Abstract
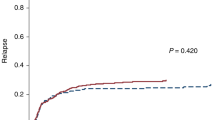
In recipients of allogeneic hematopoietic stem cell transplantation with AML in CR1, reduced intensity (RIC) conditioning regimens are usually given to older patients and myeloablative regimens (MAC) to younger patients. We analyzed whether in middle-aged patients aged 40–60 years, MAC was superior to RIC in cytogenetically higher risk AML. Among 2974 patients, 1638 had MAC and 1336 RIC transplants. Cytogenetics were high risk in 508, intermediate risk in 2297 and low risk in 169. Overall survival (OS) was higher in patients with RIC with low-risk cytogenetics but not in the intermediate- or poor-risk AML. Relapse incidence was lower with MAC in poor- and intermediate-risk AML. Nonrelapse mortality (NRM) was higher in MAC in all cytogenetic risk groups. Multivariate analysis confirmed a significant leukemia-free survival and OS advantage for RIC in low risk but no advantage of MAC in intermediate- and poor-risk leukemia. In patients aged 40–60 years, MAC has no advantage over RIC. We confirm lower relapse but higher NRM risks with MAC. MAC is not superior in patients with higher risk cytogenetics, but is inferior to RIC in the small cohort of AML patients with low-risk cytogenetics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Burnett A, Wetzler M, Löwenberg B . Therapeutic advances in acute myeloid leukemia. J Clin Oncol 2011; 29: 487–494.
Cornelissen JJ, Gratwohl A, Schlenk RF, Sierra J, Bornhäuser M, Juliusson G et al. The European LeukemiaNet AML Working Party consensus statement on allogeneic HSCT for patients with AML in remission: an integrated-risk adapted approach. Nat Rev Clin Oncol 2012; 9: 579–590.
Estey EH . Acute myeloid leukemia: 2013 update on risk-stratification and management. Am J Hematol 2013; 88: 318–327.
Magenau J, Couriel DR . Hematopoietic stem cell transplantation for acute myeloid leukemia: to whom, when, and how. Curr Oncol Rep 2013; 15: 436–444.
Ferrara F, Schiffer CA . Acute myeloid leukaemia in adults. Lancet 2013; 381: 484–495.
Forman SJ, Rowe JM . The myth of the second remission of acute leukemia in the adult. Blood 2013; 121: 1077–1082.
Schlenk RF, Döhner K, Krauter J, Fröhling S, Corbacioglu A, Bullinger L et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med 2008; 358: 1909–1918.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant 2009; 15: 1628–1633.
Gyurkocza B, Sandmaier BM . Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood 2014; 124: 344–353.
Aoudjhane M, Labopin M, Gorin NC, Shimoni A, Ruutu T, Kolb HJ et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT). Leukemia 2005; 19: 2304–2312.
Lee JH, Joo YD, Kim H, Ryoo HM, Kim MK, Lee GW et al. Randomized trial of myeloablative conditioning regimens: busulfan plus cyclophosphamide versus busulfan plus fludarabine. J Clin Oncol 2013; 31: 701–709.
Ringdén O, Erkers T, Aschan J, Garming-Legert K, Le Blanc K, Hägglund H et al. A prospective randomized toxicity study to compare reduced-intensity and myeloablative conditioning in patients with myeloid leukaemia undergoing allogeneic haematopoietic stem cell transplantation. J Intern Med 2013; 274: 153–162.
Shimoni A, Hardan I, Shem-Tov N, Yerushalmi R, Nagler A . Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: long-term follow-up. Leukemia 2010; 24: 1050–1052.
Todisco E, Ciceri F, Oldani E, Boschini C, Micò C, Vanlint MT et al. The CIBMTR score predicts survival of AML patients undergoing allogeneic transplantation with active disease after a myeloablative or reduced intensity conditioning: a retrospective analysis of the Gruppo Italiano Trapianto Di Midollo Osseo. Leukemia 2013; 27: 2086–2091.
Luger SM, Ringdén O, Zhang MJ, Pérez WS, Bishop MR, Bornhauser M et al. Similar outcomes using myeloablative vs reduced-intensity allogeneic transplant preparative regimens for AML or MDS. Bone Marrow Transplant 2012; 47: 203–211.
Martino R, de Wreede L, Fiocco M, van Biezen A, von dem Borne PA, Hamladji RM et al. Comparison of conditioning regimens of various intensities for allogeneic hematopoietic SCT using HLA-identical sibling donors in AML and MDS with <10% BM blasts: a report from EBMT. Bone Marrow Transplant 2013; 48: 761–770.
Versluis J, Labopin M, Niederwieser D, Socie G, Schlenk RF, Milpied N et al. Prediction of non-relapse mortality in recipients of reduced intensity conditioning allogeneic stem cell transplantation with AML in first complete remission. Leukemia 2014; 29: 51–57.
Bitan M, He W, Zhang MJ, Abdel-Azim H, Ayas MF, Bielorai B et al. Transplantation for children with acute myeloid leukemia: a comparison of outcomes with reduced intensity and myeloablative regimens. Blood 2014; 123: 1615–1620.
Martino R, Iacobelli S, Brand R, Jansen T, van Biezen A, Finke J et al. Retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic hematopoietic stem cell transplantation using HLA-identical sibling donors in myelodysplastic syndromes. Blood 2006; 108: 836–846.
Lioure B, Béné MC, Pigneux A, Huynh A, Chevallier P, Fegueux N et al. Early matched sibling hematopoietic cell transplantation for adult AML in first remission using an age-adapted strategy: long-term results of a prospective GOELAMS study. Blood 2012; 119: 2943–2948.
Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010; 115: 453–474.
Kaplan EL, Meier P . Nonparametric estimation from incomplete observations. J Am Statist Assoc 1958; 53: 457–481.
Gooley TA, Leisenring W, Crowley JA, Storer BE . Estimation of failure probabilities in the presence of competing risks: New representations of old estimators. Stat Med 1999; 18: 695–706.
Latouche A, Allignol A, Beyersmann J, Labopin M, Fine JP . A competing risks analysis should report results on all cause-specific hazards and cumulative incidence functions. J Clin Epidemiol 2013; 66: 648–653.
Grambsch PMTT . Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994; 81: 515–526.
Grossmann V, Schnittger S, Kohlmann A, Eder C, Roller A, Dicker F et al. A novel hierarchical prognostic model of AML solely based on molecular mutations. Blood 2012; 120: 2963–2972.
Röllig C, Bornhäuser M, Kramer M, Thiede C, Ho AD, Krämer A et al. Allogeneic stem-cell transplantation in patients with NPM1-mutated acute myeloid leukemia: results from a prospective donor versus no-donor analysis of patients after upfront HLA typing within the SAL-AML 2003 trial. J Clin Oncol 2015; 33: 403–410.
Acknowledgements
We acknowledge the many centers, data managers and patients who contributed data to make this analysis possible. MM thanks Pr JV de Melo (University of Adelaide, Australia) for critical reading of the manuscript.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This study was presented in part at the American Society of Hematology meeting in December 2013
Department of Medicine, Helsinki University Central Hospital, Helsinki, Finland; Department of Hematology—BMT, Hopital St Louis, Paris, France; Department of Hematologie, Hopital Purpan-CHU de Toulouse, Toulouse, France; Erasmus MC-Daniel den Hoed Cancer Centre, Rotterdam, The Netherlands; CHU Bordeaux, Pessac, France; Department of Hematology, First Affiliated Hospital of Soochow University, Suzhou, China; Centre for Clinical Haematology, Queen Elizabeth Hospital, Birmingham, UK; University Medical Center St Radboud, Nijmegen, The Netherlands; University Medical Centre, Utrecht, The Netherlands; Department of D'Hematologie, CHU Nantes, Nantes, France; Hopital A. Michallon—Hématologie Clinique—Pole Cancérologie, Grenoble, France; Programme de Transplantation&Therapie Cellulaire, Centre de Recherche en Cancérologie de Marseille, Institut Paoli Calmettes, Marseille, France; Hematology-Oncology and BMT Research, Shariati Hospital, Teheran, Iran; Service d'Onco Hematologie, CHU Hautepierre, Strasbourg, France; Service d'Hématologie, Hopital Jean Minjoz, Besancon, France; University Hospital Gasthuisberg, Leuven, Belgium; University Hospital, Basel, Switzerland; Hôpital HURIEZ—UAM allo-CSH—CHRU, Lille, France; Centre Hospitalier Lyon Sud—Service Hematologie, Lyon, France; Gustave Roussy, institut de cancérologie—BMT Service, Division of Hematology -Department of Medical Oncology, Villejuif, France; Leiden University Hospital, Leiden, The Netherlands; Hospital U. Marqués de Valdecilla, Santander, Spain; Hopital Saint Antoine, Paris, France; Department of Hematology, Karolinska University Hospital, Huddinge, Sweden; Hannover Medical University, Hannover, Germany; CHRU, Service des Maladies du Sang, Angers, France; Department of Hematology/Oncology, Charles University Hospital, Pilsen, Czech Republic; University Medical Center Groningen (UMCG), Groningen, The Netherlands; Nottingham City Hospital, Nottingham, UK; Hopital La Miletrie, Poitiers, France; UnitT de Transplantation Médullaire, Vandoeuvre les Nancy, France; Département d'Hématologie Clinique, CHU Lapeyronie, Montpellier, France; Rigshospitalet—BMT Unit Department of Hematology L 4042, Copenhagen, Denmark; University Hospital, Lund, Sweden; Deutsche Klinik für Diagnostik—KMT Zentrum, Wiesbaden, Germany; Department of Stem cell Transplantation, University Hospital Eppendorf, Hamburg, Germany; Department of Haematology and Stem Cell Transplant, Budapest, Hungary; Medizinische Klinik und Poliklinik, Ulm, Germany; Rome Transplant Network—‘Tor Vergata’ University of Rome—Stem Cell Transplant Unit-Policlinico Universitario Tor Vergata, Rome, Italy; Universitaetsklinikum Dresden, Dresden, Germany; Azienda Ospedaliera Papa Giovanni XXIII—Hematology and Bone Marrow Transplant Unit, Bergamo, Italy; Hôpital Necker, Paris, France; University Hospital, Linköping, Sweden; Department of Medicine-Hematology, Oncology, University of Freiburg, Freiburg, Germany; Cliniques Universitaires St Luc, Brussels, Belgium; King Faisal Specialist Hospital & Research Centre Oncology (Section of Adult Haematolgy/BMT), Riyadh, Saudi Arabia; Klinikum Nürnberg, Nürnberg, Germany; University Hospital Maastricht, Maastricht, The Netherlands; University of Liege, Liege, Belgium; Royal Victoria Infirmary, Newcastle-Upon-Tyne, UK; Leicester Royal Infirmary, Leicester, UK; Hospital Clínico, Salamanca, Spain; Centre Henri Becquerel, Rouen, France; Centre Hospitalier Universitaire, Caen, France; Department of Haematology, Oxford, UK; Hopitaux Universitaires de Geneve, Geneva, Switzerland; Hopsital Pitie-Salpetriere, Paris, France; Hôpital Henri Mondor, Creteil, France; St James Hospital Trinity College, Dublin, Ireland; Sahlgrenska University Hospital, Goeteborg, Sweden; Addenbrookes Hospital, Cambridge, UK; Turku University, Turku, Finland; VU University Medical Center, Amsterdam, The Netherlands; CHU Morvan, Brest, France; Department of Oncologico, Ospedale La Maddalena, Palermo, Italy; Hospital Santa Creu i Sant Pau, Barcelona, Spain; Ospedale San Gerardo, Monza, Italy; Universita degli Studi di Bari, Bari, Italy; University College London Hospital, London, UK; Azienda Ospedaliera S. Giovanni, Torino, Italy; Ospedale di Careggi, Firenze, Italy; Centre Hospitalier Universitaire de Rennes, Rennes, France; Hôpital Percy, Clamart, France; Institute of Portugues de Oncologia do Porto, Porto, Portugal; North Trent BMT Programme (Adults), Sheffield, UK; University of Saarland, Homburg, Germany; Charité Universitätsmedizin Berlin, Berlin, Germany; Hopital Nord, Saint Etienne Cedex 2, France; Department of Bone Marrow Transplantation, University Hospital, Essen, Germany; Hospital Universitari Germans Trias i Pujol, Barcelona, Spain; Umea University Hospital, Umeå, Sweden; Imperial College, London, UK; Bologna University, S.Orsola-Malpighi Hospital, Bologna, Italy; University Hospital, Olomouc, Czech Republic; Hospital Universitario La Fe, Valencia, Spain; Southampton General Hospital, Southampton, UK; Division of Hematology, Federico II' Medical School, University of Napoli, Napoli, Italy; Constantiaberg Medi-Clinic, Cape Town, South Africa; Institut Jules Bordet, Brussels, Belgium; Belfast City Hospital, Belfast, UK; Royal Marsden Hospital, London, UK; University of'La Sapienza', Rome, Italy; Rikshospitalet, Department of Medicine, The National Hospital, Oslo, Norway; Institute of Hematology and Blood Transfusion, Prague, Czech Republic; Tel Aviv University, Tel-Hashomer, Israel; ICO—Hospital Duran i Reynals, Barcelona, Spain; Klinikum Augsburg—II Medizinische Klinik, Augsburg, Germany; Policlinico G.B. Rossi, Verona, Italy; Ospedale Ferrarotto, Catania, Italy; Canterbury Health Laboratories, Christchurch, New Zealand; H SS. Antonio e Biagio, Alessandria, Italy; Antwerp University Hospital (UZA), Antwerp Edegem, Belgium; Haematology Department, Leeds, UK; Ospedale Maggiore di Milano, Milano, Italy; Fédération de Greffe de Moelle et de, Clermont-Ferrand, France; Policlinico San Matteo, Pavia, Italy; Royal Liverpool University Hospital, Liverpool, UK; Hôpital de l'ARCHET I, Nice, France; Manchester Royal Infirmary, Manchester, UK; University Hospital, Zürich, Switzerland; University Hospital Leipzig, Leipzig, Germany; Department of Haematological Medicine, GKT School of Medicine, London, UK; University Regensburg, Regensburg, Germany; Ospedale San Raffaele s.r.l., Milano, Italy; Military Medical Academy, Warsaw, Poland; Robert-Bosch-Krankenhaus, Stuttgart, Germany; Tel Aviv Sourasky Medical Center, Tel Aviv, Israel; Gazi Universitesi Tip Fakültesi, Ankara, Turkey; Ospedale San Martino—Department of Haematology II, Genova, Italy; Glasgow Royal Infirmary, Glasgow, UK; Hadassah University Hospital, Jerusalem, Israel; University of Est. de Campinas/TMO/UNICAMP, Campinas, Brazil; University of Heidelberg—Medizinische Klinik u. Poliklinik V, Heidelberg, Germany; Hospital Vall d'Hebron, Barcelona, Spain; Professor Dr Axel A. Fauser—Klinik für Knochenmarktransplantation, Hämatologie und Onkologie; Idar-Oberstein, Germany; Silesian Medical Academy, Katowice, Poland; Institute of Haematology and Blood Transfusion, Warsaw, Poland; Christie NHS Trust Hospital, Manchester, UK; CHRU Limoges, Limoges, France; Hospital Clinic, Barcelona, Spain; University of Wales, Cardiff, UK; Universita Cattolica S. Cuore, Rome, Italy; Medical University Graz, Graz, Austria; Makassed University Hospital, Beirut, Lebanon; Haematology Department/BMT Unit, George Papanicolaou General Hospital, Thessaloniki, Greece; University Hospital Brno, Brno, Czech Republic; University Hospital VUB, Brussels, Belgium; Azienda Ospedali Riuniti di Ancona, Ancona-Torrete, Italy; Plymouth Hospitals NHS Trust, Plymouth, UK; Hospital Guglielmo da Saliceto, Piacenza, Italy; Hacettepe University, Ankara, Turkey; Hosp. Reina Sofia, Córdoba, Spain; Academisch Ziekenhuis bij de Universiteit, Amsterdam, The Netherlands; University Hospital, Uppsala, Sweden; Bristol Royal Hospital for Children, Bristol, UK; Beilinson Hospital, Petach-Tikva, Israel; A.Z. Sint-Jan, Brugge, Belgium; Ernst-Moritz-Arndt-University of Greifswald, Greifswald, Germany; King Hussein Cancer Centre, Amman, Jordan; Hospital Aranzazu, San Sebastian, Spain; University of Milano, Milano, Italy; Adult Stem Cell Transplantation Unit, Department of Hematology, Ankara University Faculty of Medicine, Ankara, Turkey; Vilnius University Hospital 'Santariskiu Klinikos', Vilnius, Lithuania; SPb State I. Pavlov Medical University, St Petersburg, Russia; Clinica Puerta de Hierro, Madrid, Spain; University Hospital Gent, Gent, Belgium; St Bartholomew's and The Royal London Hospital, London, UK; Centre National de Greffe de Moelle, Tunis, Tunisia; Royal Free Hospital and School of Medicine, London, UK; Hopital Bretonneau, Tours, France; Institute of Portugues Oncologia, Lisboa, Portugal; Department of Hematology, Rambam Health Care Campus, Haifa, Israel; DCTK, Wroclaw, Poland; Azienda Ospedaliera, Reggio Calabria, Italy; Az. Ospedaliera S. Croce e Carle, Cuneo, Italy; Hospital Covadonga, Oviedo, Spain; Tartu University Hospital, Tartu, Estonia; St Savas Oncology Hospital, Athens, Greece; Medical University of Gdansk, Gdansk, Poland; AZ. Spedali Civili-Brescia Universitu of Brescia, Brescia, Italy; Medizinische Universitaet Wien, Vienna, Austria; Western General Hospital, Edinburgh, UK; Ospedale Civile, Pescara, Italy; Hospital Clínico Universitario, Valencia, Spain; Birmingham Heartlands Hospital, Birmingham, UK; Klinikum Karlsruhe GmbH, Karlsruhe, Germany; Hospital San Maurizio, Bolzano, Italy; University Hospital Center Rebro, Zagreb, Croatia; Hospedale Nord, Taranto, Italy; St George’s Hospital, London, UK; Evangelismos Hospital, Athens, Greece; University Medical Center, Ljubljana, Slovenia; Department of Hematology/Oncology, University of Münster, Münster, Germany; Centre Pierre et Marie Curie—Service Hématologie Greffe de Moëlle, Alger, Algeria; Allogeneic Stem Cell Transplant Center, Würzburg, Germany; Charles University Hospital, Hradec Králové, Czech Republic; Johannes-Gutenberg-University, Mainz, Germany; Hospital Gregorio Marañón, Madrid, Spain; Mazzoni Hospital, Ascoli Piceno, Italy; Hospital de la Princesa, Madrid, Spain; Division of Stem Cell Transplantation and Immunotherapy, Kiel, Germany; The Trustee of London Clinic, London, UK; Ospedale di Niguarda Ca’ Granda, Milano, Italy; University of Torino, Torino, Italy; Policlinico Le Scotte, Siena, Italy; ZNA, Antwerp, Belgium; NHS Grampian, Aberdeen, UK; Istituto Clinico Humanitas, Milano, Italy; Ospedale V. Cervello, Palermo, Italy; Gaziantep University Medical School, Gaziantep, Turkey; Ankara Bayindir Hospital, Haematology BMT, Ankara, Turkey; Albert Albert's Stem Cell Transplantation Centre-Haematology Pretoria East Hospital, Pretoria Gauteng, South Africa; Department of Medicine, University of Cologne, Cologne, Germany; Military Medical Academy, Belgrade, Serbia and Montenegro; Klinikum Bremen Mitte, Bremen, Germany; Cardarelli Hospital, Napoli, Italy; Ege University Medical School, Bornova-Izmir, Turkey; Hospital Universitario Son Espases, Palma De Mallorca, Spain; Unità Operativa Oncoematologia Pediatrica, Pisa, Italy; S. Bortolo Hospital, Vicenza, Italy; P.O. 'R. Binaghi, Cagliari, Italy; Stem Cell Transplant Unit, Medical Park Hospitals, Antalya, Turkey; Central Clinical Hospital, Warsaw, Poland.
Supplementary Information accompanies this paper on Bone Marrow Transplantation website
Supplementary information
Rights and permissions
About this article
Cite this article
Passweg, J., Labopin, M., Cornelissen, J. et al. Conditioning intensity in middle-aged patients with AML in first CR: no advantage for myeloablative regimens irrespective of the risk group–an observational analysis by the Acute Leukemia Working Party of the EBMT. Bone Marrow Transplant 50, 1063–1068 (2015). https://doi.org/10.1038/bmt.2015.121
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2015.121
This article is cited by
-
Identifying the optimal conditioning intensity for stem cell transplantation in patients with myelodysplastic syndrome: a machine learning analysis
Bone Marrow Transplantation (2023)
-
Age and allogeneic hematopoietic cell transplantation outcomes in acute myeloid leukemia
International Journal of Hematology (2023)
-
Effect of the COVID-19 pandemic on allogeneic stem cell transplantation in Japan
International Journal of Hematology (2023)
-
Predicting non-relapse mortality following allogeneic hematopoietic cell transplantation during first remission of acute myeloid leukemia
Bone Marrow Transplantation (2021)
-
Low relapse risk in poor risk AML after conditioning with 10-day decitabine, fludarabine and 2 Gray TBI prior to allogeneic hematopoietic cell transplantation
Bone Marrow Transplantation (2021)