Abstract
Background:
The follow-up after abnormal Pap smear and negative colposcopy is not clearly defined. This study aimed at investigating the role of hr-HPV testing in the management of abnormal Pap test and negative colposcopy for Cervical Intraepithelial Neoplasia grade 2 or worse (CIN2+).
Methods:
The study enroled 1029 women with abnormal screening cytology (years 2006–2010) and negative colposcopy for CIN2+, which subsequently performed a hr-HPV test. Incident CIN2+ lesions were identified through linkage with cancer registry, hospital discharge records, neoplastic pathology reports and the archive of screening programme (2006–2011).
Results:
During the follow-up, the cohort developed 133 CIN2+ lesions; only one among hr-HPV-negative women. The probability of developing CIN2+ on follow-up time was 0.44% (95% confidence interval (CI) 0.1–3.1) and 41.8% (95% CI 31.8–53.5) for hr-HPV-negative women and hr-HPV-positive women, respectively. A woman with a positive hr-HPV test had about 105 times higher probability of developing a CIN2+ lesion than a woman with a negative hr-HPV test (hazard ratio (HR)=104.5, 95% CI 14.5–755.1), adjusted for index Pap test result, age and cervix squamocolumnar junction visualisation.
Conclusion:
Our results confirm that hr-HPV testing is able to select the real group of women at risk of developing CIN2+ lesions in the follow-up of abnormal cytology and first negative colposcopy.
Similar content being viewed by others
Main
The success of any cervical cancer prevention programme relies on the interplay between the underlying risk factors and the type of screening programme: an organised programme is more effective than an opportunistic programme. Furthermore, the existence and quality of the field and laboratory facilities for screening and diagnostic follow-up, as well as the facilities available for treating diagnosed lesions, are key elements of any screening programme. Monitoring the patient path or ‘chain of action’ for each patient with an abnormal screening result is of crucial importance (Nygård, 2011). In Italy, despite the availability of well-functioning screening programmes spreading all over the country, the follow-up protocol after abnormal Pap test and negative colposcopy is not clearly defined.
While existing uniformity of indications in case of cytological abnormalities and diagnosis of Cervical Intraepithelial Neoplasia grade 2 or more severe (CIN2+) (excisional therapy), there is no uniformity of indications for the abnormal cytology. The European guidelines (Jordan et al, 2008; Arbyn et al, 2010) suggest the hr-HPV test in the follow-up after negative colposcopy only for some cytological results: Atypical Squamous Cells of Undetermined Significance (ASC-US), Low-Grade Squamous Intraepithelial Lesion (LSIL) and Atypical Squamous Cells cannot exclude a High-Grade Lesion (ASC-H). However, these indications are not universally applied in the Italian cervical cancer screening programmes and in majority of the programmes the follow-up of abnormal Pap test and negative colposcopy is usually managed according to the gynaecologist judgment.
In the Italian screening programmes, the positive predictive value (PPV) for CIN2+ lesions differs among programmes (from 2.8% to 52.7%) and in the vast majority of women, the colposcopy assessment results negative for high-grade lesions (Ancona et al, 2012). Unfortunately, colposcopy does not have optimal sensitivity for CIN2+ lesions (Milne et al, 1999; Jeronimo and Schiffman 2006). For women referred to colposcopy after abnormal screening results, it seems unlikely that a single negative colposcopy is sufficient to provide reassurance against concurrent CIN2+ over the next 3 years (Mesher et al, 2011).
The findings of long-term prospective cohort studies and randomised clinical trials have shown that hr-HPV testing is effective in reducing the incidence of cervical cancer in women aged 30 years and older (Dillner et al, 2008; Ronco et al, 2010), and in particular of adenocarcinoma, the precursors of which are often missed by cytological methods (Castle et al, 2010a). On the other hand,a woman’s risk of CIN grade 3 (CIN3) or cancer after a negative test for hr-HPV is very low for 5 years (Dillner et al, 2008; Mesher et al, 2010; Schiffman et al, 2011). Real-life clinical follow-up does not necessarily have the same outcome as a carefully controlled research trial, due to differences in sampling and analysis of specimens. Diagnostic practices also vary between countries and laboratories, and compliance with follow-up guidelines in a research study group is different from that in a population-based screening referral group (Origoni et al, 2012c). These findings lead to the introduction of hr-HPV testing in the follow-up of women with an abnormal cytology who were CIN2+ negative at the first assessment. The incorporation of hr-HPV testing in follow-up post-colposcopy can potentially reduce the colposcopy workloads (Bowring et al, 2012).
In the organised screening programme of the District of Florence, Italy, hr-HPV testing has been introduced in post-colposcopy follow-up of women with abnormal cytology and first assessment by colposcopy negative for CIN2+ lesions since 2006. According to the natural history of cervical cancer, only women with persistent hr-HPV infection are at major risk to develop a CIN. This knowledge prompted us to perform hr-HPV testing after 1 year from the initial assessment for cytological abnormalities.
The present study evaluated the efficacy of hr-HPV test in the surveillance of women with abnormal Pap test (ASC-US/hr-HPV positive, LSIL, ASC-H, High-Grade Squamous Intraepithelial Lesion (HSIL), Atypical Glandular Cells (AGC)) and negative colposcopy in current clinical practice.
Materials and methods
Study population
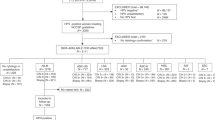
The Cancer Prevention and Research Institute (ISPO) has been running an organised population-based cervical screening in the Florence District since 1980. Cervical cancer screening is offered to all women aged 25–64 years who live in the Florence district and who are invited by mail to perform a Pap test every 3 years. Negative results are mailed to participants with a recommendation to repeat screening in 3 years. Non-respondents to the first invitation receive a reminder within 6 months. The 2001 Bethesda system terminology (TBS 2001) is used for cytologic classification (Solomon et al, 2002). Women with abnormal Pap test (Atypical Squamous Cells of Undetermined Significance or more severe, ASC-US+) are invited to perform a hr-HPV test or a colposcopy in according to the current protocol. The women diagnosed with ASC-US are invited to have a hr-HPV test; the hr-HPV-positive women are referred to colposcopy and hr-HPV-negative women returned to routine cytological screening interval. All women with other categories of abnormal cytology (ASC-H, AGC, LSIL, HSIL and cancer) are referred to immediate colposcopy at the ISPO clinic. Atypical glandular findings have been categorised following the TBS 2001 as to cell type origin (endocervical or endometrial), as the clinical management may vary significantly. Consequently, in this study we take into account only atypical endocervical or glandular cells referred to colposcopy. Women with abnormal Pap test and final histological diagnosis of CIN2+ lesions are recommended for an excisional treatment.
All data (cytology, hr-HPV test, colposcopy, histology and treatment) are recorded in ISPO’s archives.
In the present study, we selected all women aged 25–64 years with the first abnormal Pap test of screening (index Pap test) performed during the years 2006–2010 within the Florence Cervical Screening Program. Among women compliant to colposcopy, we selected all women with a negative colposcopy assessment for CIN2+ (Figure 1). We defined a woman compliant to colposcopy if it was performed within 6 months either from the abnormal, ASC-US+, index Pap Test or the positive hr-HPV triage test for ASC-US. The ISPO follow-up protocol had been tailored to the referral cytologic results. In accordance with the European guidelines (Jordan et al 2008; Arbyn et al 2010), an hr-HPV test at 12 months is recommended in case of ASC-US, ASC-H and LSIL colposcopy-negative women. Having, the European guidelines, no suggestions in the management of AGC and HSIL colposcopy-negative women, we recommended an hr-HPV test at 6 months for both these categories. Independently of initial cytologic results, if the hr-HPV test turned positive, a new colposcopy was suggested.
Women performing a hr-HPV test in the follow-up time, according to the protocol, were recruited for this cohort study. Moreover women who did not have at least one test (cytology, colposcopy or hr-HPV test) in the subsequent years of follow-up time were excluded from the cohort.
So to be included in the study cohort, each women had to have: (1) an abnormal cytology (index Pap test), (2) a first negative colposcopy assessment for CIN2+, (3) an hr-HPV test within 1 year of the negative colposcopy and at least another test (cytology, colposcopy or hr-HPV test) in the subsequent follow-up period.
For identifying incident CIN2+ lesions, the follow-up of the cohort started from the date of post-colposcopy hr-HPV test and ended with the date of occurrence of a CIN2+ lesion or with the date of last follow-up test performed. In any case, the follow-up was closed on 31 December 2011. Incident CIN2+ lesions were identified through linkage of the cohort with Tuscan Cancer Registry (2006–2008), neoplastic pathology reports (2008–2011) or the archives of the Florence Screening Program (2006–2011). Furthermore, we checked for completeness of cases through linkage with regional information flows of hospital discharge records (2006–2011).
hr-HPV testing
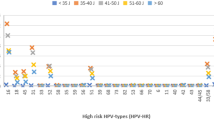
hr-HPV DNA testing was performed with Hybrid Capture 2 (HC2, Qiagen, Hilden, Germany). Only the high-risk group of probes (Probe B), designed to detect hr-HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68 was used (Terry et al, 2001). The hr-HPV testing was performed with the automated HC2 assay, Rapid Capture System and women were considered as hr-HPV positive if the relative light units/cutoff (RLU/CO) ratio was ⩾1, as recommended by the manufacturer. These RLU/CO ratios also provided an estimate of the amount of hr-HPV DNA present in the specimens, that is, the semiquantitative viral load.
In addition, the ISPO laboratory participated annually in two different external quality assessment (EQA) programmes received a certification of good quality for the hr-HC2 test results in both cases (QCMD–Quality Control for Molecular Diagnostics– and DicoCare).
Statistical analysis
We estimated the risk of developing CIN2+ lesions according to post-colposcopic hr-HPV test result using the Kaplan–Meier method. We estimated the hazard ratios (HR) either in univariate analysis or in multivariate analysis using Cox model. When the risk of developing CIN2+ lesions for hr-HPV-positive women was compared with hr-HPV-negative women a time period of 5 years of follow-up was considered, whereas when the role of some predictor covariates in hr-HPV-positive women was concerned a 3-year period of follow-up was considered.
As predictive factors for developing CIN2+ lesions, we included age, cervix squamocolumnar junction (SCJ) visualisation, semiquantitative viral load and index abnormal cytology. Age at hr-HPV test was divided in two classes <35 years or ⩾35 years. The semiquantitative viral load was classified in three categories: between 1 and 9 RLU/CO, between 10 and 99 RLU/CO or 100 or more RLU/CO. Squamocolumnar junction was classified in two categories: visible (ectocervical or endocervical) and not visible. The index Pap test was classified in ASC-US hr-HPV+, ASC-H, AGC, LSIL, HSIL or worse.
The Student's t-test was used to compare means and the chi-square test was used to compare proportions. Log-rank test was used to compare strata of covariates in Kaplan–Meier analysis. Statistically significant differences were set at P<0.05.
A receiver-operating characteristic (ROC) curve was used to evaluate, among hr-HPV-positive women, the role of semiquantitative viral load in detecting CIN2+ lesions.
Statistical analysis was performed using STATA software (http://www.stata.com) version 11.
Results
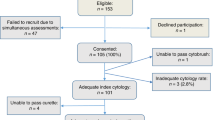
We selected 1775 women with the first abnormal Pap test of screening (ASC-US/hr-HPV positive, ASC-H, AGC, LSIL, HSIL+) and subsequent negative colposcopy for CIN2+ lesions (Figure 1). They represented 73.6% (1775 out of 2412) of all colposcopy assessment performed for the first abnormal Pap test (N=2703) performed in the period 2006–2010 in the Florence Cervical Screening Program. Treatment was recommended for 637 women with a CIN2+ lesion.
In all, 418 women were not eligible because they didn’t reach the full time necessary to evaluate compliance within the end of study. From the remaining 1357 eligible women, 328 were not compliants to hr-HPV test (328 out of 1357=24.2%) within 1 year (maximum 18 months) after the initial colposcopy and were subsequently excluded.
The hr-HPV test results by age class for the remaining cohort of 1029 women are reported in Table 1. Out of them, 63.1% were older than 34 years (649 out of 1029) and 57.7% resulted positive to hr-HPV. Positive hr-HPV test was significantly more frequent among women younger than 35 years as compared with older women (62.9% vs 54.7%; P=0.01).
Finally, we also excluded 197 (19.1%; 197 out of 1029) women who did not perform at least one test (cytology, colposcopy or hr-HPV test) after the first hr-HPV test within the end of the follow-up period.
The final cohort of 832 women was our study population. The median time from colposcopy and first hr-HPV test of follow-up was 298 days. The median age at hr-HPV test was 39 years (range 25–66 years) and 63.8% of the women were older than 34 years (531 out of 832). hr-HPV test was positive for 67.3% of them (560 out of 832). In the final cohort, as in the total cohort, positive hr-HPV test was significantly more frequent among women under 35 years of age as compared with older women (73.4% vs 63.8%; P=0.005; Table 2).
Table 2 shows the distribution of hr-HPV test results according to index Pap test and age class for the final cohort. The distribution of positive hr-HPV test was similar among index Pap test classes in women younger than 35 years (P=0.59), whereas in older women, the proportion of positive hr-HPV test was much more variable: very low (28.6%) for AGC, intermediate value (mean=63.9%) for ASC-US/hr-HPV positive, ASC-H and LSIL and high (84.8%) for HSIL. Women with the index Pap test as ASC-US/hr-HPV+ showed after 1 year a clearance of infection in 30% (46 out of 156). Moreover, ∼67% (450 out of 676) of women with the other cytological abnormalities (ASC-H, LSIL, HSIL, AGC) were hr-HPV positive 1 year after the first negative colposcopy.
Overall in 16.2% of women, the SCJ visualisation was not visible at colposcopy, without any statistically significant difference among hr-HPV-negative and hr-HPV-positive women (19.5% vs 14.6%, P=0.08).
The cohort of women positive to hr-HPV was followed for a median time of 1.25 years (0.31 and 2.35 years for 25° and 75° percentiles, respectively), whereas in women negative to hr-HPV the median follow-up time was 2.23 years (1.47 and 2.75 years for 25° and 75° percentiles, respectively). The follow-up time at 25° percentile of ‘hr-HPV-positive cohort’ was as low as 0.31 years (113 days) because the last test performed could be the colposcopy for assessment of positive hr-HPV test.
During the follow-up, the cohort developed 133 CIN2+ lesions. Only one CIN grade 2 (CIN2) occurred among hr-HPV-negative women, whereas 132 CIN2+ lesions (3 invasive carcinomas, 2 microinvasive carcinomas, 86 CIN3, 41 CIN2) occurred in hr-HPV-positive women. Distribution of CIN2+ lesions among cytologies of hr-HPV-positive women was 50% (4 out of 8) for AGC, 35.3% (54 out of 153) for ASC-H, 29.5% (18 out of 61) for HSIL, 19.1% (21 out of 110) for ASC-US/hr-HPV+ and 15.3% (35 out of 228) for LSIL. The mean age at diagnosis of women with CIN2+ lesions was 39.9 and 50.3 years for CIN2/3 lesions and microinvasive/invasive carcinomas, respectively, P=0.02. CIN2+ lesions were significantly more frequent among women aged more than 35 years (90 out of 339=26.5%) as compared with younger women (42 out of 221=19.0%), P=0.04.
At the univariate analysis (Kaplan–Meier analysis), the probability of developing a CIN2+ lesion within 5 years after hr-HPV test was 0.4% (95% confidence interval (CI) 0.1–3.1%) and 41.8% (95% CI: 31.8–53.5%) for hr-HPV-negative women and hr-HPV-positive women (Figure 2), respectively, with a highly statistically significant difference (P<0.01, log-rank test).
Table 3 reports in multivariate analysis (Cox model) the probability of developing a CIN2+ lesion according to hr-HPV test result after adjustment for index Pap test results, age class and SCJ visualisation. A woman with a positive hr-HPV test had about 105 times higher probability of developing a CIN2+ lesion than a woman with a negative hr-HPV test (hazard ratio (HR)=104.5, 95% CI 14.5–755.1). Only Pap test with ASC-H or AGC categories resulted independent predictive factors for developing CIN2+ lesions, increasing the probability by about 2 and 5 times, respectively.
In Table 4, the risk of developing a CIN2+ lesion within 3 years in hr-HPV-positive women is reported for each class of covariates. The risk was slightly higher in older women (33.5% and 27.5% for women aged ⩾35 and <35 years old, respectively, P=0.09, log-rank test). The risk was particularly high if the result of index Pap test was ASC-H or AGC (48.5% and 58.3%, respectively). In evaluating the results of AGC, the low number of observations (only 8) must be taken into account. No significant differences occurred according to the SCJ visualisation (31.8% and 27.8% for visible and not visible SCJ, respectively, P=0.63). Finally, the risk of developing a CIN2+ lesion within 3 years increased significantly according to the semiquantitative viral load and was 15.2%, 27.0% and 37.0% for <10 RLU/CO, between 10 and 99 and >99, respectively, P<0.01. In terms of HR, it corresponded to an increase of risk, as compared with RLU/CO <10 class, of more than two times and more than three times for semiquantitative viral load between 10 and 99 RLU/CO and higher than 99 RLU/CO, respectively (HR=2.4, 95% CI 1.1–5.4 and HR=3.4 95%, CI 1.7–7.1, respectively).
In hr-HPV-positive women, the mean semiquantitative viral load was 620.4 RLU/CO (range 1.0–4065.5), whereas the median of semiquantitative viral load was 167.7 RLU/CO (22.7 and 1017.5 for 25° and 75° percentiles, respectively). Figure 3 shows the ROC curve analysis of semiquantitative RLU/CO for detection of CIN2+ lesions among hr-HPV-positive women. The area under the curve (AUC) was 0.62 (95% CI 0.57–0.67). With a cutoff value of 3 RLU/CO, the sensitivity was 98.5% with a specificity of 7.5%. With a cutoff value of 10 RLU/CO, the sensitivity was 93.2% and the specificity 18.7%.
Discussion
The Pap test is a widely available screening test for detecting precursor lesions of cervical cancer and it has been greatly credited with reducing the cervical cancer incidence. Despite this effective test, every year, in Italy, many new cervical cancers are diagnosed (2200 only in 2012) and the 5-year relative survival rate is slightly increased, from 64% in 1990–1994 to 67% in 2000–2004 (Altavilla et al, 2012). There are three main factors in the relationship between Pap testing and cervical cancer incidence: lack of Pap testing, failure of the Pap test to detect an abnormality, and lack of adequate follow-up after an abnormal Pap test. The present study aims to evaluate the most appropriate protocol to follow-up for women after an abnormal Pap test and negative colposcopy.
When considering the optimal protocol, we have to keep in mind that colposcopy and directed biopsy do not have optimal sensitivity for detection of CIN2+ lesions and can lead to significant under diagnosis of cervical disease (Massad et al, 2009; Desai et al, 2010; Zuchna et al, 2010; Mesher et al, 2011). Our data confirm these observations as 16.0% (133 out of 832) of the women with negative first colposcopy developed CIN2+ lesions during the follow-up. So a negative colposcopy prompts women to do an intensive follow-up because of the high risk of developing CIN2+ in the subsequent years (Zuchna et al, 2010).
At the present time, we know that persistent infection with 12 high-risk HPV is the main cause of invasive cervical cancer (Bouvard et al, 2009). hr-HPV testing could be used as a ‘negative-triage test’ to determine if a woman can be safely returned to the regular 3-year screening schedule after a negative hr-HPV result in the follow-up of abnormal cytology and first negative assessment by colposcopy (Rijkaart et al, 2012). In our study, the hr-HPV test detected 99.2% (132 out of 133) of CIN2+ cases diagnosed during the follow-up period, with a median follow-up time of 1.6 years. These results are in agreement with earlier studies (Woodman et al, 2007; Massad et al, 2009). Moreover in the present paper, the risk of CIN2+ within 5 years with a negative hr-HC2 HPV test was 0.44% (95% CI 0.06%-3.07%) in the follow-up period, which is considered low enough to return women to the normal screening schedule (Kyrgiou et al, 2006).
This is one of the first practical studies to explore the extent to which hr-HPV testing could resolve clinical follow-up management of women with abnormal Pap test and negative assessment for high-grade disease by colposcopy and biopsy (Pretorius et al, 2006; Katki et al, 2013).
The status of hr-HPV infections at the index Pap test was known for ASC-US (triage hr-HPV), but not for the other cytologies, because in LSIL or more severe lesions the HPV positivity is very high (Carozzi et al, 2005a), so far routine hr-HPV testing for all colposcopy referrals is discouraged (Bentley, 2012). Instead the hr-HPV test could be useful after negative colposcopy.
The proportion of hr-HPV-positive test within 1 year from the negative colposcopy for index Pap test results was very low AGC (28.6%), intermediate for ASC-US/hr-HPV+, ASC-H and LSIL (mean 63.9%) and high for HSIL (84.8%). These values are very similar to those reported in the literature (Castle et al, 2010b), however, the small number of AGC (only 28) does not allow to generalise the conclusion.
We found 19% of CIN2+ lesions in ASC-US diagnosed women with persistent hr-HPV positivity and similar data were published in previous studies (Cage et al, 2010; Tropé et al, 2012).
Given the high sensitivity for CIN2+ lesions and the high reproducibility of hr-HPV testing (Carozzi et al, 2005b), its role in surveillance seems pivotal for a standardised follow-up protocol in which Pap test, whose low reproducibility rates among centres are well known (Confortini et al, 2006; Tinacci et al, 2011), should be progressively substituted. As any other test, hr-HPV testing may be affected by some false-negative results (Negri et al, 2007) and, even if only a few of those results have potential clinical significance, most of them are due to a sporadic human error in performing the test. However, it is expected that the introduction of complete automation of the procedure may reduce at least in part the occurrence of such false-negative test results.
Our findings in the multivariate analysis confirm the strong increased risk of CIN2+ lesions (105 times higher: HR=104.5 95% CI 14.5–755.1) among hr-HPV-positive women when compared with hr-HPV-negative women in the follow-up of abnormal Pap test and negative colposcopy, adjusted by age class, index Pap test results and cervix SCJ visualisation at colposcopy. We found independent predictive factors of risk of developing a CIN2+ lesion in follow-up such as results of index Pap test (ASC-H or AGC), but not age class or cervix SCJ visualisation. Women with initial ASC-H and AGC cytologic lesions appear at greater risk of progression probably due to the intrinsic characteristics of these borderline categories. The term ASC-H, as illustrated by Sherman et al (2004) apparently pertains to small dysplastic cells that should have been recognised as malignant and to metaplastic cells with enlarged nuclei that may be the precursor of high-grade lesions. The first cellular type generally replaces the epithelium lining, the endocervical canal and frequently extends to endocervical glands. In many instances, the lesion is beyond the reach of colposcopy.
Among hr-HPV-positive women, the risk of developing a CIN2+ lesion increases significantly with increase of semiquantitative viral load. Compared with 1–9 RLU/CO, the risk becomes more than two times higher in the class 10–99 RLU/CO and more than three times higher in the class >99 RLU/CO. The area under the curve of the ROC curve of semiquantitative viral load among hr-HPV-positive women observed in the present study (0.62) is rather good but, from a clinical point of view, it is difficult to identify a single point of cutoff capable to discriminate efficiently the risk of CIN2+ lesion. For example, with a cutoff of 3 RLU/CO, the sensitivity remains quite high (98.5%) but the gain in specificity is scarce (7.5%). Anyway, several recent papers show that the semiquantitave hr-HPV viral load in ASC-US cases significantly correlates with the severity of cervical cancer precursors (Origoni et al, 2012a) and that increasing semiquantitative hr-HPV viral load significantly correlates with increasing prevalence of CIN2/CIN3 in ASC-US follow-up cases (Origoni et al, 2012b). However, the clinical significance of viral load is very controversial (Berkhof et al, 2006; Manawapat et al, 2012; Sundström et al, 2013). In our study, neverthless the risk to develop a high-grade lesion increases with high semiquantitative viral load but most of subjects with high semiquantitative viral load (⩾100 RLU/CO) do not develop CIN2+ lesions (231 out of 324=71%). Moreover, semiquantitative viral load shows a certain degree of correlation with the increase of the degree of cytological abnormality, especially in the cytogical classes most associated with productive HPV infection (LSIL, ASC-US, ASC-H) and it doesn’t correlate with age class (data not shown). The data of semiquantitative viral load used in the present study are not correlated with the number of cells present in the sample and it is a cumulative measure for all HPV types identified by HC2 probe B. Generally, the application in a context of screening is related to the complexity and costs of the methods able to perform an absolute quantitative measurement, to the lack of technologies that allow for a quantitative measurement of all high-risk HPV types and the presence of co-infections in the same sample.
The present study confirm that hr-HPV testing in women with ASC-US or greater cytological lesions negative at the first colposcopy examination is able to select the real group of women at risk of developing CIN2+ lesions in the follow-up: all the risk is concentrated in women with positive hr-HPV test. This evidence allows us to re-enrol the women with negative hr-HPV in the routine screening interval and to focus on follow-up procedures in the hr-HPV-positive women, thus reducing the workload of the colposcopy services and the anxiety of women.
Perfoming a hr-HPV testing within 1 year could avoid ∼30% (46 out of 156) follow-up colposcopies in women with ASC-US and 33% (226 out of 676) in women with the other cytological abnormalities (ASC-H, LSIL, HSIL, AGC). The future challenge is to determine which hr-HPV-positive women are at future clinical risk and to identify robust markers of disease progression (Woodman et al, 2007). Benevolo et al (2011) showed that PreTect HPVProofer is a good reflex test for triage to reduce colposcopy referral, but with a sensitivity comparable to that of repeat cytology, and they therefore recommended a strict follow-up despite a negative test result (Jeronimo and Schiffman 2006). Persistence of the same genotype may give an even better PPV than testing positive once by hr-HPV HC2 test, but this was not investigated in the present study. Newer HPV DNA tests, including direct genotyping with 16 out of 18 (Castle et al, 2011) or CINtec p16INK4a, have been shown to be other promising triage test methods (Carozzi et al, 2008, 2013), so with the introduction of new biomarkers for cervical cancer, more screening options will be available.
Change history
01 October 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Altavilla G, Bernardo G, Bracarda S, Cascinu S, Di Maio M, Federico M, Gori S, Ionta MT, Pignata S, Pinto C, Tonini G, Torri V, Ferretti S, Crocetti E, Falcini F, Buzzoni C, Serraino D (2012) I numeri del cancro in Italia. Intermedia editore: Brescia.
Ancona C, Bertozzi N, Caranci N, Costa G, Fano V, Gini R, Gnavi R, Michelozzi P, Zocchetti C, Berrino F, Biggeri A, Comba P, Gatta G, Mara L, Martinelli A, Merler E, Merletti F, Panico S, Piffer S, Barbone F, Bertazzi PA, Bianchi F, Borgia P, Candela S, Carnevale F, Fedeli F, Ferretti S, Finarelli A, Giordano L, Grilli R, Kriebel D, Micheli A, Pirastu R, Pizzuti R, Ricciardi W, Romizi R, Salmaso S, Saracci R, Scondotto S, Vineis P, Zappa M (2012) The italian national Center for screening monitoring–Tenth Report. Epidemiol Prev Inferenze edizioni: Milan.
Arbyn M, Anttila A, Jordan J, Ronco G, Schenck U, Segnan N, Wiener H, Herbert A, von Karsa L (2010) European Guidelines for Quality Assurance in Cervical Cancer Screening. Second edition–summary document. Ann Oncol 21: 448–458.
Benevolo M, Vocaturo A, Caraceni D, French D, Rosini S, Zappacosta R, Terrenato I, Ciccocioppo L, Frega A, Giorgi Rossi P (2011) Sensitivity, specificity, and clinical value of human papillomavirus (HPV) E6/E7 mRNA assay as a triage test for cervical cytology and HPV DNA test. J Clin Microbiol 49: 2643–2650.
Bentley J (2012) Colposcopic management of abnormal cervical cytology and histology. J Obstet Gynaecol Can 34: 1188–1202.
Berkhof J, Bulkmans NW, Bleeker MC, Bulk S, Snijders PJ, Voorhorst FJ, Meijer CJ (2006) Human papillomavirus type-specific 18-month risk of high-grade cervical intraepithelial neoplasia in women with a normal or borderline/mildly dyskaryotic smear. Cancer Epidemiol Biomarkers Prev 15: 1268–1273.
Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V WHO International Agency for Research on Cancer Monograph Working Group (2009) A review of human carcinogens-Part B: biological agents. Lancet Oncol 10: 321–322.
Bowring J, Albrow R, Fisher A, Downey G, Cullimore J, Patnick J, Walker PG, Kitchener HC (2012) A prospective study of human papillomavirus (HPV) testing to resolve uncertainty in colposcopy. Cytopathology doi:10.1111/j.1365-2303.2012.01003.x.
Cage JC, Schiffman M, Solomon D, Wheeler CM, Castle PE (2010) Comparison of measurements of human papillomavirus persistence for postcolposcopic surveillance for cervical cancer lesions. Cancer Epidemiol Biomarkers 19: 1668–1674.
Carozzi FM, Confortini M, Cecchini S, Bisanzi S, Cariaggi MP, Pontenani G, Raspollini MR, Sani C, Zappa M, Ciatto S (2005a) Triage with human papilloma virus testing of women with cytologic abnormalities prompting referral for colposcopy assessment. Cancer Cytopathol 105: 2–7.
Carozzi FM, Del Mistro A, Confortini M, Sani C, Puliti D, Trevisan R, De Marco L, Tos AG, Girlando S, Palma PD, Pellegrini A, Schiboni ML, Crucitti P, Pierotti P, Vignato A, Ronco G (2005b) Reproducibility of HPV DNA testing by hybrid capture 2 in a screening setting. Am J Clin Pathol 124: 716–721.
Carozzi F, Confortini M, Dalla Palma P, Del Mistro A, Gillio-Tos A, De Marco L, Giorgi-Rossi P, Pontenani G, Rosso S, Sani C, Sintoni C, Segnan N, Zorzi M, Cuzick J, Rizzolo R, Ronco G New Technologies for Cervival Cancer Screening (NTCC) Working Group (2008) Use of p16-INK4A overexpression to increase the specificity of human papillomavirus testing: a nested substudy of the NTCC randomised controlled trial. Lancet Oncol 9: 937–945.
Carozzi F, Gillio-Tos A, Confortini M, Del Mistro A, Sani C, De Marco L, Girlando S, Rosso S, Naldoni C, Dalla Palma P, Zorzi M, Giorgi-Rossi P, Segnan N, Cuzick J, Ronco G NTCC working group (2013) Risk of high-grade cervical intraepithelial neoplasia during follow-up in HPV-positive women according to baseline p16-INK4A results: a prospective analysis of a nested substudy of the NTCC randomised controlled trial. Lancet Oncol 14: 168–176.
Castle PE, Fetterman B, Poitras N, Lorey T, Shaber R, Kinney W (2010a) Relationship of atypical glandular cell cytology, age, and human papillomavirus detection to cervical and endometrial cancer risks. Obstet Gynecol 115: 243–248.
Castle PE, Fetterman B, Thomas Cox J, Shaber R, Poitras N, Lorey T, Kinney W (2010b) The age-specific relationships of abnormal cytology and human papillomavirus DNA results to the risk of cervical precancer and cancer. Obstet Gynecol 116: 76–84.
Castle PE, Stoler MH, Wright TC Jr, Sharma A, Wright TL, Behrens CM (2011) Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. Lancet Oncol 12: 880–890.
Confortini M, Di Bonito L, Carozzi F, Ghiringhello B, Montanari G, Parisio F, Prandi S GISCi Working Group for Cervical Cytology (2006) Interlaboratory reproducibility of atypical glandular cells of undetermined significance: a national survey. Cytopathology 17: 353–360.
Desai M, Hadden P, Kitchener H, Martin-Hirsch P, Prendiville W, Redman C, Shafi M, Tidy J (2010) Colposcopy and Programme Management. Guidelines for the NHS Cervical Screening Programme 2nd edn NHSCSP Publication: Sheffield.
Dillner J, Rebolj M, Birembaut P, Petry KU, Szarewski A, Munk C, de Sanjose S, Naucler P, Lloveras B, Kjaer S, Cuzick J, van Ballegooijen M, Clavel C, Iftner T Joint European Cohort Study (2008) Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 337: a1754.
Jeronimo J, Schiffman M (2006) Colposcopy at a crossroads. Am J Obstet Gynecol 195: 349–353.
Jordan J, Arbyn M, Martin-Hirsch P, Schenck U, Baldauf JJ, Da Silva D, Anttila A, Nieminen P, Prendiville W (2008) European guidelines for quality assurance in cervical cancer screening: recommendations for clinical management of abnormal cervical cytology, part 1. Cytopathology 19: 342–354.
Katki HA, Gage JC, Schiffman M, Castle PE, Fetterman B, Poitras NE, Lorey T, Cheung LC, Raine-Bennett T, Kinney WK (2013) Follow-up testing after colposcopy: five-year risk of CIN 2+ after a colposcopic diagnosis of CIN 1 or less. J Low Genit Tract Dis 17 (5 Suppl 1): S69–S77.
Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E (2006) Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet 367: 489–498.
Manawapat A, Stubenrauch F, Russ R, Munk C, Kjaer SK, Iftner T (2012) Physical state and viral load as predictive biomarkersfor persistence and progression of HPV16-positive cervical lesions: results from a population based long-term prospective cohort study. Am J Cancer Res 2: 192–203.
Massad LS, Jeronimo J, Katki HA, Schiffman M (2009) The accuracy of colposcopic grading for detection of high-grade cervical intraepithelial neoplasia. J Low Genit Tract Dis 13: 137–144.
Mesher D, Szarewski A, Cadman L, Cubie H, Kitchener H, Luesley D, Menon U, Hulman G, Desai M, Ho L, Terry G, Williams A, Sasieni P, Cuzick J (2010) Long-term follow-up of cervical disease in women screened by cytology and HPV testing: results from the HART study. Br J Cancer 102: 1405–1410.
Mesher D, Tristram A, Castanono A, Beer H, Ashman S, Fielder H, Fiander A, Sasieni P (2011) Single negative colposcopy: it is enough to rule out high grade disease? J Med Screen 18: 160–161.
Milne DS, Wadehra V, Mennim D, Wagstaf TI (1999) A prospective follow up study of women with colposcopically unconfirmed positive cervical smears. Br J Obstet Gynaecol 106: 38–41.
Negri G, Rigo B, Vittadello F, Mian C, Egarter-Vigl E (2007) Abnormal cervicovaginal cytology with negative human papillomavirus testing. Cancer 111: 280–284.
Nygård M (2011) Screening for cervical cancer: when theory meets reality. BMC Cancer 11: 240.
Origoni M, Carminati G, Rolla S, Clementi M, Sideri M, Sandri MT, Candiani M (2012a) Human papillomavirus semiquantitative viral load expressed as relative light units (RLU) correlates with the presence and grade of preneoplastic lesions of the uterine cervix in atypical squamous cells of undetermined significance (ASCUS) cytology. Eur J Clin Microbiol Infect Dis 31: 2401–2406.
Origoni M, Carminati G, Sideri M, Clementi M, Rolla S, Candiani M (2012b) "Low-grade positivity" of HPV viral load after atypical squamous cells of undetermined significance (ASC-US) cytology identifies women at low-risk for cervical intraepithelial neoplasia grade 2 and 3. Eur J Gynaecol Oncol 33: 261–264.
Origoni M, Cristoforoni P, Costa S, Mariani L, Scirpa P, Lorincz A, Sideri M (2012c) HPV-DNA testing for cervical cancer precursors: from evidence to clinical practice. Ecancermedicalscienc 6: 258.
Pretorius RG, Peterson P, Azizi F, Burchette RJ (2006) Subsequent risk and presentation of cervical intraepithelial neoplasia (CIN) 3 or cancer after a colposcopic diagnosis of CIN 1 or less. Am J Obstet Gynecol 195: 1260–1265.
Rijkaart DC, Berkhof J, van Kemenade FJ, Coupe VM, Hesselink AT, Rozendaal L, Heideman DA, Verheijen RH, Bulk S, Verweij WM, Snijders PJ, Meijer CJ (2012) Evaluation of 14 triage strategies for HPV DNApositive women in population-based cervical screening. Int J Cancer 130: 602–610.
Ronco G, Giorgi-Rossi P, Carozzi F, Confortini M, Dalla Palma P, Del Mistro A, Ghiringhello B, Girlando S, Gillio-Tos A, De Marco L, Naldoni C, Pierotti P, Rizzolo R, Schincaglia P, Zorzi M, Zappa M, Segnan N, Cuzick J New Technologies for Cervical Cancer screening (NTCC) Working Group (2010) Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol 11: 249–257.
Schiffman M, Glass AG, Wentzensen N, Rush BB, Castle PE, Scott DR, Buckland J, Sherman ME, Rydzak G, Kirk P, Lorincz AT, Wacholder S, Burk RD (2011) A long-term prospective study of type-specific human papillomavirus infection and risk of cervical neoplasia among 20,000 women in the Portland Kaiser Cohort Study. [Published erratum appears in Cancer Epidemiol Biomarkers Prev (2011); 20: 1398–1409]. Cancer Epidemiol Biomarkers Prev 21: 1390–1391.
Sherman ME, Abdul Karim FW, Berek JS (2004) Atypical squamous cells. In: The Bethesda System for Reporting Cervical Cytology Solomon D, Nayar R (eds) Springer-Verlag: New York. pp 67–87.
Solomon D, Davey D, Kurman R, Moriarty A, O’Connor D, Prey M, Raab S, Sherman M, Wilbur D, Wright T (2002) The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA 287: 2114–2119.
Sundström K, Ploner A, Dahlström LA, Palmgren J, Dillner J, Adami HO, Ylitalo N, Sparén P (2013) Prospective study of HPV16 viral load and risk of in situ and invasive squamous cervical cancer. Cancer Epidemiol Biomarkers Prev 22: 150–158.
Terry G, Ho L, Londesborough P, Cuzick J, Mielzynska-Lohnas I, Lorincz A (2001) Detection of high-risk HPV types by the hybrid capture 2 test. J Med Virol 65: 155–162.
Tinacci G, Biggeri A, Pellegrini A, Cariaggi MP, Schiboni ML, Confortini M (2011) The use of digital images to evaluate the interobserver agreement on cervical smear readings in Italian cervical cancer screening. Cytopathology 22: 75–81.
Tropé A, Sjøborg KD, Nygård M, Røysland K, Campbell S, Alfsen GC, Jonassen CM (2012) Cytology and human papillomavirus testing 6 to 12 months after ASCUS or LSIL cytology in organized screening to predict highgrade cervical neoplasia between screening rounds. J Clin Microbiol 50: 1927–1935.
Woodman CB, Collins SI, Young LS (2007) The natural history of cervical HPV infection: unresolved issues. Nat Rev Cancer 7: 11–22.
Zuchna C, Hager M, Tringler B, Georgoulopoulos A, Ciresa-Koenig A, Volgger B, Widschwendter A, Staudach A (2010) Diagnostic accuracy of guided cervical biopsies: a prospective multicenter study comparing the histopathology of simultaneous biopsy and cone specimen. Am J Obstet Gynecol 203: 321–326.
Acknowledgements
We thank Stefano Ciatto and Silvia Cecchini for initiating the study; Stefania Capassoni, Carmelina Di Pierro, Nicaela Aspite, Luciana Rossi and Cristina Sani for excellent technical assistance, Carlotta Buzzoni for suggestion in statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Carozzi, F., Visioli, C., Confortini, M. et al. hr-HPV testing in the follow-up of women with cytological abnormalities and negative colposcopy. Br J Cancer 109, 1766–1774 (2013). https://doi.org/10.1038/bjc.2013.519
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.519
Keywords
This article is cited by
-
Biomarkers for detection of human papillomavirus (HPV)
Indian Journal of Gynecologic Oncology (2021)
-
Detection and Clinical Management of Cervical Pathology in the Era of HPV
Current Obstetrics and Gynecology Reports (2014)
-
Role of Human Papillomavirus Testing in Screening of Cervical Neoplasia
Current Obstetrics and Gynecology Reports (2014)