Abstract
Background
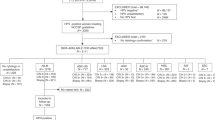
Twenty percent of women referred to colposcopy have a type 3 transformation zone—where colposcopic assessment for high-grade dysplasia (CIN2+) is not possible. This study examines the effectiveness of HPV biomarkers and genotyping in combination with techniques that sample an endocervical TZ.
Methods
A prospective diagnostic accuracy study. Women booked for large-loop excision (LLETZ) with squamous dyskaryosis, high-risk HPV and a TZ3 were recruited. Immediately prior to LLETZ samples were collected for p16/Ki-67 dual-stained cytology, HPV genotyping and H&E, p16- and Ki-67-stained endocervical curettings.
Results
In women with low-grade screening (n = 64), 35.9% had CIN2+; dual-stained cytology had the greatest effect on the PPV of routine screening (76.1% vs 35.9%) and perfectly predicted the absence of CIN2+. In women with a high-grade screening result (n = 37); 75.6% had CIN2+ and dual-stained curettings improved the PPV (96.5 vs 75.6%).
Conclusions
With high-grade screening and a TZ3, LLETZ appears safest as three quarters have CIN2+ . Women with low-grade screening and a TZ3 have a twofold increased risk of CIN2+ when compared to women where the TZ is visible. The use of dual-stained cytology may help identify those women who can be safely offered surveillance and those who require treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Data supporting the results reported in the paper cannot be found on publicly available databases. Data can be requested on reasonable request from the authors.
References
Bruni L, Diaz M, Castelsague X, Ferrer E, Bosch F, de Sanjose S, et al. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202:1789–99.
Kelly RS, Patnick J, Kitchener HC, Moss SM. HPV testing as a triage for borderline or mild dyskaryosis on cervical cytology: results from the Sentinel Sites study. Br J Cancer. 2011;105:983–8.
Massad LS, Einstein M, Huh W, Katki H, Kinney W, Schiffman M, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Trac Dis. 2013;17:S1–27.
Public Health England. NHS CSP Publication 20. Cervical Screening: programme and colposcopy management. Third Edition (14th March 2016). Cervical screening: programme and colposcopy management - GOV.UK (www.gov.uk). Accessed 26 October 2021.
Castanon A, Landy R, Brocklehurst P, Evans H, Peebles D, Singh N, et al. Risk of preterm delivery with increasing depth of excision for cervical intraepithelial neoplasia in England: nested case-control study. BMJ. 2014;349:g6223.
Baldauf JJ, Dreyfus M, Ritter J, Meyer P, Philippe E. Risk of cervical stenosis after large loop excision or laser conization. Obstet Gynecol. 1996;88:933–8.
Suh-Burgmann EJ, Whall-Strojwas D, Chang Y, Hundley D, Goodman A. Risk factors for cervical stenosis after loop electrocautery excision procedure. Obstet Gynecol. 2000;96:657–60.
Manley KM, Simms B, Platt S, Patel A, Bahl R. Unsatisfactory colposcopy: clinical decision-making in conditions of uncertainty. BMC Med Inform Decis Mak. 2017;17:125.
Manley KM, Wills AK, Crouch N, Lopez Bernal A, Patel A, Current practices in the management of a type 3 transformation zone: results of a UK survey. Abstract. Cardiff: The British Society of Colposcopy and Cervical Pathology Annual Scientific Meeting; 2017.
Manley KM, Wills AK, Morris GC, Hogg JL, Lopez-Bernal A, Murdoch JB. The impact of HPV cervical screening on negative large loop excision of the transformation zone (LLETZ): a comparative cohort study. Gynecol Oncol. 2016;141:485–91.
Pretorius RG, Zhang W, Belinson JL, Huang M, Wu L, Zhang X, et al. Colposcopically directed biopsy, random cervical biopsy, and endocervical curettage in the diagnosis of cervical intraepithelial neoplasia II or worse. Am J Obstet Gynecol. 2004;191:430–4.
Williams DL, Dietrich C, McBroom J. Endocervical curettage when colposcopic examination is satisfactory and normal. Obstet Gynecol. 2000;95:801–3.
Franco EL, Villa LL, Sobrinho JP, Prado JM, Rousseau MC, Desy M, et al. Epidemiology of acquisition and clearance of cervical human papillomavirus infection in women from a high-risk area for cervical cancer. J Infect Dis. 1999;180:1415–23.
Molano M, den Brule A, Plummer M, Weiderpass E, Posso H, Arslan A, et al. Determinants of clearance of human papillomavirus infections in Colombian women with normal cytology: a population-based, 5-year follow-up study. Am J Epidemiol. 2003;158:486–94.
Galgano MT, Castle PE, Atkins KA, Brix WK, Nassau SR, Stoler MH. Using biomarkers as objective standards in the diagnosis of cervical biopsies. Am J Surg Pathol. 2010;34:1077–87.
von Knebel Doeberitz M. New markers for cervical dysplasia to visualise the genomic chaos created by aberrant oncogenic papillomavirus infections. Eur J Cancer. 2002;38:2229–42.
Wentzensen N, Schwartz L, Zuna R, Smith K, Mathews C, Gold M, et al. Performance of p16/Ki-67 immunostaining to detect cervical cancer precursors in a colposcopy referral population. Clin Cancer Res. 2012;18:4154–62.
Schmidt D, Bergeron C, Denton K, Ridder R. European CINtec Cytology Study Group). p16/ki-67 dual-stain cytology in the triage of ASCUS and LSIL Papanicolaou cytology: results from the European equivocal or mildly abnormal Papanicolaou cytology study. Cancer Cytopathol. 2011;119:158–66.
Loghavi S, Walts AE, Bose S. CINtec(R) PLUS dual immunostain: a triage tool for cervical pap smears with atypical squamous cells of undetermined significance and low grade squamous intraepithelial lesion. Diagn Cytopathol. 2013;41:582–7.
Waldstrom M, Christensen RK, Ornskov D. Evaluation of p16(INK4a)/Ki-67 dual stain in comparison with an mRNA human papillomavirus test on liquid-based cytology samples with low-grade squamous intraepithelial lesion. Cancer Cytopathol. 2013;121:136–45.
Kelly RS, Patnick J, Kitchener HC, Moss SM, for the NHSCSP HPV Special Interest Group. HPV testing as a triage for borderline or mild dyskaryosis on cervical cytology: results from the Sentinel Sites Study. Br J Cancer. 2011;105:983–8
Jordan J, Arbyn M, Martin-Hirsch P, Schenck U, Balduff JJ, da Silva D, et al. European guidelines for quality assurance in cervical cancer screening: recommendations for clinical management of abnormal cervical cytology, part 1. Cytopathology. 2008;19:342–54.
Stillson T, Knight AL, Elswick RK Jr. The effectiveness and safety of two cervical cytologic techniques during pregnancy. J Fam Pr. 1997;45:159–63.
Royal College of Obstetricians and Gynaecologists. Induction of Labour at Term in Older Mothers: Scientific Impact Paper No. 34. 2013. www.rcog.org.uk/en/guidelines-research-services/guidelines/sip34/. Accessed 27 October 2021.
Public Health England. Cervical screening: primary HPV screening implementation. Appendix 2: Cervical Screening Colposcopy Management Recommendation Algorithms. 2019. Available from: https://www.gov.uk/government/publications/cervical-screening-primary-hpv-screening-implementation#history. Accessed 27 November 2020.
Eisenberger D, Hernandez E, Tener T, Atkinson BF. Order of endocervical and ectocervical cytologic sampling and the quality of the Papanicolaou smear. Obstet Gynecol. 1997;90:755–8.
Zhao C, Austin RM. Human papillomavirus DNA detection in ThinPrep Pap test vials is independent of cytologic sampling of the transformation zone. Gynecol Oncol. 2007;107:231–5.
Denton KJ, Herbert A, Turnbull LS, Waddell C, Desai MS, Rana DN, et al. The revised BSCC terminology for abnormal cervical cytology. Cytopathology. 2008;19:137–57.
Lesnikova I, Lidang M, Hamilton-Dutoit S, Koch J. p16 as a diagnostic marker of cervical neoplasia: a tissue microarray study of 796 archival specimens. Diagnostic Pathol. 2009;4:22–28.
Van Niekerk D, Guillaud M, Matisic J, Benedet J, Freeberg JA, Follen M, et al. p16 and MIB1 improve the sensitivity and specificity of the diagnosis of high grade squamous intraepithelial lesions: methodological issues in a report of 447 biopsies with consensus diagnosis and HPV HCII testing. Gynecol Oncol. 2007;107:S233–40.
NHS CSP Cervical Screening Programme, England. Statistics for 2019-20. Published 26th November 2020. England: Cervical Screening Programme, 2019-20. www.digital.nhs.uk/data-and-information/publications/statistical/cervical-screening-annual/england---2019-20. Accessed 17 May 2021.
Cancer Research UK. Cervical Cancer Incidence Statistics. Cervical cancer|Cancer Research UK. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/cervical-cancer/incidence. Accessed 18 May 2021.
Mercer CH, Tanton C, Prah P, Erens B, Sonnenberg P. Clifton Soazig. Changes in sexual attitudes and lifestyles in Britain through the life course and over time: findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal). Lancet. 2013;382:1781–94.
Nakamura Y, Matsumoto K, Satoh T, Nishide K, Nozue A, Shimabukuro K, et al. HPV genotyping for triage of women with abnormal cervical cancer screening results: a multicenter prospective study. Int J Clin Oncol. 2015;20:974–81.
Khan M, Castle P, Lorincz A, Wacholder S, Sherman M, Scott D, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97:1072–9.
Peralta R, Vargas-de-Leon C, Cabrera A, Miramontes P. Dynamics of high-risk nonvaccine human papillomavirus types after actual vaccination scheme. Comput Math Methods Med. 2014;542923:1–8.
Hellsten C, Sjostrom K, Lindqvist PG. A prospective Swedish cohort study on psychosocial factors influencing anxiety in women referred for colposcopy. BJOG. 2007;114:32–8.
Bonevski B, Sanson-Fisher R, Girgis A, Perkins J. Women’s experiences of having a colposcopic examination: self-reported satisfaction with care, perceived needs and consequences. J Obstet Gynecol. 1998;18:462–70.
Gray NM, Sharp L, Cotton SC, Masson LF, Little J, Walker LG, et al. Psychological effects of a low-grade abnormal cervical smear test result: anxiety and associated factors. Br J Cancer. 2006;94:1253–62.
Manley KM, Wills AK, Villeneuve N, Hunt K, Patel A, Glew S. Comparison of the Cervex-Brush alone to Cytobrush plus Cervex-Brush for detection of cervical dysplasia in women with a transformation zone type 3. Cytopathol. 2019;30:157–63.
Bergeron C, Ikenberg H, Sideri M, Denton K, Bogers J, Schmidt D, et al. Prospective evaluation of p16/Ki-67 dual-stained cytology for managing women with abnormal Papanicolaou cytology: PALMS study results. Cancer Cytopathol. 2015;123:373–81.
Boonstra H, Aalders JG, Koudstaal J, Oosterhuis JW, Janssens J. Minimum extension and appropriate topographic position of tissue destruction for treatment of cervical intraepithelial neoplasia. Obstet Gynecol. 1990;75:227–31.
Mangham LJ, Petrou S, Doyle LW, Draper ES, Marlow N. The cost of preterm birth throughout childhood in England and Wales. Pediatrics. 2009;123:e312–27.
Davey DD, Cox JT, Austin RM, Birdsong G, Colgan TJ, Howell LP, et al. Cervical cytology specimen adequacy: patient management guidelines and optimizing specimen collection. J Low Genit Trac Dis. 2008;12:71–81.
Acknowledgements
The authors would like to thank all the women who participated in the study and donated their samples and data for research. The research group would also like to thank Professor Henry Kitchener, Dr. Alex Sargent, Professor David Cahill, Dr. Naomi Crouch, Mrs. Yvonne Higgins, Miss. Karen Shaw and the Colposcopy Administration Department at University Hospitals Bristol for their advice and support during the running of this study.
Funding
The study was funded by the David Telling Charitable Trust and the Above & Beyond Charity Bristol; they did not have a role in the design or conduct of the study.
Author information
Authors and Affiliations
Contributions
Study concept and design: KM, AW, JP, SG, AP. Acquisition, processing and reporting of samples: KM, JP, KH, NV, PM, HH, ST. Analysis and interpretation of the data: KM and AW. Drafting of the manuscript: KM. Critical revision of the manuscript: KM, AW, SG, AP, PM, JP, NV, AL-B.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the ethical principles as outlined in the Declaration of Helsinki. Ethical approval was granted by NRES Committee South West—Frenchay on May 25, 2014, (ref OG/SW/0028). All patients provided written informed consent.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Manley, K., Patel, A., Pawade, J. et al. The use of biomarkers and HPV genotyping to improve diagnostic accuracy in women with a transformation zone type 3. Br J Cancer 126, 91–99 (2022). https://doi.org/10.1038/s41416-021-01539-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-021-01539-y