Abstract
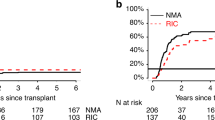
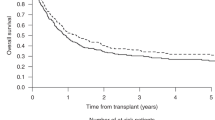
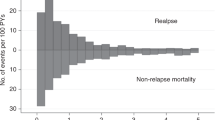
We investigated the impact of the number of induction/consolidation cycles on outcomes of 3113 adult AML patients who received allogeneic hematopoietic cell transplantation (allo-HCT) between 2008 and 2019. Patients received allo-HCT using myeloablative (MAC) or reduced-intensity (RIC) conditioning in first complete remission (CR) or with primary induction failure (PIF). Patients who received MAC allo-HCT in CR after 1 induction cycle had 1.3-fold better overall survival (OS) than 2 cycles to CR and 1.47-fold better than ≥3 cycles. OS after CR in 2 or ≥3 cycles was similar. Relapse risk was 1.65-fold greater in patients receiving ≥3 cycles to achieve CR. After RIC allo-HCT, the number of induction cycles to CR did not affect OS. Compared to CR in 1 cycle, relapse risk was 1.24-1.41-fold greater in patients receiving 2 or ≥3 cycles. For patients receiving only 1 cycle to CR, consolidation therapy prior to MAC allo-HCT was associated with improved OS vs. no consolidation therapy. Detectable MRD at the time of MAC allo-HCT did not impact outcomes while detectable MRD preceding RIC allo-HCT was associated with an increased risk of relapse. For allo-HCT in PIF, OS was significantly worse than allo-HCT in CR after 1–3 cycles.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The final analysis dataset will be posted to the CIBMTR website at https://www.cibmtr.org/ReferenceCenter/PubList/PubDsDownload/Pages/default.aspx. CIBMTR supports accessibility of research in accord with the National Institutes of Health (NIH) Data Sharing Policy and the National Cancer Institute (NCI) Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases de-identified datasets that comply with all relevant global regulations regarding privacy and confidentiality.
Change history
22 March 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41375-023-01814-2
References
Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47.
Short NJ, Rytting ME, Cortes JE. Acute myeloid leukaemia. Lancet. 2018;392:593–606.
Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301:2349–61.
Dholaria B, Savani BN, Hamilton BK, Oran B, Liu HD, Tallman MS, et al. Hematopoietic cell transplantation in the treatment of newly diagnosed adult acute myeloid leukemia: an evidence-based review from the american society of transplantation and cellular therapy. Transpl Cell Ther. 2021;27:6–20.
Duval M, Klein JP, He W, Cahn JY, Cairo M, Camitta BM, et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol. 2010;28:3730–8.
Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15:367–9.
Michelis FV, Gupta V, Zhang MJ, Wang HL, Aljurf M, Bacher U, et al. Cytogenetic risk determines outcomes after allogeneic transplantation in older patients with acute myeloid leukemia in their second complete remission: a Center for International Blood and Marrow Transplant Research cohort analysis. Cancer. 2017;123:2035–42.
Weisdorf DJ, Millard HR, Horowitz MM, Hyare PS, Champlin R, Ho V, et al. Allogeneic transplantation for advanced acute myeloid leukemia: the value of complete remission. Cancer. 2017;123:2025–34.
Rowe JM, Kim HT, Cassileth PA, Lazarus HM, Litzow MR, Wiernik PH, et al. Adult patients with acute myeloid leukemia who achieve complete remission after 1 or 2 cycles of induction have a similar prognosis: a report on 1980 patients registered to 6 studies conducted by the Eastern Cooperative Oncology Group. Cancer. 2010;116:5012–21.
Wheatley K, Burnett AK, Goldstone AH, Gray RG, Hann IM, Harrison CJ, et al. A simple, robust, validated and highly predictive index for the determination of risk-directed therapy in acute myeloid leukaemia derived from the MRC AML 10 trial. United Kingdom Medical Research Council’s Adult and Childhood Leukaemia Working Parties. Br J Haematol. 1999;107:69–79.
Othus M, Estey EH, Garcia-Manero G, Wood BL, Stirewalt DL, Godwin JE, et al. Second cycle remission achievement with 7+3 and survival in adults with newly diagnosed acute myeloid leukemia: analysis of recent SWOG trials. Leukemia. 2019;33:554–8.
Walter RB, Sandmaier BM, Storer BE, Godwin CD, Buckley SA, Pagel JM, et al. Number of courses of induction therapy independently predicts outcome after allogeneic transplantation for acute myeloid leukemia in first morphological remission. Biol Blood Marrow Transplant. 2015;21:373–8.
Keating S, Suciu S, de Witte T, Mandelli F, Willemze R, Resegotti L, et al. Prognostic factors of patients with acute myeloid leukemia (AML) allografted in first complete remission: an analysis of the EORTC-GIMEMA AML 8A trial. The European Organization for Research and Treatment of Cancer (EORTC) and the Gruppo Italiano Malattie Ematologiche Maligne dell’ Adulto (GIMEMA) Leukemia Cooperative Groups. Bone Marrow Transplant. 1996;17:993–1001.
Nagler A, Labopin M, Huang XJ, Blaise D, Arcese W, Araujo MC, et al. Non-T depleted haploidentical stem cell transplantation in AML patients achieving first complete remission after one versus two induction courses: a study from the ALWP/EBMT. Bone Marrow Transplant. 2022;57:572–8.
Mayer RJ, Davis RB, Schiffer CA, Berg DT, Sarno E, Frei E 3rd. Intensive post-remission therapy with Ara-C in adults with acute myeloid leukemia: initial results of a CALGB phase III trial. The Cancer and Leukemia Group B. Leukemia. 1992;6:66–7.
McCormack SE, Cao Q, Oran B, Weisdorf DJ, Warlick ED. Pre-transplant consolidation chemotherapy may not improve outcomes after reduced intensity conditioning hematopoietic stem cell transplantation for acute myeloid leukemia in first complete remission. Leuk Res. 2011;35:757–61.
Warlick ED, Paulson K, Brazauskas R, Zhong X, Miller AM, Camitta BM, et al. Effect of postremission therapy before reduced-intensity conditioning allogeneic transplantation for acute myeloid leukemia in first complete remission. Biol Blood Marrow Transplant. 2014;20:202–8.
Yeshurun M, Labopin M, Blaise D, Cornelissen JJ, Sengeloev H, Vindelov L, et al. Impact of postremission consolidation chemotherapy on outcome after reduced-intensity conditioning allogeneic stem cell transplantation for patients with acute myeloid leukemia in first complete remission: a report from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Cancer. 2014;120:855–63.
Tallman MS, Rowlings PA, Milone G, Zhang MJ, Perez WS, Weisdorf D, et al. Effect of postremission chemotherapy before human leukocyte antigen-identical sibling transplantation for acute myelogenous leukemia in first complete remission. Blood. 2000;96:1254–8.
Cahn JY, Labopin M, Sierra J, Blaise D, Reiffers J, Ferrant A, et al. No impact of high-dose cytarabine on the outcome of patients transplanted for acute myeloblastic leukaemia in first remission. Acute Leukaemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT). Br J Haematol. 2000;110:308–14.
Ferguson P, Hills RK, Grech A, Betteridge S, Kjeldsen L, Dennis M; UK NCRI AML Working Group, et al. An operational definition of primary refractory acute myeloid leukemia allowing early identification of patients who may benefit from allogeneic stem cell transplantation. Haematologica. 2016;101:1351–8.
Heuser M, Freeman SD, Ossenkoppele GJ, Buccisano F, Hourigan CS, Ngai LL, et al. 2021 Update on MRD in acute myeloid leukemia: a consensus document from the European LeukemiaNet MRD Working Party. Blood. 2021;138:2753–67.
Schuurhuis GJ, Heuser M, Freeman S, Bene MC, Buccisano F, Cloos J, et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood. 2018;131:1275–91.
Hourigan CS, Dillon LW, Gui G, Logan BR, Fei M, Ghannam J, et al. Impact of conditioning intensity of allogeneic transplantation for acute myeloid leukemia with genomic evidence of residual disease. J Clin Oncol. 2020;38:1273–83.
Percival ME, Wang HL, Zhang MJ, Saber W, de Lima M, Litzow M, et al. Impact of depth of clinical response on outcomes of acute myeloid leukemia patients in first complete remission who undergo allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2021;56:2108–17.
Buckley SA, Wood BL, Othus M, Hourigan CS, Ustun C, Linden MA, et al. Minimal residual disease prior to allogeneic hematopoietic cell transplantation in acute myeloid leukemia: a meta-analysis. Haematologica. 2017;102:865–73.
Craddock C, Jackson A, Loke J, Siddique S, Hodgkinson A, Mason J, et al. Augmented reduced-intensity regimen does not improve postallogeneic transplant outcomes in acute myeloid leukemia. J Clin Oncol. 2021;39:768–78.
Acknowledgements
The authors thank the patients and families for their courage and confidence in their medical teams; and the data management staff at the transplant Centers for their dedicated work in submitting data to the CIBMTR. This work was supported in part by the Intramural Research Program of the National Heart, Lung, and Blood Institute (CSH).
Funding
The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); HHSH250201700006C from the Health Resources and Services Administration (HRSA); and N00014-20-1-2832 and N00014-21-1-2954 from the Office of Naval Research; support is also provided by Be the Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, and from the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc.; Adaptimmune; Adaptive Biotechnologies Corporation; ADC Therapeutics; Adienne SA; Allogene; Allovir, Inc.; Amgen, Inc.; Angiocrine; Anthem; Astellas Pharma US; AstraZeneca; Atara Biotherapeutics; BeiGene; bluebird bio, inc.; Bristol Myers Squibb Co.; CareDx Inc.; CRISPR; CSL Behring; CytoSen Therapeutics, Inc.; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Gamida Cell, Ltd.; Gilead; GlaxoSmithKline; HistoGenetics; Incyte Corporation; Iovance; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc.; Kadmon, a Sanofi Company; Karius; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Mallinckrodt Pharmaceuticals; Medac GmbH; Medexus Pharma; Merck & Co.; Mesoblast; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; MorphoSys; Novartis Pharmaceuticals Corporation; Omeros Corporation; OptumHealth; Orca Biosystems, Inc.; Ossium Health, Inc.; Pfizer, Inc.; Pharmacyclics, LLC, An AbbVie Company; Pluristem; PPD Development, LP; Sanofi; Sobi, Inc.; Stemcyte; Takeda Pharmaceuticals; Talaris Therapeutics; Terumo Blood and Cell Technologies; TG Therapeutics; Vertex Pharmaceuticals; Vor Biopharma Inc.; Xenikos BV.
Author information
Authors and Affiliations
Contributions
The study was proposed by MB, revised by MB and DJW, statistical analysis by KC and M-JZ. All authors reviewed the analysis, revised the manuscript and approved the final submission. Additional contributing co-authors from the Writing Committee: A. Samer Al-Homsi, Hassan B. Alkhateeb, Yasuyuki Arai, Jean-Yves Cahn, Moussab Damlaj, Firas El Chaer, Jong Wook Lee, Hongtao Liu, Leland Metheny, Fotios V. Michelis, Pashna Munshi, Alberto Mussetti, David Rizzieri, Sachiko Seo, Leo F. Verdonck.
Corresponding author
Ethics declarations
Competing interests
MB reports employment with Genentech. TB reports Mayo Clinic Comprehensive Cancer Center Developmental Fund to conduct research P30 CA015083; and honoraria with Pfizer hematology and Oncology. MB reports research support for unrelated project paid to Novartis. NB served as a consultant or had an advisory role with Medexus Pharmaceuticals, Magenta Therapeutics, CareDx Pharma, Sanofi and CTI BioPharma. VB reports institutional grants or contracts from Abbvie, Pfizer, Incyte, Jazz, Tolero Pharmaceuticals, Inc., and National Marrow Donor Program; consulting fees to self from Genentech, Rigel, Agios, Incyte, Servier Pharmaceuticals LLC, Omeros, Takeda, Partnership for health analytic research, LLC (which in turn, receives funds from Jazz Pharmaceuticals) and Abbvie; safety monitoring committee member for Protagonist; and drug support (institutional) for a trial from Chimerix. VB reports as member of the American Board of Pediatrics Pediatric Hematology/Oncology subboard; National Leader of Pediatric and AYA oncology disease section with Caris Precision Oncology Alliance. JC reports as a member Data Safety Monitoring Board with Allovir, consulting fees with advisory board membership with Jazz Pharmaceuticals, Pfizer, and Amgen; and stocks with Actinium Pharmaceuticals, Bluebird Bio/2Seventy, Dynavax Technologies, aTyr Pharma, Gamida Cell, Miragen Therapeutics, Mustang Bio, Novavax, Ovid Therapeutics, Sorrento Therapeutics, TG Therapeutics, Vaxart, and Veru. RPG reports consulting fees with Ascentage Pharma Group, BeiGene Ltd., Kite Pharma, Inc., Fusion Pharma LLC, LaJolla NanoMedical, Inc., Mingsight Pharma, Inc., Prolacta Bioscience, Inc., CStone Pharmaceuticals. Board participation on FFF Enterprises, Azca, Inc., RakFond Foundation, Antegene Biotech LLC, Stem Rad LTD; and stock or stock options with Bristol Myers. SG reports participation on advisory board with Astellas, Daiichi Sankyo, Bristol Myers Squibb, Sanofi Aventis, AstraZeneca, and Kite Pharma. MRGr reports consulting fees and/or advisory board with Abbvie, Agios/Servier, Amgen, Astellas, Blueprint Medicines, Bristol Myers Squibb, Cardinal Health, CTI Biopharma, Daiichi Sankyo, Gamida Cell, Gilead, Incyte, Invitae, Karius, Novartis, Ono Pharmaceutical, Pfizer, Pharmacosmos, Premier, Sierra Oncology, and Stemline; stock ownership in Medtronic; medical writing with Incyte, Amgen, Jazz, Janssen, Genentech/Roche; and support with Incyte, Genetech/Roche, Janssen. Shahrukh Hashmi reports honorarium for educational talks (all non-promotional, majority on “quality of life” or “survivorship”) in CME accredited educational seminars/conferences. This is over the past 10 years: Pfizer, Novartis, Gilead, Sanofi, Therakos, Janssen, MSD; Relationships: No financial but only volunteer: ASTCT, WBMT, CIBMTR. GH reports grants or contracts from Incyte, Acerta/Astra Zeneca, Takeda, Jazz Pharmaceuticals, and Pharmacyclics; consulting fees from Incyte, Morphosys, Alexio n Pharmaceutcials, Karyopharm, Seattle Genetics, Janssen; participation on a Data Safety Monitoring Board or Advisory Board with Rapa Therapauetics, serves as Vice President, Kentucky Society for Clinical Oncology; Axim Biotechnologies, GW Pharmaceuticals, Cardinal Health, Clovis Oncology, Pfizer, Cellectis, CVS Health, Bluebird Bio, Biogen, Charlottes Webb, AImmune Therapeutics Inc, Medical PPTYS TR Inc, Caretrust Reit Inc, Moderna Therapeutics, Zoom. MK-D reports consulting fees with Darrchi Sankyo. HML reports honoraria for consulting fees from Jazz Pharmaceuticals, CSL Behring, Partner Therapeutics, Actinium, Pluristem, Seattle Genetics; payment or honoraria from speakers’ bureau with Jazz Pharmaceuticals, Seattle Genetics, AstraZeneca; travel reimbursement from Jazz Pharmaceuticals and Seattle Genetics; honoraria for participation on a Data Safety Monitoring Board or Advisory Board with BMS-Celgene and Biosight; and stock options with Partner Therapeutics. DM reports honoraria for AstraZeneca; advisory board for Morphosys and Seagen; and research funding with Genentech, ADC therapeutics, and Karyopharm. AS is the St. Jude Children’s Research Hospital site principal investigator of clinical trials sponsored by Vertex Pharmaceuticals/CRISPR Therapeutics (NCT03745287) and by Novartis (NCT04443907). The industry sponsors provide funding for the clinical trial, which includes salary support paid to AS’s institution. AS has received consultant fee from Spotlight Therapeutics, Medexus Inc. and Vertex Pharmaceuticals. He has also received research funding from CRISPR Therapeutics and honoraria from Vindico Medical Education. None of these are related to the work presented in this manuscript. Kirsten Williams reports grants for immunotherapy in AML unrelated to current project with Leukemia Lymphoma Society; payment for educational seminar in lung complications of HCT with ASH; ASTCT board of directors meeting; ASH for educational seminar; patents pending for MRD test for AML and ALL unrelated to this work; and leadership roles with ASTCT, CIBMTR, and PTCTC. CH reports the National Heart, Lung, and Blood Institute receives research funding for the laboratory of Dr Hourigan from Sellas; and the National Heart, Lung, and Blood Institute receives research funding for the laboratory of Dr Hourigan from the Foundation of the NIH AML MRD Biomarkers Consortium. PK reports participation on advisory board with Kite, Pfizer, and Jazz. Marcos de Lima reports research agreements (no direct payments) with Miltenyi Biotec; payment to Pfizer; and consulting fees from BMS/Pfizer/Incyte.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Authors Wael Saber and Amer Zeidan have been added. Furthermore, the Author Contributions section was corrected.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Boyiadzis, M., Zhang, MJ., Chen, K. et al. Impact of pre-transplant induction and consolidation cycles on AML allogeneic transplant outcomes: a CIBMTR analysis in 3113 AML patients. Leukemia 37, 1006–1017 (2023). https://doi.org/10.1038/s41375-022-01738-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-022-01738-3