Abstract
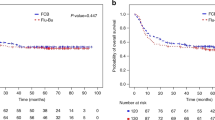
Allogeneic hematopoietic cell transplantation offers improved survival in patients with ALL, but with regimens containing TBI, the nonrelapse mortality is 20–40%. Efforts to lessen transplant toxicities by reducing conditioning regimen intensity have led to increased relapse risk. Therefore, there is a need for less toxic regimens that maintain an anti-leukemia effect. We report here a retrospective review of 65 patients with ALL in first remission receiving grafts from allogeneic donors after fludarabine 40 mg/m2/day for 4 days and i.v. BU targeted to a median daily area under the concentration–time curve below 6000 μmoles min/L. At 2 years after transplantation, OS was 65% (95% confidence interval (CI): 52–77%), relapse-free survival was 61% (95% CI: 48–73%), cumulative incidence of relapse was 26% (95% CI: 17–39%) and cumulative incidence of nonrelapse mortality was 14% (95% CI: 8–26%). Age over 35 years, Ph chromosome positivity and minimal residual disease at transplant did not adversely affect outcomes. Pharmacokinetically targeted BU and fludarabine can provide intensive pre-transplant conditioning for adults with ALL in first remission, with promising relapse-free and OS rates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Goldstone AH, Richards SM, Lazarus HM, Tallman MS, Buck G, Fielding AK et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993). Blood 2008; 111: 1827–1833.
Fielding AK, Rowe JM, Richards SM, Buck G, Moorman AV, Durrant IJ et al. Prospective outcome data on 267 unselected adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia confirms superiority of allogeneic transplantation over chemotherapy in the pre-imatinib era: results from the International ALL Trial MRC UKALLXII/ECOG2993. Blood 2009; 113: 4489–4496.
Lee HJ, Thompson JE, Wang ES, Wetzler M . Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer 2011; 117: 1583–1594.
Mohty M, Labopin M, Volin L, Gratwohl A, Socie G, Esteve J et al. Reduced-intensity versus conventional myeloablative conditioning allogeneic stem cell transplantation for patients with acute lymphoblastic leukemia: a retrospective study from the European Group for Blood and Marrow Transplantation. Blood 2010; 116: 4439–4443.
Marks DI, Wang T, Perez WS, Antin JH, Copelan E, Gale RP et al. The outcome of full-intensity and reduced-intensity conditioning matched sibling or unrelated donor transplantation in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia in first and second complete remission. Blood 2010; 116: 366–374.
Zwaan CM, Kaspers GJ, Pieters R, Ramakers-Van Woerden NL, den Boer ML, Wunsche R et al. Cellular drug resistance profiles in childhood acute myeloid leukemia: differences between FAB types and comparison with acute lymphoblastic leukemia. Blood 2000; 96: 2879–2886.
Bunin N, Aplenc R, Kamani N, Shaw K, Cnaan A, Simms S . Randomized trial of busulfan vs total body irradiation containing conditioning regimens for children with acute lymphoblastic leukemia: a Pediatric Blood and Marrow Transplant Consortium study. Bone Marrow Transplant 2003; 32: 543–548.
Kalaycio M, Bolwell B, Rybicki L, Absi A, Andresen S, Pohlman B et al. BU- vs TBI-based conditioning for adult patients with ALL. Bone Marrow Transplant 2011; 46: 1413–1417.
Davies SM, Ramsay NKC, Klein JP, Weisdorf DJ, Bolwell B, Cahn JY et al. Comparison of preparative regimens in transplants for children with acute lymphoblastic leukemia. J Clin Oncol 2000; 18: 340.
Slattery JT, Buckner CD, Schaffer RL, Lambert KW, Langer FP, Anasetti C et al. Graft-rejection and toxicity following bone marrow transplantation in relation to busulfan pharmacokinetics. Bone Marrow Transplant 1995; 16: 31–42.
Slattery JT, Clift RA, Buckner CD, Radich J, Storer B, Bensinger WI et al. Marrow transplantation for chronic myeloid leukemia: the influence of plasma busulfan levels on the outcome of transplantation. Blood 1997; 89: 3055–3060.
Andersson BS, Thall PF, Madden T, Couriel D, Wang X, Anderlini P et al. Busulfan systemic exposure relative to regimen-related toxicity and acute graft-versus-host disease: Defining a therapeutic window for i.v. BuCy2 in chronic myelogenous leukemia. Biol Blood Marrow Transplant 2002; 8: 477–485.
de Lima M, Couriel D, Thall PF, Wang X, Madden T, Jones R et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood 2004; 104: 857–864.
Ljungman PHM, Békássy AN, Ringden O, Öberg G . High busulfan concentrations are associated with increased transplant-related mortality in allogeneic bone marrow transplant patients. Bone Marrow Transplant 1997; 20: 909–913.
Perkins J, Field T, Kim J, Kharfan-Dabaja MA, Ayala E, Perez L et al. Pharmacokinetic targeting of i.v. BU with fludarabine as conditioning before hematopoietic cell transplant: the effect of first-dose area under the concentration time curve on transplant-related outcomes. Bone Marrow Transplant 2011; 46: 1418–1425.
Andersson BS, de Lima M, Thall PF, Wang X, Couriel D, Korbling M et al. Once Daily i.v. Busulfan and Fludarabine (i.v. Bu-Flu) Compares Favorably with i.v. Busulfan and Cyclophosphamide (i.v. BuCy2) as Pretransplant Conditioning Therapy in AML/MDS. Biol Blood Marrow Transplant 2008; 14: 672–684.
Bredeson CN, Zhang MJ, Agovi MA, Bacigalupo A, Bahlis NJ, Ballen K et al. Outcomes following HSCT using fludarabine, busulfan, and thymoglobulin: a matched comparison to allogeneic transplants conditioned with busulfan and cyclophosphamide. Biol Blood Marrow Transplant 2008; 14: 993–1003.
Pidala J, Kim J, Anasetti C, Kharfan-Dabaja MA, Nishihori T, Field T et al. Pharmacokinetic targeting of intravenous busulfan reduces conditioning regimen related toxicity following allogeneic hematopoietic cell transplantation for acute myelogenous leukemia. J Hematol Oncol 2010; 3: 36.
Santarone S, Pidala J, Di Nicola M, Field T, Alsina M, Ayala E et al. Fludarabine and pharmacokinetic-targeted busulfan before allografting for adults with acute lymphoid leukemia. Biol Blood Marrow Transplant 2011; 17: 1505–1511.
Perkins JB, Kim J, Anasetti C, Fernandez HF, Perez LE, Ayala E et al. maximally tolerated busulfan systemic exposure in combination with fludarabine as conditioning before allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2011; 18: 1099–1107.
Perkins J, Field T, Kim J, Kharfan-Dabaja MA, Fernandez H, Ayala E et al. A randomized phase ii trial comparing tacrolimus and mycophenolate mofetil to tacrolimus and methotrexate for acute graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant 2010; 16: 937–947.
Pidala J, Kim J, Jim H, Kharfan Dabaja MA, Nishihori T, Fernandez HF et al. A randomized phase II study to evaluate tacrolimus in combination with sirolimus or methotrexate after allogeneic hematopoietic cell transplantation. Haematologica 2012; 97: 1882–1889.
Lobashevsky AL, Senkbeil RW, Townsend JE, Mink CA, Thomas JM . Quantitative analysis of chimerism using a short tandem repeat method on a fluorescent automated DNA sequencer. Clin Lab Haematol 2006; 28: 40–49.
McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med 1993; 118: 255–267.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant 1995; 15: 825–828.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005; 11: 945–956.
Gray RJ . A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988; 16: 1141–1154.
Choudhury JB . Non-parametric confidence interval estimation for competing risks analysis: application to contraceptive data. Stat Med 2002; 21: 1129–1144.
Doney K, Gooley TA, Deeg HJ, Flowers ME, Storb R, Appelbaum FR . Allogeneic hematopoietic cell transplantation with full-intensity conditioning for adult acute lymphoblastic leukemia: results from a single center, 1998-2006. Biol Blood Marrow Transplant 2011; 17: 1187–1195.
Daly A, Savoie ML, Geddes M, Chaudhry A, Stewart D, Duggan P et al. Fludarabine, busulfan, antithymocyte globulin, and total body irradiation for pretransplantation conditioning in acute lymphoblastic leukemia: Excellent Outcomes in all but older patients with comorbidities. Biol Blood Marrow Transplant 2012; 18: 1921–1926.
Kebriaei R, Basset R, Ledesma C, Ciurea S, Parmar S, Shpall EJ et al. Clofarabine combined with busulfan provides excellent disease control in adult patients with acute lymphoblastic leukemia undergoing allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2012; 18: 1819–1826.
Geddes M, Kangarloo SB, Naveed F, Quinlan D, Chaudhry MA, Wu J et al. High busulfan exposure is associated with worse outcomes in a daily i.v. busulfan and fludarabine allogeneic transplant regimen. Biol Blood Marrow Transplant 2008; 14: 220–228.
Ram R, Storb R, Sandmaier BM, Maloney DG, Wolfry A, Flowers MD et al. Non-myeloablative conditioning with allogeneic hematopoietic cell transplantation for the treatment of high-risk acute lymphoblastic leukemia. Haematologica 2011; 96: 1113–1120.
Pfeifer H, Wassmann B, Bethge W, Dengler J, Bornhauser M, Stadler M et al. Randomized comparison of prophylactic and minimal residual disease-triggered imatinib after allogeneic stem cell transplantation for BCR-ABL1 positive acute lymphoblastic leukemia. Leukemia 2012; 27: 1254–1262.
Sive JI, Buck G, Fielding A, Lazarus HM, Litzow MR, Luger S et al. Outcomes in older adults with acute lymphoblastic leukaemia (ALL): results from the international MRC UKALL XII/ECOG2993 trial. Br J Haematol 2012; 157: 463–471.
Brüggemann M, Gökbuget N, Kneba M . Acute lymphoblastic leukemia: monitoring minimal residual disease as a therapeutic principle. Sem Oncol 2012; 39: 47–57.
Oyekunle A, Haferlach T, Kröger N, Klyuchnickov E, Zander AR, Schnittger S et al. Molecular diagnostics, targeted therapy, and the indication for allogeneic stem cell transplantation in acute lymphoblastic leukemia. Adv Hematol 2011; 2011: 154745.
Patel B, Rai L, Buck G, Richards SM, Mortuza Y, Mitchell W et al. Minimal residual disease is a significant predictor of treatment failure in non T-lineage adult acute lymphoblastic leukaemia: final results of the international trial UKALL XII/ECOG2993. Br J Haematol 2010; 148: 80–89.
Kantarjian H, Thomas D, O’Brien S, Certes J, Giles F, Jeha S et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose intensive regimen, in adult acute lymphocytic leukemia. Cancer 2004; 101: 2788–2801.
Sorror ML, Maris MB, Storb R, Baron F, Sandmeier BM, Maloney DG et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005; 106: 2912–2919.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
The study abstract was presented in oral session at the Meeting of the American Society of Hematology, December 10-13, 2011, San Diego, California.
Rights and permissions
About this article
Cite this article
Kunter, G., Perkins, J., Pidala, J. et al. Pharmacokinetically-targeted BU and fludarabine as conditioning before allogeneic hematopoietic cell transplantation for adults with ALL in first remission. Bone Marrow Transplant 49, 11–16 (2014). https://doi.org/10.1038/bmt.2013.121
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2013.121
Keywords
This article is cited by
-
Resistance of bone marrow stroma to genotoxic preconditioning is determined by p53
Cell Death & Disease (2021)
-
Who Should Receive a Transplant for Acute Lymphoblastic Leukaemia?
Current Hematologic Malignancy Reports (2017)