismal prognosis of MRD. These data provide reinforcing evidence that our haplo-HSCT system could dramatically improve the survival of high-risk acute leukemia with low relapse and acceptable transplantation-related mortality, and might be a promising therapeutic option for high-risk patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Yahng SA, Kim JH, Jeon YW, Yoon JH, Shin SH, Lee SE et al. A well-tolerated regimen of 800cGy TBI-fludarabine-busulfan-ATG for reliable engraftment after unmanipulated haploidentical peripheral blood stem cell transplantation in adult patients with acute myeloid leukemia. Biol Blood Marrow Transplant 2015; 21: 119–129.
Ciurea SO, Bayraktar UD . “No donor”? Consider a haploidentical transplant. Blood Rev 2015; 29: 63–70.
Xuan L, Fan Z, Zhang Y, Zhou H, Huang F, Dai M et al. Sequential intensified conditioning followed by prophylactic DLI could reduce relapse of refractory acute leukemia after allo-HSCT. Oncotarget 2016; 7: 32579–32591.
Kawashima N, Inamoto Y, Sato T, Nakashima M, Kagaya Y, Watakabe K et al. Long-term outcomes of allogeneic hematopoietic cell transplantation with intensified myeloablative conditioning for refractory myeloid malignancy. Bone Marrow Transplant 2016; 51: 869–871.
Fang J, Zhang R, Wang H, Hong M, Wu Q, Nie D et al. Idarubicin-intensified BUCY2 conditioning regimen improved survival in high-risk acute myeloid, but not lymphocytic leukemia patients undergoing allogeneic hematopoietic stem cell transplantation: a retrospective comparative study. Leuk Res 2016; 46: 61–68.
Wu Q, Zhang R, Wang H, You Y, Zhong Z, Hong M et al. Comparison of outcomes of idarubicin intensified TBI-CY and traditional TBI-CY conditioning regimen for high-risk acute lymphoblastic leukemia undergoing allogeneic hematopoietic stem cell transplantation: a single center experience. Leuk Res 2015; 39: 1192–1200.
Bashey A, Zhang X, Sizemore CA, Manion K, Brown S, Holland HK et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol 2013; 31: 1310–1316.
Baker M, Wang H, Rowley SD, Cai L, Pecora AL, Skarbnik A et al. Comparative outcomes after haploidentical or unrelated donor bone marrow or blood stem cell transplantation in adult patients with hematological malignancies. Biol Blood Marrow Transplant 2016; 22: 2047–2055.
Solomon SR, Solh M, Morris LE, Holland HK, Bashey A . Myeloablative conditioning with PBSC grafts for T cell-replete haploidentical donor transplantation using posttransplant cyclophosphamide. Adv Hematol 2016; 2016: 9736564.
Arcese W, Picardi A, Santarone S, De Angelis G, Cerretti R, Cudillo L et al. Haploidentical, G-CSF-primed, unmanipulated bone marrow transplantation for patients with high-risk hematological malignancies: an update. Bone Marrow Transplant 2015; 50 (Suppl 2): S24–S30.
Apperley J, Niederwieser D, Huang XJ, Nagler A, Fuchs E, Szer J et al. Haploidentical hematopoietic stem cell transplantation: a global overview comparing Asia, the European Union, and the United States. Biol Blood Marrow Transplant 2016; 22: 23–26.
Chang YJ, Huang XJ . Haploidentical stem cell transplantation: anti-thymocyte globulin-based experience. Semin Hematol 2016; 53: 82–89.
Wang Y, Fu HX, Liu DH, Xu LP, Zhang XH, Chang YJ et al. Influence of two different doses of antithymocyte globulin in patients with standard-risk disease following haploidentical transplantation: a randomized trial. Bone Marrow Transplant 2014; 49: 426–433.
Crocchiolo R, Esterni B, Castagna L, Furst S, El-Cheikh J, Devillier R et al. Two days of antithymocyte globulin are associated with a reduced incidence of acute and chronic graft-versus-host disease in reduced-intensity conditioning transplantation for hematologic diseases. Cancer 2013; 119: 986–992.
Devillier R, Crocchiolo R, Castagna L, Furst S, El CJ, Faucher C et al. The increase from 2.5 to 5mg/kg of rabbit anti-thymocyte-globulin dose in reduced intensity conditioning reduces acute and chronic GVHD for patients with myeloid malignancies undergoing allo-SCT. Bone Marrow Transplant 2012; 47: 639–645.
Siddiqi T, Blaise D . Does antithymocyte globulin have a place in reduced-intensity conditioning for allogeneic hematopoietic stem cell transplantation? Hematology Am Soc Hematol Educ Program 2012; 2012: 246–250.
Toso C, Edgar R, Pawlick R, Emamaullee J, Merani S, Dinyari P et al. Effect of different induction strategies on effector, regulatory and memory lymphocyte sub-populations in clinical islet transplantation. Transpl Int 2009; 22: 182–191.
Hendrikx TK, Klepper M, Ijzermans J, Weimar W, Baan CC . Clinical rejection and persistent immune regulation in kidney transplant patients. Transpl Immunol 2009; 21: 129–135.
Fang J, Hu C, Hong M, Wu Q, You Y, Zhong Z et al. Prophylactic effects of interleukin-2 receptor antagonists against graft-versus-host disease following unrelated donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant 2012; 18: 754–762.
Bonnefoy-Berard N, Verrier B, Vincent C, Revillard JP . Inhibition of CD25 (IL-2R alpha) expression and T-cell proliferation by polyclonal anti-thymocyte globulins. Immunology 1992; 77: 61–67.
Di Bartolomeo P, Santarone S, De Angelis G, Picardi A, Cudillo L, Cerretti R et al. Haploidentical, unmanipulated, G-CSF-primed bone marrow transplantation for patients with high-risk hematologic malignancies. Blood 2013; 121: 849–857.
Hire K, Ngo DK, Stewart-Maynard KM, Hering B, Bansal-Pakala P . FoxP3+, and not CD25+, T cells increase post-transplant in islet allotransplant recipients following anti-CD25+ rATG immunotherapy. Cell Immunol 2012; 274: 83–88.
Hong M, Wu Q, Hu C, Fang J, You Y, Zhong Z et al. Idarubicin-intensified BUCY2 regimens may lower relapse rate and improve survival in patients undergoing allo-SCT for high-risk hematological malignancies: a retrospective analysis. Bone Marrow Transplant 2012; 47: 196–202.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus Conference on acute GVHD grading. Bone Marrow Transplant 1995; 15: 825–828.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005; 11: 945–956.
Shabbir-Moosajee M, Lombardi L, Ciurea SO . An overview of conditioning regimens for haploidentical stem cell transplantation with post-transplantation cyclophosphamide. Am J Hematol 2015; 90: 541–548.
McCurdy SR, Kanakry JA, Showel MM, Tsai HL, Bolanos-Meade J, Rosner GL et al. Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood 2015; 125: 3024–3031.
Raiola AM, Dominietto A, Ghiso A, Di Grazia C, Lamparelli T, Gualandi F et al. Unmanipulated haploidentical bone marrow transplantation and posttransplantation cyclophosphamide for hematologic malignancies after myeloablative conditioning. Biol Blood Marrow Transplant 2013; 19: 117–122.
Ciceri F, Labopin M, Aversa F, Rowe JM, Bunjes D, Lewalle P et al. A survey of fully haploidentical hematopoietic stem cell transplantation in adults with high-risk acute leukemia: a risk factor analysis of outcomes for patients in remission at transplantation. Blood 2008; 112: 3574–3581.
Fabricius WA, Ramanathan M . Review on haploidentical hematopoietic cell transplantation in patients with hematologic malignancies. Adv Hematol 2016; 2016: 5726132.
Wang Y, Liu DH, Xu LP, Liu KY, Chen H, Chen YH et al. Superior graft-versus-leukemia effect associated with transplantation of haploidentical compared with HLA-identical sibling donor grafts for high-risk acute leukemia: an historic comparison. Biol Blood Marrow Transplant 2011; 17: 821–830.
Lee DA, Denman CJ, Rondon G, Woodworth G, Chen J, Fisher T et al. Haploidentical natural killer cells infused before allogeneic stem cell transplantation for myeloid malignancies: a Phase I Trial. Biol Blood Marrow Transplant 2016; 22: 1290–1298.
Solomon SR, Sizemore CA, Sanacore M, Zhang X, Brown S, Holland HK et al. Haploidentical transplantation using T cell replete peripheral blood stem cells and myeloablative conditioning in patients with high-risk hematologic malignancies who lack conventional donors is well tolerated and produces excellent relapse-free survival: results of a prospective phase II trial. Biol Blood Marrow Transplant 2012; 18: 1859–1866.
Luznik L, O'Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant 2008; 14: 641–650.
Solomon SR, Sizemore CA, Sanacore M, Zhang X, Brown S, Holland HK et al. Total body irradiation-based myeloablative haploidentical stem cell transplantation is a safe and effective alternative to unrelated donor transplantation in patients without matched sibling donors. Biol Blood Marrow Transplant 2015; 21: 1299–1307.
Sedlacek P, Mejstrikova E, Formankova R, Keslova P, Dobrovolna M, Vrana M et al. Allo-SCT in children with high-risk leukemia using unmanipulated grafts from alternative donors. Bone Marrow Transplant 2008; 42 (Suppl 2): S10–S15.
Zhou Y, Othus M, Araki D, Wood BL, Radich JP, Halpern AB et al. Pre- and post-transplant quantification of measurable ('minimal') residual disease via multiparameter flow cytometry in adult acute myeloid leukemia. Leukemia 2016; 30: 1456–1464.
Araki D, Wood BL, Othus M, Radich JP, Halpern AB, Zhou Y et al. Allogeneic hematopoietic cell transplantation for acute myeloid leukemia: time to move toward a minimal residual disease-based definition of complete remission? J Clin Oncol 2016; 34: 329–336.
Raj K, Pagliuca A, Bradstock K, Noriega V, Potter V, Streetly M et al. Peripheral blood hematopoietic stem cells for transplantation of hematological diseases from related, haploidentical donors after reduced-intensity conditioning. Biol Blood Marrow Transplant 2014; 20: 890–895.
Mitsuhashi K, Kako S, Shigematsu A, Atsuta Y, Doki N, Fukuda T et al. Comparison of cyclophosphamide combined with total body irradiation, oral busulfan, or intravenous busulfan for allogeneic hematopoietic cell transplantation in adults with acute lymphoblastic leukemia. Biol Blood Marrow Transplant 2016; 22: 2194–2200.
Hamadani M, Blum W, Phillips G, Elder P, Andritsos L, Hofmeister C et al. Improved nonrelapse mortality and infection rate with lower dose of antithymocyte globulin in patients undergoing reduced-intensity conditioning allogeneic transplantation for hematologic malignancies. Biol Blood Marrow Transplant 2009; 15: 1422–1430.
Campara M, Tzvetanov IG, Oberholzer J . Interleukin-2 receptor blockade with humanized monoclonal antibody for solid organ transplantation. Expert Opin Biol Ther 2010; 10: 959–969.
Acknowledgements
This work was supported by grant from the National Natural Sciences foundation of China (Grant number: 81370668 for Ling-Hui Xia) and Collaborative Innovation Center of Hematology of China.
Author contributions
L-HX and Q-LW designed the protocol; RZ and WS contributed patients, analyzed the data and wrote the manuscript; H-FW, YY, Z-DZ, W-ML and CZ contributed patients and critically reviewed the manuscript; XL and PZ performed the statistical analysis; JF and MH provided clinical and laboratory data, revised and corrected the manuscript, and all authors read and approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Bone Marrow Transplantation website
Rights and permissions
About this article
Cite this article
Zhang, R., Shi, W., Wang, HF. et al. Idarubicin-intensified haploidentical HSCT with GvHD prophylaxis of ATG and basiliximab provides comparable results to sibling donors in high-risk acute leukemia. Bone Marrow Transplant 52, 1253–1260 (2017). https://doi.org/10.1038/bmt.2017.100
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2017.100
This article is cited by
-
Comparative analysis of Decitabine intensified BUCY2 and BUCY2 conditioning regimen for high-risk MDS patients undergoing allogeneic hematopoietic stem cell transplantation
Bone Marrow Transplantation (2022)
-
The consensus from The Chinese Society of Hematology on indications, conditioning regimens and donor selection for allogeneic hematopoietic stem cell transplantation: 2021 update
Journal of Hematology & Oncology (2021)
-
Impact of prophylactic/preemptive donor lymphocyte infusion and intensified conditioning for relapsed/refractory leukemia: a real-world study
Science China Life Sciences (2020)
-
Everyone has a donor: contribution of the Chinese experience to global practice of haploidentical hematopoietic stem cell transplantation
Frontiers of Medicine (2019)
-
The consensus on indications, conditioning regimen, and donor selection of allogeneic hematopoietic cell transplantation for hematological diseases in China—recommendations from the Chinese Society of Hematology
Journal of Hematology & Oncology (2018)