Abstract
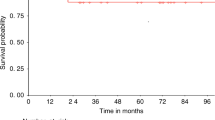
Graft engineering procedures for hematopoietic SCT (HSCT) may improve the chance of success in matched unrelated donor (MUD) and haploidentical donor transplantations. Successful donor immune reconstitution is important to mediate GVL effects in reduced-intensity conditioning (RIC) HSCT. We prospectively investigated early immune reconstitution and clinical outcome in 30 CD3/CD19-depleted MUD (n=15) or HP (n=15) HSCTs for high-risk childhood leukemia using a fludarabine-based RIC without serotherapy. The graft consisted of a mean of 10.5 × 106/kg CD34+, 77 × 103/kg CD3+ and 39 × 106/kg CD56+ cells. After transplantation, 86% of the patients engrafted. In all, 13% of patients had >grade 3 acute GVHD. Natural killer (NK) cell, DC and T-cell recovery achieved normal values within the first 60 days after transplantation. DC recovery was dominated by the DC2− subset. NK-cell phenotype was altered and cytotoxicity was lower compared with their donors. EFS was 50±9% (73±11% for those in CR1 and 26±11% for those with advanced disease). Faster DC2− recovery was associated with better outcome, especially in the MUD setting. In summary, CD3/CD19-depleted HSCT with fludarabine-based RIC without serotherapy resulted in favorable patient survival, and rapid NK, DC and T-cell recovery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Aversa F, Reisner Y, Martelli MF . Hematopoietic stem cell transplantation from alternative sources in adults with high-risk acute leukemia. Blood Cells Mol Dis 2004; 33: 294–302.
Cairo MS, Rocha V, Gluckman E, Hale G, Wagner J . Alternative allogeneic donor sources for transplantation for childhood diseases: unrelated cord blood and haploidentical family donors. Biol Blood Marrow Transplant 2008; 14: 44–53.
Pérez A, González-Vicent M, Ramírez M, Sevilla J, Madero L, Díaz MA . Intentional induction of mixed hematopoietic chimerism as platform for cellular therapy after HLA-matched allogeneic stem cell transplantation in childhood leukemia patients. Br J Haematol 2008; 140: 340–343.
González-Vicent M, Pérez A, Abad L, Sevilla J, Ramirez M, Diaz MA . Graft manipulation and reduced-intensity conditioning for allogeneic hematopoietic stem cell transplantation from mismatched unrelated and mismatched/haploidentical related donors in pediatric leukemia patients. J Pediatr Hematol Oncol 2010; 32: e85–e90.
Weimer R, Staak A, Süsal C, Streller S, Yildiz S, Pelzl S et al. ATG induction therapy: long-term effects on Th1 but not on Th2 responses. Transpl Int 2005; 18: 226–236.
Locatelli F, Pende D, Maccario R, Mingari MC, Moretta A, Moretta L . Haploidentical hemopoietic stem cell transplantation for the treatment of high-risk leukemias: how NK cells make the difference. Clin Immunol 2009; 133: 171–178.
Gassas A, Sung L, Saunders EF, Doyle J . Graft-versus-leukemia effect in hematopoietic stem cell transplantation for pediatric acute lymphoblastic leukemia: significantly lower relapse rate in unrelated transplantations. Bone Marrow Transplant 2007; 40: 951–955.
Pfeiffer MM, Feuchtinger T, Teltschik HM, Schumm M, Müller I, Handgretinger R et al. Reconstitution of NK cell receptors influences NK activity and relapse rate after haploidentical transplantation of T and B cell depleted grafts in children. Haematologica 2010; 95: 1381–1388.
Dulphy N, Haas P, Busson M, Belhadj S, Peffault de Latour R, Robin M et al. An unusual CD56(bright) CD16(low) NK cell subset dominates the early post transplant period following HLA-matched hematopoietic stem cell transplantation. J Immunol 2008; 181: 2227–2237.
Vakkila J, Thomson AW, Hovi L, Vettenranta K, Saarinen-Pihkala UM . Circulating dendritic cell subset levels after allogeneic stem cell transplantation in children correlate with time post transplant and severity of acute graft-versus-host disease. Bone Marrow Transplant 2005; 35: 501–507.
Fagnoni FF, Oliviero B, Giorgiani G, De Stefano P, Dehò A, Zibera C et al. Reconstitution dynamics of plasmacytoid and myeloid dendritic cell precursors after allogeneic myeloablative hematopoietic stem cell transplantation. Blood 2004; 104: 281–289.
Talarn C, Urbano-Ispizua A, Martino R, Pérez-Simón JA, Batlle M, Herrera C et al. Kinetics of recovery of dendritic cell subsets after reduced-intensity conditioning allogeneic stem cell transplantation and clinical outcome. Haematologica 2007; 92: 1655–1663.
Sevilla J, González-Vicent M, Lassaletta A, Ramírez M, Pérez-Martínez A, Madero L et al. Peripheral blood progenitor cell collection adverse events for childhood allogeneic donors: variables related to the collection and safety profile. Br J Haematol 2009; 144: 909–916.
Blomberg K, Hautala R, Lovgren J, Mukkala VM, Lindqvist C, Akerman K . Time-resolved fluorometric assay for natural killer activity using target cells labeled with a fluorescence enhancing ligand. J Immunol Methods 1995; 193: 199–206.
Park SH, Choi SM, Lee DG, Choi JH, Yoo JH, Kim SH et al. Infectious complications associated with alemtuzumab use for allogeneic hematopoietic stem cell transplantation: comparison with anti-thymocyte globulin. Transpl Infect Dis 2009; 11: 413–423.
Ciurea SO, Saliba R, Rondon G, Pesoa S, Cano P, Fernandez-Vina M et al. Reduced-intensity conditioning using fludarabine, melphalan and thiotepa for adult patients undergoing haploidentical SCT. Bone Marrow Transplant 2010; 45: 429–436.
Westerhuis G, Maas WG, Toes RE, Fibbe WE . Persisting NK cell alloreactivity in the presence of long-term stable hematopoietic chimerism. Exp Hematol 2009; 37: 739–743.
Westerhuis G, Maas WG, Willemze R, Toes RE, Fibbe WE . Long-term mixed chimerism after immunologic conditioning and MHC-mismatched stem-cell transplantation is dependent on NK-cell tolerance. Blood 2005; 106: 2215–2220.
Larsen CP, Page A, Linzie KH, Russell M, Deane T, Stempora L et al. An MHC-defined primate model reveals significant rejection of bone marrow after mixed chimerism induction despite full MHC matching. Am J Transplant 2010; 10: 2396–2409.
Kean LS, Hamby K, Koehn B, Lee E, Coley S, Stempora L et al. NK cells mediate costimulation blockade-resistant rejection of allogeneic stem cells during nonmyeloablative transplantation. Am J Transplant 2006; 6: 292–304.
Brunstein CG, Hirsch BA, Miller JS, McGlennen RC, Verfaillie CM, McGlave PB et al. Non-leukemic autologous reconstitution after allogeneic bone marrow transplantation for Ph-positive chronic myelogenous leukemia: extended remission preceding eventual relapse. Bone Marrow Transplant 2000; 26: 1173–1177.
De Somer L, Sprangers B, Fevery S, Rutgeerts O, Lenaerts C, Boon L et al. Recipient lymphocyte infusion in MHC-matched bone marrow chimeras induces a limited lymphohematopoietic host-versus-graft reactivity but a significant antileukemic effect mediated by CD8+ T cells and natural killer cells. Haematologica 2011; 96: 424–431.
Ball LM, Bernardo ME, Roelofs H, Lankester A, Cometa A, Egeler RM et al. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood 2007; 110: 2764–2767.
Lang P, Mueller I, Greil J, Bader P, Schumm M, Pfeiffer M et al. Retransplantation with stem cells from mismatched related donors after graft rejection in pediatric patients. Blood Cells Mol Dis 2008; 40: 33–39.
Chakraverty R, Sykes M . The role of antigen-presenting cells in triggering graft-versus-host disease and graft-versus-leukemia. Blood 2007; 110: 9–17.
Reddy P, Maeda Y, Liu C, Krijanovski OI, Korngold R, Ferrara J . A crucial role for antigen-presenting cells and alloantigen expression in graft-versus-leukemia responses. Nat Med 2005; 11: 1244–1249.
Miller JS, Warren EH, van den Brink MR, Ritz J, Shlomchik WD, Murphy WJ et al. NCI First International Workshop on The Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: Report from the Committee on the Biology Underlying Recurrence of Malignant Disease following Allogeneic HSCT: Graft-versus-Tumor/Leukemia Reaction. Biol Blood Marrow Transplant 2010; 16: 565–586.
Perez-Martinez A, Iyengar R, Gan K, Chotsampancharoen T, Rooney B, Holladay M et al. Blood dendritic cells suppress NK cell function and increase the risk of leukemia relapse after hematopoietic cell transplantation. Biol Blood Marrow Transplant 2011; 17: 598–607.
Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002; 295: 2097–2100.
Triplett BM, Horwitz EM, Iyengar R, Turner V, Holladay MS, Gan K et al. Effects of activating NK cell receptor expression and NK cell reconstitution on the outcomes of unrelated donor hematopoietic cell transplantation for hematologic malignancies. Leukemia 2009; 23: 1278–1287.
Federmann B, Hägele M, Pfeiffer M, Wirths S, Schumm M, Faul C et al. Immune reconstitution after haploidentical hematopoietic cell transplantation: impact of reduced intensity conditioning and CD3/CD19 depleted grafts. Leukemia 2011; 25: 121–129.
Nguyen S, Dhedin N, Vernant JP, Kuentz M, Al Jijakli A, Rouas-Freiss N et al. NK-cell reconstitution after haploidentical hematopoietic stem-cell transplantations: immaturity of NK cells and inhibitory effect of NKG2A override GvL effect. Blood 2005; 105: 4135–4142.
Spaner DE, Masellis A . Toll-like receptor agonists in the treatment of chronic lymphocytic leukemia. Leukemia 2007; 21: 53–60.
Link BK, Ballas ZK, Weisdorf D, Wooldridge JE, Bossler AD, Shannon M et al. Oligodeoxynucleotide CpG 7909 delivered as intravenous infusion demonstrates immunologic modulation in patients with previously treated non-Hodgkin lymphoma. J Immunother 2006; 29: 558–568.
Zhang X, Munegowda MA, Yuan J, Wei Y, Xiang J . Optimal TLR9 signal converts tolerogenic CD4-8- DCs into immunogenic ones capable of stimulating antitumor immunity via activating CD4+ Th1/Th17 and NK cell responses. J Leukoc Biol 2010; 88: 393–403.
Leung W, Iyengar R, Turner V, Lang P, Bader P, Conn P et al. Determinants of anti leukemia effects of allogeneic NK cells. J Immunol 2004; 172: 644–650.
Stern M, Ruggeri L, Mancusi A, Bernardo ME, de Angelis C, Bucher C et al. Survival after T cell-depleted haploidentical stem cell transplantation is improved using the mother as donor. Blood 2008; 112: 2990–2995.
Leung W, Campana D, Yang J, Pei D, Coustan-Smith E, Gan K et al. High success rate of hematopoietic cell transplantation regardless of donor source in children with very high-risk leukemia. Blood 2011; 118: 223–230.
Acknowledgements
We would like to acknowledge Dr W Leung from St Jude Children's Research Hospital for the critical review of the manuscript. We would also like to thank Hannah Maxwell from Cardiff University for the English edition of the manuscript. This work was supported in part by National Health Service of Spain grant FIS PS09/02393, Fundación Mutua Madrileña and ‘La hucha de Tomás’ project (http://www.lahuchadetomas.com/).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Bone Marrow Transplantation website
Supplementary information
Rights and permissions
About this article
Cite this article
Pérez-Martínez, A., González-Vicent, M., Valentín, J. et al. Early evaluation of immune reconstitution following allogeneic CD3/CD19-depleted grafts from alternative donors in childhood acute leukemia. Bone Marrow Transplant 47, 1419–1427 (2012). https://doi.org/10.1038/bmt.2012.43
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2012.43
Keywords
This article is cited by
-
Comparison of clinical outcomes between unrelated single umbilical cord blood and “ex-vivo” T-cell depleted haploidentical transplantation in children with hematological malignancies
World Journal of Pediatrics (2021)
-
Possible role of highly activated mucosal NK cells against viral respiratory infections in children undergoing haematopoietic stem cell transplantation
Scientific Reports (2019)
-
Prognostic factors and outcomes for pediatric patients receiving an haploidentical relative allogeneic transplant using CD3/CD19-depleted grafts
Bone Marrow Transplantation (2016)
-
Refinement of treatment strategies in ex vivo T-cell-depleted haploidentical SCT for pediatric patients
Bone Marrow Transplantation (2015)
-
The immune response to cytomegalovirus in allogeneic hematopoietic stem cell transplant recipients
Cellular and Molecular Life Sciences (2015)