Abstract
Background:
Ultraviolet radiation (UVR) is the major risk factor for development of malignant melanoma. Fibroblast activation protein (FAP)-α is a serine protease expressed on the surface of activated fibroblasts, promoting tumour invasion through extracellular matrix (ECM) degradation. The signalling mechanism behind the upregulation of FAP-α is not yet completely revealed.
Methods:
Expression of FAP-α was analysed after UVR exposure in in vitro co-culture systems, gene expression arrays and artificial skin constructs. Cell migration and invasion was studied in relation to cathepsin activity and secretion of transforming growth factor (TGF)-β1.
Results:
Fibroblast activation protein-α expression was induced by UVR in melanocytes of human skin. The FAP-α expression was regulated by UVR-induced release of TGF-β1 and cathepsin inhibitors prevented such secretion. In melanoma cell culture models and in a xenograft tumour model of zebrafish embryos, FAP-α mediated ECM degradation and facilitated tumour cell dissemination.
Conclusions:
Our results provide evidence for a sequential reaction axis from UVR via cathepsins, TGF-β1 and FAP-α expression, promoting cancer cell dissemination and melanoma metastatic spread.
Similar content being viewed by others
Main
Ultraviolet radiation (UVR) is the major risk factor for the development of cutaneous malignant melanoma, but the role of UVR in melanoma progression and its tendency to metastasise is not studied in detail (Venza et al, 2015). Nevi consist of growth-arrested melanocytes that share similarities with senescent cells, and senescence could be referred to as a tumour suppressor mechanism. Histologically, melanocytic nevi precursors can be identified in at least half of all cutaneous melanomas (Tsao et al, 2003). Importantly, several of the features distinguish benign nevi from melanoma lies in the senescence pathways (Ross et al, 2011). Thus, comparing the gene and protein expression between nevi and melanoma might provide important information on which signalling pathways and proteins that need to be activated to accomplish the transition of growth-arrested cells into a (pre)malignant state (Orfanidis et al, 2016). The determinants of tumour invasion and metastasis include changes in the attachment of cells to their microenvironment, activation of extracellular proteases and modification of the surrounding stromal tissue through direct contact or cell-to-cell crosstalk by soluble mediators. Fibroblast activation protein-α (seprase/antiplasmin-cleaving enzyme/dipeptidyl prolyl peptidase 5) is a plasma membrane serine protease and has been detected in the reactive stroma of melanocytic nevi and melanoma (Scanlan et al, 1994; Huber et al, 2003). Fibroblast activation protein-α exhibits both protease and collagenase activity, and is important for extracellular matrix (ECM) degradation and modification (O'Brien and O'Connor, 2008; Wäster et al, 2011).
Previously, we presented the novel finding that UVR upregulates the expression of FAP-α in melanocytes and melanoma cells in vitro, which facilitates ECM degradation (Wäster et al, 2011). Moreover, we have established that growth and invasion of primary melanoma cells is dependent on secretion of the lysosomal cathepsins B and D (Eding et al, 2015). Overexpression of cathepsins is associated with increased metastatic potential as well as poor prognosis (Vasiljeva et al, 2007; Palermo and Joyce, 2008). Yin et al (2012) revealed that melanoma invasion was enhanced by cathepsin B-induced TGF-β1 expression, resulting in activated fibroblasts with increased ability to promote invasion of melanoma. Transforming growth factor-β subtypes are constitutively expressed by primary melanoma and metastatic melanoma cells (Lazar-Molnar et al, 2000). Furthermore, TGF-β1 released from melanoma cells stimulates FAP-α expression in surrounding fibroblasts (Wäster et al, 2011).
Given that UVR is important for the initiation and progression of melanoma, the aim of the current study is to identify signalling pathways that contribute to these processes. Specifically, we investigate the relationship between cathepsins, TGF-β1 and FAP-α during UVR exposure using in vitro co-culture systems, ex vivo skin, artificial skin constructs and a xenograft tumour model of zebrafish embryos.
Materials and methods
Cell cultures and additions
All experiments were performed according to the ethical principles of the Helsinki declaration and were approved by the Ethical Review Board at Linköping University, Sweden. Primary melanocytes, keratinocytes and fibroblasts were obtained from Caucasian donors by means of foreskin circumcisions (0–3 years of age; parental written informed consent) as described previously (Larsson et al, 2005). Four primary melanoma cell lines WM115, WM793, WM278, and FM55P (Wistar Institute, Philadelphia, PA, USA) were used. The fibroblasts and melanoma cells were cultivated in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin. Prior to experiments, cells were trypsinated and seeded at 2.5 × 104 cells per cm2.
The following antibodies from Santa Cruz Biotechnology (Santa Cruz, CA, USA) were used for inhibition when indicated; anti-TGF-β1 (200 ng ml−1), anti-FAP-α (20.6 μg ml−1), or anti-cathepsin D (42 μg ml−1; Athens Research and Technology Inc., Athens, GA, USA). Inhibitors of cathepsin B (CA-074 Me; 1 μ M) and cathepsin K (inhibitor II; 10 μ M) were from Calbiochem (San Diego, CA, US) and preincubated for 16 h. Lysosomal acidity was neutralised by NH4Cl (10 mM, 30 min, Sigma, St Louis, MO, USA). Fibroblast activation protein-α activity was blocked by addition of Gly-PhP(OPh)2 (H2N-Gly-Pro diphenylphosphonate; 100 μ M; Gilmore et al, 2006). In selected experiments, recombinant TGF- β1 (10 ng ml−1, Sigma) was added.
Reconstructed skin and ex vivo skin
Fibroblasts, keratinocytes and melanocytes from the same donors were isolated (Larsson et al, 2005). Skin constructs consisting of a fibroblast/collagen matrix in the bottom with a layer of melanocytes and keratinocytes allowed to form an epidermis-like layer with cornified keratinocytes covering the construct were prepared according to Li et al, 2011. Ex vivo skin (biopsies of 4 mm diameter) was obtained from excess skin from reduction plastic surgery of breast. The biopsies were placed in inserts with reconstructed skin medium II (Li et al, 2011). After exposure to UV, the samples were fixed, embedded in paraffin and subjected to immunohistochemical staining according to standard methods using a monoclonal anti-mouse primary antibody FAP-α (1:100, sc65398, Santa Cruz Biotechnology) and polyclonal anti-rabbit primary antibody tyrosinase (1:50, ab175997, Abcam, Cambridge, UK).
Whole human genome microarray analysis
Microarray analysis was performed in melanocytes from four different donors as described in Orfanidis et al (2016). In short, young and senescent cell cultures were sampled 3 and 30 days after seeding, respectively. After isolation of total RNA, labelling and hybridisation were performed with the Affymetrix Human Genome Microarray (WT GeneTitan ST1.1; Plate type, HuGene-1_1-st-v1–16; Array type, HuGene-1_1-st-v1; Affymetrix Inc., CA, USA) and analysed at BEA (Karolinska Institutet, Stockholm, Sweden). The raw.CEL files were processed using the Agilent GeneSpring GX 13 software package (Agilent Technologies, Waldbronn, Germany). For the gene-level experiment, the data were normalised using a quantile algorithm (Bolstad et al, 2003). The data is deposited at Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/); accession number GSE83922. Hierarchical clustering and heat map was determined in R (version 3.3.1, https://www.r-project.org) using Euclidean similarity measure with Wards linkage rule. GSE3189 identifier was used to import the microarray data from a public data set (Talantov et al, 2005). The intersection of the common differentially expressed genes (adjusted P-value of 0.05 and minimum fold change of 1.5) from the above data sets (melanoma vs benign nevi and young vs senescent melanocytes) was used to identify lysosome-associated genes that are up/downregulated in young melanocytes and melanomas. The gene set of interest (including cathepsins, TGF-β1, FAP-α, lysosome-associated genes) was used in the ingenuity pathway analysis (IPA) software (Ingenuity Systems, Qiagen, Hilden, Germany) to generate the network of direct relationships between molecules.
Gene silencing
Depletion of FAP-α was performed with two siRNA sequences (SI00064050; SI00064057, 1 μg of each) and 6 μl RNAiFect (all from Qiagen, Germantown, MD, USA). Optimal transfection conditions were determined by Alexa Fluor 555 labelled non-silencing siRNA, with a scrambled sequence, which was also used as negative control. siRNA targeting Lamin A/C (SI03650332) served as positive control.
UV radiation
The UVB source was Philips TL20W/12 tubes emitting in the spectral range 280–370 nm with a main output between 305–320 nm. For UVA, a Medisun 2000-L tube (Dr Gröbel UV-Elektronik GmbH, Ettlingen, Germany; 340–400 nm) with Schott WG 305 cutoff filter (Mainz, Germany) was used. Exposure was performed in phosphate buffered saline (w/o NaHCO3). Sublethal radiation doses of UVA 6 J cm−2 (80 mW cm−2) and UVB 60 mJ cm−2 (1.44 mW cm−2) were used (Larsson et al, 2005). Non-irradiated controls (sham) were analysed in parallel.
Immunocytochemistry
Cells were fixed in 4% paraformaldehyde for 20 min at 4 °C, permeabilised in PBS (without Ca2+/Mg2+) containing 0.1% saponin and 5% fetal bovine serum (Appelqvist et al, 2011). The primary antibodies used were monoclonal anti-mouse FAP-α (1:50, Santa Cruz Biotechnology) and lactate dehydrogenase (LDH, 1:100, ab52488, Abcam) followed by a secondary Alexa Fluor-488 or -594 conjugated antibody (Molecular Probes, Eugene, OR, USA). Fibroblast activation protein-α positivity was quantified in 200 cells from randomly selected areas using a confocal microscope (LSM700, Zeiss, Heidelberg, Germany). Negative controls incubated without primary antibody did not stain.
Western blot analysis
Cell cultures were prepared for immunoblot as previously described (Appelqvist et al, 2011). Immunodetection was performed with monoclonal primary antibodies: FAP-α (1:1000, Santa Cruz Biotechnology), and TGF-β1 (1:1000, sc52893; Santa Cruz Biotechnology) followed by HRP-conjugated corresponding secondary antibodies. GAPDH (1:50000, NB300–328, Novus Biologicals, Littleton, CO, USA) was used as loading control and densitometric quantification was performed with Gel-Pro Analyzer 3.1 (Media Cybernetics, Rockville, MO, USA).
To analyse proteins secreted into the medium, supernatants were collected and concentrated using Amicon Ultra-4 Centrifugal filter (5 kDa; Millipore Corporation, Billerica, MA, USA) at 4000 × g for 30 min at 4 °C.
Cell invasion assay
Cells (5 × 103 per well) were cultured in the top chamber of Cultrex 96-well basement membrane extract (BME) Invasion Assay, coated with 0.5% BME solution consisting of laminin, collagen IV, entactin, and heparin sulfate proteoglycan. Conditioned medium from UVR exposed cells was obtained by collecting the PBS from irradiated cultures and diluting it 1:1 with 2 × culture medium. Invaded cells were dissociated, treated with Calcein-AM and the fluorescence was analysed at λex 485/λem 520 nm. The data were correlated to standard curves. In order to identify different cell types in the co-culture setup, fibroblasts and melanoma cells were pre-stained with Celltracker Green CMFDA (λex 490/λem 520 nm) or Celltracker Red CMTPX (λex 580/λem 600 nm) supplemented in medium (5 or 10 mol l−1, respectively; Invitrogen Molecular Probes, Carlsbad, CA, USA) at 37 °C for 45 min.
Migration assay
Confluent cultures were scratched using a p200 pipet tip (Liang et al, 2007). Debris were removed by washing the cells with PBS and fresh culture medium was added. Closure of the scratch was followed during 24 h and images captured and analysed.
Xenograft tumour model
Zebrafish embryos, 2 days post-fertilisation, were anestetised and dechorionated. Melanoma cells, prelabeled with DiI dye, were microinjected (∼100–500 cells per embryo) in the perivitelline space. Embryos with fluorescent cells outside the perivitelline space were discarded. The embryos were irradiated with (UVA 20 J cm−2, UVB 200 mJ cm−2) and incubated at 28 °C for 3 days in the presence of anti-FAP-α (1:50, Santa Cruz Biotechnology) when indicated. The metastatic ability was assessed by quantification of distally disseminated tumour cells. Bright field and fluorescent images were captured.
Nascent protein assay
Cells were pre-incubated in methionine- and serum-free media for 60 min and then incubated with 25 μ M Click-iT AHA (L-azidohomoalaine, C10102, Molecular Probes) with or without addition of recombinant TGF-β1 (10 ng ml−1) for 4 h, 37 °C. After cell lysis and sonication, the samples were centrifuged 15 000 × g for 5 min, 4 °C, followed by Click-IT ligation using Biotin conjugate and precipitation according to the manufacturer’s protocol (B10184, B10185, Molecular Probes, Waltham, MA, USA). The samples were further processed for immunoprecipitation of biotin using Pierce Protein Streptavidin beads (20357, Thermo Scientific, Waltham, MA, USA) according to Pierce Classic IP Kit manual (26146, Thermo Scientific).
Subcellular fractionation
Cells were lysed in subcellular fractionation buffer (10 mM KCl, 2 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 M DTT, PI cocktail (III), Hepes pH 7.4) on ice and passed through a 27-gauge needle, followed by centrifugation at 720 × g for 5 min to pellet nuclei. The supernatant was centrifuged 10 000 × g, 4 °C for 5 min to pellet mitochondria, which was discarded, and the supernatant was further centrifuged at 100 000 × g, 4 °C for 1 h, to collect the cytosol. The remaining pellet (containing the membrane fraction) was passed through a 25-gauge needle and re-centrifuged at 100 000 × g, 4 °C for 1 h, followed by resuspension in fractionation buffer. The cytosolic fraction was concentrated using Amicon Ultra 3 K, 4000 × g, 4 °C, 40 min.
Statistics
Statistical evaluations were performed with one-way or two-way ANOVA, followed by Dunnettś or Tukeyś multiple comparison post-test, respectively, for comparisons between groups using GraphPad Prism 6 Software (Version 6.05, La Jolla, CA, USA). All P-values below 0.05 were considered significant.
Results
Fibroblast activation protein-α is induced by UV radiation and downregulated during senescence in human melanocytes
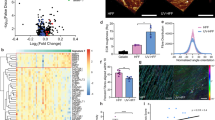
In reconstructed skin, both UVA and UVB clearly induced FAP-α expression in melanocytes (tyrosinase positive cells), whereas the surrounding keratinocytes were unaffected (Figure 1A). In line, ex vivo skin shows UV-induced FAP-α expression. Noteworthy, in ex vivo skin, the radiation-induced increase of FAP-α declined and was back at control levels after 24 h (Figure 1B). In mono-cultures of human melanocytes, essentially no FAP-α was located on the plasma membrane of non-irradiated cells (Figure 1C). Four hours after UVA or UVB radiation, the protein level of FAP-α was increased (Figure 1D) and located at the plasma membrane (Figure 1C). By subcellular fractionations, the location of FAP-α was confirmed to the membrane fraction (Figure 1E). However, no soluble form or release of FAP-α was detected in the supernatants of non-irradiated or irradiated melanocytes (Figure 1D). As presented in Figure 1F, the radiation-induced expression of FAP-α declined time dependently, which is in line with the results from ex vivo skin (Figure 1B).
UV radiation augments FAP- α expression. Samples were irradiated with UVA (6 J cm−2) or UVB (60 mJ cm−2). (A) Artificial skin constructs stained for FAP-α (green) and the melanocytic marker tyrosinase (red) in combination with DAPI-stained nuclei (blue) and bright field in the merged image 4 h after UVR. (B) Normal ex vivo skin stained 2 and 24 h after radiation showing representative merged images of FAP-α (green), the melanocytic marker tyrosinase (red) and DAPI-stained nuclei (blue). (C) Representative merged images of immunofluorescence of FAP-α (green), cytosolic marker protein (LDH, red) and bright field, and (D) immunoblotting of active and secreted FAP-α 4 h after UVR. GAPDH is used as loading control. (E) Immunoblotting of FAP-α, E-cadherin (E-cad, plasma membrane marker), and GAPDH (cytosolic marker) in subcellular fractions after radiation. (F) Quantification of FAP-α-positive melanocytes following radiation (mean±s.d.; n=4; *P<0.05).
Previously, we have established a model showing that the gene expression in growth-arrested senescent melanocytes resembles gene expression in nevi. Using this model, we were able to identify gene expression that marks a transition toward a malignant state (Orfanidis et al, 2016). Gene expression profiling of four matched young and senescent melanocytes, indicated 351 differentially expressed genes (minimum fold change of 2 and an adjusted P-value ⩽0.01), including downregulation of FAP-α (fold change 2.5 and adjusted P-value<0.01), in the senescent cultures (Figure 2A). The senescent state was confirmed by flattened cell morphology and positive β-galactosidase staining in senescent compared to young melanocytes (Figure 2B). Comparison of the FAP-α protein expression of young and senescent cultures confirmed decreased levels of FAP-α in the senescent cultures (Figure 2C).
Fibroblast activation protein- α is downregulated in senescence and found in a TGFB1-centered gene network. Human melanocytes from 4 donors were cultured for 3 days (young) and until they stopped dividing and displayed senescent morphology (minimum of 30 days). Gene expression was analysed using Affymetrix microarray. (A) Heat map presenting hierarchical clustering results (using Euclidean similarity measure with Wards linkage rule) from the microarray expression profile data and the expression of 351 selected genes. Gene expression ratios are displayed by applying progressively brighter shades of red (upregulated) or green (downregulated). (B) Staining of β-galactosidase in young and senescent melanocytes. Representative images are presented. (C) Immunoblotting of FAP-α in four young (Y) and the corresponding senescent (S) cultures. GAPDH used as an internal loading control. (D) Generated network of relationships between FAP, TGFB1 and cathepsins (CTS) using Ingenuity Pathway Analysis software. The network entitled ‘post-translational modifications, protein degradation, protein synthesis’ show TGFB1 as the most interconnected gene.
Bioinformatic comparison of gene expression in nevi and malignant melanoma
Using the data from a previously published data set of gene expression from 45 primary melanomas and 18 benign skin nevi (Talantov et al, 2005), we found upregulated expression of FAP-α (fold change; 2.8, P=1.48E-06, adjusted P-value=8.85E-06), cathepsin B (fold change; 5.9, P=5.71E-23, adjusted P-value=9.36E-20), cathepsin D (fold change; 2.2, P=9.88E-06, adjusted P-value=4.96E-05), and TGF-β1 (fold change; 1.6, P=0.003, adjusted P-value =0.007) in melanoma as compared to nevi. The intersection of the common differentially expressed genes (adjusted P-value of 0.05 and minimal fold change 1.5) from the above data sets (melanoma vs benign nevi and young vs senescent melanocytes) was used to identify lysosome-associated genes that are commonly up- or downregulated in young melanocytes and melanomas. Applying IPA software of our gene set of interest generated a network of relationships between FAP, TGFB1 and cathepsins. The network category ‘post-translational modifications, protein degradation, protein synthesis’ showed TGFB1 as one of the most interconnected genes (Figure 2D).
TGF-ß1 promotes cell migration and invasion through FAP-α expression
To identify TGF-β1-producing cell types, conditioned media was collected and immunoblotted. UVA and UVB stimulated extracellular release of TGF-β1 from both young and growth arrested melanocyte cultures, whereas TGF-β1 levels were below detection limits in the respective controls. In melanoma cell lines, we found spontaneous production from control cultures that was enhanced after UVR (Table 1). However, no TGF-β1 was released from human keratinocytes or fibroblasts either spontaneously or after radiation (Table 1). To study the relationship between TGF-β1 and FAP-α expression, conditioned media from four different radiated primary melanoma cell lines was added to the corresponding non-irradiated cultures. Such treatment stimulated the expression of FAP-α (Figure 3A). By adding anti-TGF-β1 antibodies, it was demonstrated that the increase was TGF-β1 dependent (Figure 3A). Supplementation of recombinant TGF-β1 to non-irradiated cultures also increased FAP-α expression (Supplementary Figure S1A and B). We found that the rise in FAP-α level was due to TGF-β1-controlled de novo synthesis of the protein (Supplementary Figure S1C). In a similar manner, conditioned media from irradiated melanoma cultures added to fibroblasts stimulated TGF-β1-dependent FAP-α expression in these cultures (Figure 3B).
Fibroblast activation protein- α expression is regulated by TGF- β 1 and stimulates cell migration and invasion. Primary melanoma cells (FM55P, WM115, WM278, WM793) were irradiated with UVA (6 J cm−2) or UVB (60 mJ cm−2) and when indicated treated with anti-TGF-β1 (200 ng ml−1). Conditioned media were collected and supplemented to the respective non-irradiated primary melanoma cells (FM55P, WM115, WM278, WM793) or normal skin fibroblasts (FB1–4). Quantification of FAP-α expression in (A) primary melanoma cells and (B) fibroblasts (n=4; *P<0.05). Estimation of migration in (C) primary melanoma and (D) fibroblast. The results are presented as the percentage of cells that invaded the scratch during 24 h (n=4; *P<0.05). Invasion of (E) primary melanoma and (F) fibroblasts using Cultrex BME. The results are presented as the percentage of invading cells relative to the total number of cells (n=4; *P<0.05). (G) Time-dependent invasion of co-cultures of primary melanoma cells (labelled with Celltracker Red CMTPX) and fibroblasts (labelled with Celltracker Green CMFDA) (n=4). *P<0.05 compared to time 0 h.
Next, we assessed the effect of conditioned media from radiated cultures on cell migration and invasion. Melanoma cell lines, as well as fibroblasts, were found to enhance cell growth and migration upon addition of conditioned media (Figure 3C and D). The effect was reduced by inhibition of TGF-β1. Invasion through BME-coated inserts was also stimulated in a TGF-β1-dependent manner when melanoma cultures were exposed to conditioned media from irradiated cultures (Figure 3E). Accordingly, the invasive capacity of fibroblasts cultured in conditioned media was increased (Figure 3F). These results were confirmed by addition of recombinant TGF-β1, which augmented migration and invasion in melanoma cell lines as well as in fibroblasts (Supplementary Figure S2A–D). In order to investigate if senescent melanocytes could elude the growth-arrested state by UVR, senescent melanocytes cultures were irradiated. No significant effect on cell migration or invasion was detected after 24 h or after 1 week (Supplementary Figure S3A–D). However, increased migration and invasion was detected 1 week after addition of recombinant TGF-β1, implying its ability to break senescence in the model system (Supplementary Figure S3).
Finally, we compared the time dependence for invasion in co-cultures of fibroblasts and melanoma cells. To distinguish between the two cell types, fibroblasts and melanoma cells were pre-stained with Celltracker Green and Celltracker Red, respectively. The fibroblasts launched the invasion and penetrated BME several hours before the melanoma cells (Figure 3G) and cell invasion was significantly stimulated following both UVA and UVB radiation (Figure 3G).
TGF-β1 secretion is controlled by cathepsin activity
To assess if release and activation of TGF-β1 is cathepsin-dependent, we inhibited the cysteine cathepsins B and K using Ca-074 Me and Calbiochem inhibitor II, respectively, and inhibited the aspartic cathepsin D using anti-cathepsin D antibodies. Cathepsin inhibition caused reduced release of TGF-β1 from all four primary melanoma cell lines tested (Figure 4A). Altering the lysosomal function by increasing the lysosomal pH using NH4Cl also diminished TGF-β1 release (Supplementary Figure S2E). Moreover, conditioned media from melanoma cells, pretreated with cathepsin inhibitors and irradiated before added to fibroblasts, caused diminished invasion as compared to controls (Figure 4B). Thus, cathepsins participate in the activation and release of TGF-β1.
Cathepsins act upstream of TGF- β 1 and UVR stimulates FAP- α -dependent invasion. (A and B) Primary melanoma cells (FM55P, WM115, WM278, WM793) were irradiated with UVA (6 J cm−2) or UVB (60 mJcm−2) and when indicated, treated with inhibitors of cathepsin B (CA-074 Me; 1 μ M), anti-cathepsin D (anti-cat D; 42 μg ml−1), cathepsin K (Inh II; 10 μ M). The conditioned media were collected. (A) TGF-β1 release to the medium was analysed by immunoblot. Samples were collected from equal number of cells and representative blots are shown (mean±s.d.; n=4; *P<0.05). (B) Invasion of fibroblasts (FB1–4) through Cultrex BME after supplementation with the conditioned media from melanoma cultures. (C and (D) FAP-α was depleted in primary melanomas (WM115, WM793, WM278 and FM55P) with two different siRNA sequences (S1 and S2; 1 μg of each). (C) Immunoblotting of FAP-α. One representative blot out of four is shown. (D) Invasion assay *P<0.05. (E and F) FAP-α was depleted in human skin fibroblasts (FB1–4) with two different siRNA sequences (S1 and S2; 1 μg of each). (E) Immunoblotting of FAP-α. One representative blot out of four is shown. (F) Invasion assay *P<0.05. (G and H) Invasion assay in Zebra fish embryos injected with primary melanoma cells (FM55P; 100–500 cells, labelled in vitro with DiI dye) in the perivitelline space and when indicated anti-FAP-α (20.6 μg ml−1) was added. After microinjection, the embryos were irradiated with UVA (20 J/cm−2) or UVB (200 mJ cm−2) and the distally posterior disseminated tumour cells were quantified after 3 days. (G) Bright field and fluorescent images illustrating tumour cell dissemination *P<0.05. (H) Quantification of distally posterior fluorescent cells. Values shown are mean±s.d. of 3 independent experiments.
FAP-α promotes cell migration and invasion
To establish that FAP-α is the primary actor during TGF-β1 stimulated invasion, we depleted FAP-α expression in melanoma cells using siRNA (Figure 4C). Conditioned media from irradiated cultures (not treated with siRNA) were added to the siRNA-treated cultures, which resulted in decreased invasion of FAP-α-depleted cultures (Figure 4D). In line, the invasion decreased when inhibiting FAP-α with Gly-PhP(OPh)2 or anti-FAP-α (Supplementary Figure S4A). The corresponding experiment performed in fibroblasts from four different individuals showed equivalent results (Figure 4E and F and Supplementary Figure S4B). Finally, to establish the importance of UVR in invasion mediated by FAP-α, we performed invasion experiments using Zebra fish embryos in a xenograft tumour model. Primary melanoma cells (FM55P) were injected in the perivitelline space, and following UV radiation, the melanoma cells were allowed to invade for 48 h with or without addition of anti-FAP-α. The UVR significantly increased the number of disseminated melanoma cells to the posterior part in a FAP-α-dependent manner (Figure 4G and H). Taken together, our results show that cathepsins are upstream the activation of TGF-β1 in malignant melanoma cell lines. Transformation growth factor-β1 is a central factor responsible for the increase of FAP-α protein level both by signalling in an autocrine manner to melanoma cells and a paracrine manner to fibroblasts. Fibroblast activation protein-α expression has great impact on melanoma invasiveness.
Discussion
The initial steps of metastasis involves invasion through neighboring tissues, which in turn requires the degradation of the ECM and the basal membrane. In this study, the impact of UVR for increased melanoma dissemination was investigated. We present an axis of cathepsin activity, release of TGF-β1 and the expression of FAP-α, responsible for UVR-stimulated cell migration and invasion. The expression and subcellular localisation of cathepsins changes during cancer progression and they have been shown to be causally involved in various aspects of tumourigenesis, including ECM remodelling and metastasis (Fonovic and Turk, 2014; Olson and Joyce, 2015). Moreover, cathepsin overexpression is often associated with tumour aggressiveness and poor prognosis (Vasiljeva et al, 2007; Palermo and Joyce, 2008). We have previously demonstrated that melanoma is associated with altered lysosomal function, cathepsin-dependent invasion and proliferative stimulation (Eding et al, 2015). Herein, inhibition of cathepsins B, D or K in primary melanoma cells, caused decreased TGF-β1 release. In accordance, Yin et al (2012), previously showed that cathepsin B could regulate TGF-β signalling in melanoma cells. These results indicate that the main tumour-promoting function of cathepsins in the melanoma cells is not proteolysis of ECM per se, but rather activation of TFG-β1, promoting the upregulation of FAP-α-mediated cancer cell dissemination.
Interactions between cancer cells and stromal cells are important for the development and progression of many cancers. We found that the normal fibroblasts alone did not invade BME-matrices but needed the initiation from the melanoma cells. Transforming growth factor-β1 is a well-known activator of cancer associated fibroblasts (Löhr et al, 2001) and we found TGF-β1-dependent FAP-α expression as a sign of fibroblast activation in co-culture with melanoma cells and after supplementation of conditioned media from melanoma cultures. Previous studies have suggested that cancer cell growth could be inhibited by co-culture with normal fibroblasts (Alkasalias et al, 2014). Our results indicated that UVR interrupts such preventive capacity of the fibroblasts. Interestingly, it has been shown that UVR impairs the TGF-β signalling in human skin fibroblasts by reducing TGF-β receptor II gene expression, which results in decrease of type I procollagen synthesis (He et al, 2014). Such mechanism would promote a switch in the fibroblast characteristics from synthesising ECM components into a state where FAP-α expression mediates the degradation of ECM. Although both melanoma cells and fibroblasts expressed FAP-α, we found that fibroblasts led and facilitated the invasion of melanoma cells, which is in accordance with previous findings (Yin et al, 2012). A similar mechanism for invasion has been reported for squamous cell carcinoma (Gaggioli et al, 2007). Moreover, we have previously shown that UVR exposed melanoma cells produce several soluble factors including TGF-β1 (Wäster et al, 2011). A TGF-β-responsive cis-regulatory element was recently identified in the FAP promoter, indicating the central role of TGF-β for FAP-α expression (Tulley and Chen, 2014), and here we confirm de novo synthesis of FAP-α. Melanoma cells secrete TGF-β1 spontaneously, while melanocytes only showed secretion after UVR. Thus, release of TGF-β1 from melanocytes suggests ability to affect surrounding cells in the case of tissue damage. Accordingly, increased FAP-α expression facilitates ECM degradation in keloid scars (benign fibroproliferative reticular dermal lesions), indicating a general role for FAP-α during ECM remodelling (Dienus et al, 2010).
Although UVR is a well-documented risk factor for development of malignant melanoma, very few experimental studies have addressed the relationship between UVR and induction of epigenetic changes. Nevi are growth arrested melanocytic lesions often harboring mutations in BRAF or NRAS (Ross et al, 2011). The initiation of a malignant transformation is therefore hypothesised to include the epigenetic breakage of the senescent state (Tsao et al, 2003). Recently, we showed that the gene expression in growth-arrested senescent melanocytes resembles that found in nevi and created a cell culture model system to identify gene expression that marks a transition toward a malignant state (Orfanidis et al, 2016). The ability of recombinant TGF-β1 to stimulate cell growth and invasion in senescent melanocyte cultures implies the potential involvement of the studied mechanism for evasion of senescence in nevi. When the gene expression in melanomas was compared with that of nevi, a network involving TGF-β1, FAP-α and lysosome-associated genes was found using IPA. Transforming growth factor-β1 was identified as the most interconnected gene. Interestingly, Tas et al (2014) have suggested that the serum levels of TGF-β1 have diagnostic, predictive and possible prognostic roles in melanoma patients (), indicating its clinical significance.
The UV exposure of reconstructed skin, as well as skin ex vivo, showed FAP-α upregulation in melanocytes, indicating that the finding has physiological relevance. Consequently, sun exposure of a primary melanoma of the skin could initiate invasion and further progression of the tumour, emphasising the necessity of research in the area. Future strategies for melanoma prevention need to focus on more efficient protection against UV-induced damage. Extracellular matrix degrading enzymes, including matrix metalloproteinases and cathepsins have received much attention as potential future therapeutic targets (Deryugina and Quigley, 2006). However, their applicability is limited due to their widespread expression. In contrast, targeting of FAP-α might be an attractive alternative since it is highly expressed in tumour stroma and absent in normal adult tissue (O'Brien and O'Connor, 2008). The clinical relevance of FAP-α inhibition for possible identification and treatment of advanced disease is underlined by our findings on tumour dissemination in zebrafish embryos. So far, FAP-α inhibitors have not demonstrated sufficient efficacy in clinical trials (reviewed in Jiang et al, 2016). Instead, the development of better FAP-α blockers are required, and combination strategies addressing the heterogeneity of melanomas. Recently, anticancer drugs, encapsulated by cleavable amphiphilic peptide assembled into nanocarriers, were shown to disassemble upon cleavage by FAP-α, resulting in rapid and efficient release of encapsulated drugs specifically at the tumour sites (Ji et al, 2016).
In conclusion, our study indicates UVR as an initiating factor engaging an axis from cathepsins via TGF-β1 release, which results in increased protein expression of FAP-α in both malignant cells and the surrounding stroma (Figure 5). Fibroblast activation protein-α causes ECM degradation enabling cell migration and invasion as well as tumour dissemination in zebrafish. Thus, UV exposure might be involved in the early migration and invasion process of melanoma cells, emphasising the important role of UV protection not only to prevent melanoma development, but also to avoid migration and invasion of an established disease.
Intercellular signalling between melanoma cells and stromal fibroblasts. UV initiates an axis from cathepsins that stimulates release and activation of TGF-β1. Transforming growth factor-β1 transactivates the FAP-α gene and increase the expression of FAP-α, which causes ECM degradation enabling cell migration and invasion.
Change history
08 August 2017
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Alkasalias T, Flaberg E, Kashuba V, Alexeyenko A, Pavlova T, Savchenko A, Szekely L, Klein G, Guven H (2014) Inhibition of tumor cell proliferation and motility by fibroblasts is both contact and soluble factor dependent. Proc Natl Acad Sci USA 111: 17188–17193.
Appelqvist H, Nilsson C, Garner B, Brown AJ, Kagedal K, Ollinger K (2011) Attenuation of the lysosomal death pathway by lysosomal cholesterol accumulation. Am J Pathol 178 (2): 629–639.
Bolstad BM, Irizarry RA, Astrand M, Speed TP (2003) A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19 (2): 185–193.
Deryugina EI, Quigley JP (2006) Matrix metalloproteinases and tumour metastasis. Cancer Metastasis Rev 25 (1): 9–34.
Dienus K, Bayat A, Gilmore BF, Seifert O (2010) Increased expression of fibroblast activation protein-alpha in keloid fibroblasts: implications for development of a novel treatment option. Arch Dermatol Res 302 (10): 725–731.
Eding CB, Domert J, Wäster P, Jerhammar F, Rosdahl I, Ollinger K (2015) Melanoma growth and progression after ultraviolet A irradiation: impact of lysosomal exocytosis and cathepsin proteases. Acta Derm Venereol 95 (7): 792–797.
Fonovic M, Turk B (2014) Cysteine cathepsins and extracellular matrix degradation. Biochim Biophys Acta 1840 (8): 2560–2570.
Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, Sahai E (2007) Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol 9 (12): 1392–1400.
Gilmore BF, Lynas JF, Scott CJ, McGoohan C, Martin L, Walker B (2006) Dipeptide proline diphenyl phosphonates are potent, irreversible inhibitors of seprase (FAPalpha). Biochem Biophys Res Commun 346 (2): 436–446.
He T, Quan T, Fisher GJ (2014) Ultraviolet irradiation represses TGF-beta type II receptor transcription through a 38-bp sequence in the proximal promoter in human skin fibroblasts. Exp Dermatol 23 (Suppl 1): 2–6.
Huber MA, Kraut N, Park JE, Schubert RD, Rettig WJ, Peter RU, Garin-Chesa P (2003) Fibroblast activation protein: differential expression and serine protease activity in reactive stromal fibroblasts of melanocytic skin tumours. J Invest Dermatol 120 (2): 182–188.
Ji T, Zhao Y, Ding Y, Wang J, Zhao R, Lang J, Qin H, Liu X, Shi J, Tao N, Qin Z, Nie G, Zhao Y (2016) Transformable peptide nanocarriers for expeditious drug release and effective cancer therapy via cancer-associated fibroblast activation. Angew Chem Int Ed Engl 55 (3): 1050–1055.
Jiang GM, Xu W, Du J, Zhang KS, Zhang QG, Wang XW, Liu ZG, Liu SQ, Xie WY, Liu HF, Liu JS, Wu BP (2016) The application of the fibroblast activation protein α-targeted immunotherapy strategy. Oncotarget 7 (22): 33472–33482.
Larsson P, Andersson E, Johansson U, Ollinger K, Rosdahl I (2005) Ultraviolet A and B affect human melanocytes and keratinocytes differently. A study of oxidative alterations and apoptosis. Exp Dermatol 14 (2): 117–123.
Lazar-Molnar E, Hegyesi H, Toth S, Falus A (2000) Autocrine and paracrine regulation by cytokines and growth factors in melanoma. Cytokine 12 (6): 547–554.
Li L, Fukunaga-Kalabis M, Herlyn M (2011) The three-dimensional human skin reconstruct model: a tool to study normal skin and melanoma progression. J Vis Exp 54: pii: 2937.
Liang CC, Park AY, Guan JL (2007) In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2: 329–333.
Löhr M, Schmidt C, Ringel J, Kluth M, Müller P, Nizze H, Jesnowski R (2001) Transforming growth factor-beta1 induces desmoplasia in an experimental model of human pancreatic carcinoma. Cancer Res 61: 550–555.
O'Brien P, O'Connor BF (2008) Seprase: an overview of an important matrix serine protease. Biochim Biophys Acta 1784 (9): 1130–1145.
Olson OC, Joyce JA (2015) Cysteine cathepsin proteases: regulators of cancer progression and therapeutic response. Nat Rev Cancer 15 (12): 712–729.
Orfanidis K, Wäster P, Lundmark K, Rosdahl I, Öllinger K (2016) Evaluation of tubulin β-3 as a novel senescence-associated gene in melanocytic malignant transformation. Pigment Cell Melanoma Res 26: 243–254.
Palermo C, Joyce JA (2008) Cysteine cathepsin proteases as pharmacological targets in cancer. Trends Pharmacol Sci 29 (1): 22–28.
Ross AL, Sanchez MI, Grichnik JM (2011) Nevus senescence. ISRN Dermatol 2011: 642157.
Scanlan MJ, Raj BK, Calvo B, Garin-Chesa P, Sanz-Moncasi MP, Healey JH, Old LJ, Rettig WJ (1994) Molecular cloning of fibroblast activation protein alpha, a member of the serine protease family selectively expressed in stromal fibroblasts of epithelial cancers. Proc Natl Acad Sci USA 91 (12): 5657–5661.
Talantov D, Mazumder A, Yu JX, Briggs T, Jiang Y, Backus J, Atkins D, Wang Y (2005) Novel genes associated with malignant melanoma but not benign melanocytic lesions. Clin Cancer Res 11 (20): 7234–7242.
Tas F, Karabulut S, Yasasever CT, Duranyildiz D (2014) Serum transforming growth factor-beta 1 (TGF-beta1) levels have diagnostic, predictive, and possible prognostic roles in patients with melanoma. Tumour Biol 35 (7): 7233–7237.
Tsao H, Bevona C, Goggins W, Quinn T (2003) The transformation rate of moles (melanocytic nevi) into cutaneous melanoma: a population-based estimate. Arch Dermatol 139 (3): 282–288.
Tulley S, Chen WT (2014) Transcriptional regulation of seprase in invasive melanoma cells by transforming growth factor-beta signaling. J Biol Chem 289 (22): 15280–15296.
Vasiljeva O, Reinheckel T, Peters C, Turk D, Turk V, Turk B (2007) Emerging roles of cysteine cathepsins in disease and their potential as drug targets. Curr Pharm Des 13 (4): 387–403.
Venza M, Visalli M, Beninati C, De Gaetano GV, Teti D, Venza I (2015) Cellular mechanisms of oxidative stress and action in melanoma. Oxid Med Cell Longev 2015: 481782.
Wäster P, Rosdahl I, Gilmore BF, Seifert O (2011) Ultraviolet exposure of melanoma cells induces fibroblast activation protein-alpha in fibroblasts: Implications for melanoma invasion. Int J Oncol 39 (1): 193–202.
Yin M, Soikkeli J, Jahkola T, Virolainen S, Saksela O, Holtta E (2012) TGF-beta signaling, activated stromal fibroblasts, and cysteine cathepsins B and L drive the invasive growth of human melanoma cells. Am J Pathol 181 (6): 2202–2216.
Acknowledgements
The melanoma cells were kindly provided by Professor Meenhard Herlyn, Wistar Institute, Philadelphia, USA and the human skin samples were from Capio-Lund Clinic, Lund, Sweden. The zebrafish embryos were kindly provided by Lasse Jensen and Zaheer Ali, Division of Cardiovascular Medicine, Linköping University, Sweden. This study was supported by grants from the Swedish Research Council, the Welander-Finsen Foundation, Östgötaregionens Cancer Foundation, the Swedish Cancer Society, the County Council of Östergötland, and from the Olle Engkvist Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Wäster, P., Orfanidis, K., Eriksson, I. et al. UV radiation promotes melanoma dissemination mediated by the sequential reaction axis of cathepsins–TGF-β1–FAP-α. Br J Cancer 117, 535–544 (2017). https://doi.org/10.1038/bjc.2017.182
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2017.182
Keywords
This article is cited by
-
Melanocytic nevi in sentinel lymph nodes: association with cutaneous nevi and clinical relevance in patients with cutaneous melanomas
Journal of Cancer Research and Clinical Oncology (2022)
-
Application of adipose-derived stem cells in photoaging: basic science and literature review
Stem Cell Research & Therapy (2020)
-
Pro-tumorigenic roles of fibroblast activation protein in cancer: back to the basics
Oncogene (2018)