Abstract
Background:
The aim of this current study was to assess the expression and activity of Src family kinases, focal adhesion kinase (FAK), caveolin (Cav-1) and RhoGD12 in bladder cancer.
Methods:
Fifty-eight patients with a new diagnosis of bladder cancer undergoing transurethral resection were included. Immunohistochemical staining was utilised to assess expression of c-Src, dephosphorylated (SrcY530), phosphorylated Src (Y419), phosphorylated FAK (FAK Y861), Cav-1 and RhoGD12. Expression was assessed using the weighted histoscore method.
Results:
High expression of dephosphorylated Y527, phosphorylated Y416 and phosphorylated FAK Y861 in the membrane were associated with increased cancer-specific survival (P=0. 01, P=0.001, P=0.008, respectively) and expression of Y416 in the membrane was an independent factor on multivariate analysis when combined with known clinical parameters (P=0.008, HR 0.288, 95% CI 0.11–0.72).
Conclusion:
These results demonstrate that in contrast to other solid tumours, activation of the Src family members and downstream signalling proteins are associated with a good prognosis in transitional cell carcinoma of the bladder, and activated Src has a positive relationship with RhoGD12.
Similar content being viewed by others
Main
Worldwide, there are around 360 000 new cases of bladder cancer diagnosed each year (www.cancerresearchuk.org). In the UK alone, bladder cancer is the fifth most common solid non-cutaneous malignancy with approximately 10 000 new cases and 5000 deaths annually (www.cancerresearchuk.org). Overall survival is variable; those with non-muscle invasive disease have an 80–90% 5-year survival, whereas in those with muscle invasive disease, the 3-year survival is between 25–50% depending on the degree of invasive disease (www.cancerresearchuk.org).
In those with non-muscle invasive disease, the recurrence and progression rate is strongly associated with tumour stage and grade. These factors can be categorised to show the likelihood for recurrence and progression using EORTC risk tables (Sylvester et al, 2006). Even in those considered low risk, the 5-year recurrence is 31%, increasing to 78% in those considered to be in the risk high category (Sylvester et al, 2006). The 5-year progression rate in those at high risk is 45% (Sylvester et al, 2006).
In those with muscle invasive disease undergoing radical cystectomy, the 5-year survival is only 50% (Dalbagni et al, 2001; Stein et al, 2001; Stein and Skinner, 2006). Despite neoadjuvant chemotherapy in this patient group, published data has only shown a 5% absolute improvement in survival at 5 years (Advanced Bladder Cancer (ABC) Meta-analysis Collaboration, 2005). The role of neoadjuvant radiotherapy has shown a significant increase in survival in randomised studies (Slack et al, 1977; Smith Jr et al, 1997). For those with non-resectable disease, palliation of symptoms is the only treatment option.
One potential molecular target is the non-receptor tyrosine kinase Src, the first identified human proto-oncogene. Src kinase has a role in signal transduction of multiple oncogenic cellular processes, including migration, adhesion, invasion, angiogenesis, proliferation and differentiation, and has significant interactions with other cellular proteins such as growth factor receptors (Kopetz et al, 2007). The c-Src (commonly referred to as Src) is the prototypical member of the Src kinase family (SFK), with a total of eight members expressed in mammalian cells (c-Src, Blk, Fgr, Fyn Yes, Hck, Lck and Lyn). Src is composed of a C-terminal tail, kinase domain, two protein–protein interaction domains (SH2, SH3) and a unique amino-terminal domain that varies between the Src family members. Src is activated by a number of pathways. Src kinase activation involves dephosphorylation of a conserved tyrosine residue in the C-terminal negative regulatory tail region (Y530) and subsequent autophosphorylation of the Y419 site in the kinase domain (Cooper and Howell, 1993; Engen et al, 2008). Consequently, antibodies to the dephosphorylation of Y530 and phosphorylation of Src kinase at the Y419 sites can be used as markers for activated Src kinase (Campbell et al, 2008). Activated Src kinase translocates to the cell membrane (Frame, 2002), and therefore, cellular location may also be employed as a surrogate marker of activation (Luo et al, 2008). Also, when Src is activated, several downstream signalling proteins such as focal adhesion kinase (FAK) are phosphorylated and could therefore act as biomarkers for Src activation (Nam et al, 2005). FAK is phosphorylated at several sites by Src such as Y397, Y576 and Y577, but it has been reported that Y861 is the major site in the carboxyl-terminal domain of FAK (Schaller et al, 1994; Calalb et al, 1995, 1996). It has been shown that Src expression and activity decreases with bladder tumour stage (Fanning et al, 1992; Blaveri et al, 2005; Sanchez-Carbayo et al, 2006; Wu et al, 2009; Thomas et al, 2011), although a positive correlation has been shown with breast and prostate cancers (Elsberger et al, 2009; Tatarov et al, 2009).
Caveolin-1 (Cav-1) is the major coat protein of caveolae, regulating the activity of signalling molecules in cancer (Liu et al, 2002), and can make both a negative and positive impact on cancer progression. Cav-1 has been implicated in preventing cell transformation (Galbiati et al, 1998) and promoting cell cycle arrest and senescence (Galbiati et al, 2001; Volonte et al, 2002). Cav-1 expression has been shown to be elevated in various malignancies such as colon (Fine et al, 2001), thyroid (Ito et al, 2002), lung (Ho et al, 2002) and bladder (Fong et al, 2003) cancers. In bladder cancer, it has previously been reported that Cav-1 expression is elevated at the onset of oncogenesis (Fong et al, 2003) and these levels rise further with progression of the disease in terms of stage and grade (Rajjayabun et al, 2001; Fong et al, 2003; Kunze et al, 2006). Caveolin-1 expression has been demonstrated to be associated with increased cytoplasmic expression in colon cancer but was not associated with tumour stage (Fine et al, 2001), whereas membranous expression in ovarian cancer was associated with shorter survival (Davidson et al, 2001). Cav-1 was first described as the major substrate for Src in v-Src-transformed cell lines (Glenney Jr and Zokas, 1989; Lee et al, 2000), and phosphorylated Cav-1 can either inhibit Src through recruitment of the C-terminal (Li et al, 1996; Cao et al, 2002) or promote Src activation through an unknown mechanism (Grande-Garcia et al, 2007). In addition, high expression of Cav-1 and low expression of Src were associated with metastasis and poor survival in bladder cancer and suggested that these both control bladder metastases through the Rho–ROCK pathway (Thomas et al, 2011).
RhoGD12 is known to have a negative and positive impact on cancer. It has previously been demonstrated that RhoGD12 positively correlates with tumour progression and metastases in gastric, ovarian and breast cancers (Tapper et al, 2001; Zhang and Zhang, 2006; Cho et al, 2009), whereas others have reported it to be a metastatic suppressor in malignancies, including prostate, breast, lung and bladder cancers (Gildea et al, 2002; Theodorescu et al, 2004; Moon et al, 2010; Niu et al, 2010). There is controversy in the literature regarding RhoGD12 expression; it has been reported to be both more prominent in the nuclei and cytoplasm of tumour cells (Theodorescu et al, 2004; Cho et al, 2009). Interestingly, a negative relationship was observed between RNA expression of Src and RhoGD12; it was further suggested that membrane expression of RhoGD12 is elevated when co-transfected with Src Y527 (Wu et al, 2009). Given that Src is still inactive in this state, this would suggest that RhoGD12 expression has a positive relationship with Src inactivity and not Src activity as previously reported.
We hypothesise that it is the activity of Src that drives the relationship with Cav-1 and RhoGD12 expression. The aims of this study were to assess expression of Src, Cav-1 and RhoGD12 in relationship to cancer-specific survival, and to examine the potential interrelationships between these markers.
Patients and methods
Patients with organ-confined bladder cancer were included for this study. All patients had transitional cell carcinoma. These patients had undergone resection based on investigations in the North Glasgow NHS Trust. Only those patients were included, whose initial CT scans at the time of diagnosis showed no evidence of regional or metastatic disease, patients who had undergone instillation of intravesical chemotherapy post operation, and whose only subsequent treatment was transurethral resection of recurrences and intravesical therapy. Those patients that subsequently underwent further treatment by way of radical surgery or radiotherapy were excluded. Patients were staged pathologically according to the TNM classification and graded according to the World Health Organisation/International Society of Urological Pathology criteria (Epstein et al, 1998). Cancer-specific survival rate was the time from diagnosis until time of death or last follow-up. The cause of death was determined by linkage through the Scottish Cancer Registry. In those who were deceased, if the primary cause of death was of bladder cancer, these were classed as cancer specific, and all other causes were non-cancer specific deaths. The Research Ethics Committee of West of Scotland has approved the study.
Immunohistochemical staining was utilised to assess the expression of c-Src, dephosphorylated Src at Y530, phosphorylated Src at Y419, FAK at Y861, Cav-1 and RhoGD12. Both antibodies for dephosphorylated Src (Y530) and phosphorylated Src (Y419) are not specific for c-Src because of sequence homogeneity between family members, and can therefore also detect other family members, including Fyn, Yes and Fgr.
Src kinase and activated Src kinase expression (c-Src and Src Y419) were investigated using antibodies for c-Src (36D10, Cell Signalling Technology, Beverly, MA, USA) and Y416Src (Cell Signalling Technology). Dephosphorylated Src and phosphorylated FAK were investigated using antibodies for Src dephosphorylated at Y527 (dephosphorylated Y527) and FAK Y861 (Invitrogen, Paisley, UK). In humans, the activated phosphorylations that were investigated in the current study are amino acids Y530 and Y419. Antibodies used relate to the rabbit sequence and not the human sequence. Expression for Cav-1 and RhoGD12 were investigated using an anti-Caveolin 1 antibody (Abcam, Cambridge, UK) and D4-GDI (Spring Bioscience, Pleasanton, CA, USA), respectively.
Tissue sections were dewaxed and rehydrated through graded alcohol. Antigen retrieval was performed by heating tissue sections under pressure for 5 min in a pressure cooker, using citrate buffer pH 6 for c-Src, dephosphorylated Y527, FAK Y861, Cav-1, RhoGD12 and EDTA buffer pH 9 for Src Y416. Endogenous peroxidase activity was blocked by incubation in 3% hydrogen peroxide (H2O2). To reduce non-specific binding, the tissue sections were then incubated with 5% normal horse serum (Vector Laboratories, Burlingame, CA, USA) in antibody dilutent (DAKO Cytomation, Glostrup, Denmark) for 20 min at room temperature. Incubation with primary antibody was performed with c-Src (1 : 200) for 60 min at room temperature and overnight at 4 °C for antibodies for dephosphorylated Src Y527 (1 : 3000), phosphorylated Src Y416 (1 : 25), FAK Y861 (1 : 200), Cav-1 (1 : 500) and RhoGD12 (1 : 200).
Signal was amplified and visualised using the DAKO Envision Kit (DAKO Cytomation) and the chromagen 3,3′-diaminobenzidine (Vector Laboratories).
Sections were counterstained, dehydrated and mounted. In each run, a positive and negative isotype-matched control was included to ensure no false-positive staining.
Scoring
Protein expression was assessed using the weighted histoscore method (H score method; Kirkegaard et al, 2006). The weighted histoscore grades staining intensity as negative (0), weak (1), moderate (2) and strong (3), then multiplication of the percentage of tumour cells within each category. Agreement between observers was excellent and measured in interclass correlation coefficient, respectively.
Statistical analysis was undertaken using SPSS (Chicago, IL, USA). Cancer-specific survival rates were generated using the Kaplan–Meier method. The log rank test was utilised to compare significant differences between subset groups using univariate analysis. Multivariate analysis was carried out based on the results of the univariate analysis. Multivariate Cox regression analysis was performed to identify those factors that were independently associated with cancer-specific death. A stepwise backward procedure was utilised to ascertain which of the variables had a significant independent relationship with survival. A χ2-analysis was utilised to assess relationships between pathological parameters and the biomarkers at the various locations. Pearson's correlation was utilised to assess if relationships could be identified between the various proteins at the various cellular locations. P-values <0.0003 were deemed significant according to Bonferronis correction.
Results
Analysis was based on 58 bladder transitional cell carcinoma patients with full clinical follow-up. Median age at diagnosis was 69 years (range 43–91). Median follow-up was 33 months (range 1–180). In all, 27 patients died of their disease.
Initial analysis was based on known clinico-pathological features, which are known to be prognostic indicators for survival in bladder cancer. T-stage (Figure 1A) and tumour grade (Figure 1B) were significantly associated with poor prognosis, thus validating our cohort for use in a biomarker study (Table 1).
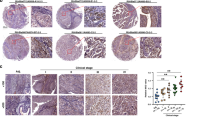
Each cellular location was independently assessed for expression levels (Figure 2). Tumours were divided into those with high (above median) or low (below or equal to the median) expression.
c-Src kinase
Of the tumours investigated, 74% showed some nuclear expression, 100% cytoplasmic expression, and 93% membrane expression. Tumours were subdivided into with high (above median) and low (below median) expression. The χ2-analysis demonstrated that membrane c-Src negatively correlated with tumour grade (P=0.024, Table 2), but no correlation was demonstrated with age, T-stage, recurrence or metastases. These results suggested that when located in the membrane, c-Src conferred a good prognosis; however, on univariate analysis, expression of c-Src at the different cellular locations did not show significance (Table 1), therefore no correlation with cancer-specific survival was observed for this marker. Pearson's correlation demonstrated that membrane c-Src expression positively correlated with cytoplasmic c-Src (P<0.0001, Table 3).
Y527 Src kinase
Of the tumours investigated, 3% showed some degree of nuclear expression, 98% cytoplasmic expression, and 93% membrane expression. The χ2-analysis demonstrated that expression of membrane-dephosphorylated Y527 negatively correlated with T-stage and grade (P=0.021, P=0.011, Table 2), but no correlation was demonstrated with age, recurrence or metastases. On univariate analysis, high expression of membrane-dephosphorylated Y527 was associated with increased cancer-specific survival (P=0.01, Table 1). In contrast, high expression of nuclear-dephosphorylated Y527 was associated with decreased cancer-specific survival, P=0.008; however, this result should be viewed with caution, as only 3% of tumours exhibited high nuclear-dephosphorylated Y527 expression, (Table 1). Pearson's correlation demonstrated expression of membrane Y527 positively correlated with membrane c-Src expression (P<0.0001, Table 3), suggesting that both surrogate markers of c-Src activation correlate with each other, and therefore validating their use in this manner.
Y416 Src kinase
Of the tumours investigated, 90% showed some degree of nuclear expression, 100% cytoplasmic expression, and 100% membrane expression. The χ2-analysis demonstrated that expression of membrane Y416 negatively correlated with tumour grade and evidence of metastasis at follow-up (P=0.005, P=0.001, Table 2), but no correlation was demonstrated with age, T-stage and recurrence. On univariate analysis, high expression of membrane Y416 was associated with increased cancer-specific survival (P=0.001, Table 1, Figure 3A) and was shown to be an independent factor on multivariate analysis (P=0.008, HR 0.288, CI 0.11–0.72, Table 1).
FAK Y861
Of the tumours investigated, 10% showed some degree of nuclear expression, 34% cytoplasmic expression, and 91% membrane expression. The χ2-analysis demonstrated that expression of membrane Y861 negatively correlated with recurrence and evidence of metastases (P=0.026, P=0.045, Table 2), but no correlation was demonstrated with age, T-stage or grade. On univariate analysis, high expression of membrane Y861 was associated with increased cancer-specific survival (P=0.008, Table 1, Figure 3B). Pearson's correlation demonstrated the expression of cytoplasmic FAK Y861 demonstrated a positive correlation with cytoplasmic Y527 (P<0.0001, Table 3). Expression of membrane FAK Y861 demonstrated a positive correlation with membrane c-Src, Y527 and Y416 (P<0.0001, P<0.0001, P=0.0001, Table 3). Therefore, all markers of Src activation correlated with activation of the downstream marker FAK Y861.
Caveolin-1
Of the tumours investigated, 2% showed degree of nuclear expression, 97% cytoplasmic expression, and 24% membrane expression. The χ2-analysis demonstrated that expression of membrane Cav-1 positively correlated with tumour grade (P=0.026, Table 2), but no correlation was demonstrated with age, T-stage, recurrence or metastases. On univariate analysis, however, high expression of nuclear Cav-1 was associated with decreased cancer-specific survival (P=0.042, Table 1), but as only 2% of tumours exhibited high nuclear expression, these results need to be viewed with caution.
RhoGD12
Of the tumours investigated, 91% showed degree of nuclear expression, 91% cytoplasmic expression, and 71% membrane expression. The χ2-analysis demonstrated that expression of nuclear RhoGD12 had a negative correlation with metastases (P=0.036, Table 2), but no correlation was demonstrated with age, T-stage, grade or recurrence. Pearson's correlation demonstrated expression of cytoplasmic RhoGD12 demonstrated a positive correlation with nuclear RhoGD12 (P<0.0001, Table 3). Expression of membrane RhoGD12 demonstrated a positive correlation with cytoplasmic and membrane c-Src (P<0.0001, P<0.0001, Table 3) and cytoplasmic RhoGD12 expression (P<0.0001, Table 3). This suggests that RhoGD12 expression is associated with levels of c-Src in bladder cancer.
Discussion
To our knowledge, this is the only study investigating the role of Src kinase, dephosphorylation status (Y530), autophosphorylation status (Y419), the downstream signalling protein FAK Y861, Cav-1 and RhoGD12 expression in one cohort of bladder cancers.
This study demonstrates that when c-Src is active (utilising membrane expression as a surrogate marker of activation), an inverse correlation with tumour grade is observed. These results are consistent with those that have previously reported a negative correlation of Src expression with bladder aggressiveness (Fanning et al, 1992; Blaveri et al, 2005; Sanchez-Carbayo et al, 2006; Wu et al, 2009; Thomas et al, 2011), and further reiterates that Src inhibitors should be utilised with much caution for cancer prevention in transitional cell carcinoma.
It has been suggested that a biomarker for prediction of Src kinase activity would be to measure phosphorylation of the protein at a site associated with activity (Bolen et al, 1987; Masaki et al, 1998), and Src kinase, when active, translocates to the membrane (Frame, 2002). When examining the expression of various phosphorylated statuses of Src, we demonstrated that expression of dephosphorylated Src (dephosphorylated Y527) and autophosphorylated Src (Y416) negatively correlated with tumour grade. Furthermore, increased expression of membrane Src Y416 was an independent predictor of improved cancer-specific survival. The antibody for Y419 is not specific for one particular Src family member, but because of sequence homogeneity, cross reacts with all Src family members that are phosphorylated at this site. Therefore, as c-Src itself in this study is not associated with clinical outcome measures, we hypothesise that expression of another of the SFK members is driving the association with improved outcome observed in the current study. Further evidence supporting the observation that activation of Src family members confers an improved prognosis is by assessing the relationships of the downstream signalling protein FAK Y861 to outcome. Expression of FAK Y861 was observed to be negatively associated with recurrence, presence of metastases and increased cancer-specific survival.
Cav-1 expression can have both a positive and negative effect on cancer progression. In this study, when assessing expression of Cav-1, it was shown that presence in the membrane positively correlated with nuclear grade. This is also in keeping with previous work which has shown that Cav-1 expression was associated with grade (Rajjayabun et al, 2001; Fong et al, 2003; Kunze et al, 2006). It has also been demonstrated that Cav-1 expression was more membranous in those with ovarian cancer and had short-term survival (Fine et al, 2001), which is a similar finding to what we have shown that membranous expression has a positive correlation with grade, and this factor is associated with poorer survival. Previous work has shown that Cav-1 and Src have a reciprocal relationship in bladder cancer (Thomas et al, 2011). In this study, no such relationship reached significance, but it was demonstrated that Cav-1 and c-Src had a positive correlation and Cav-1 had a negative correlation with Y416. This also suggests that it is another of the SFK member, which is associated with a reciprocal relationship with Cav-1. It has been suggested that Cav-1 inhibits Src through recruitment of the C-terminal (Li et al, 1996; Cao et al, 2002), but in this study, there was no correlation between Cav-1 and Y527.
RhoGD12 expression has been thought of as being a suppressor of cancer progression and metastases, but has also been shown to positively correlate with cancer progression in various malignancies (Tapper et al, 2001; Zhang and Zhang, 2006; Cho et al, 2009). We have observed that nuclear expression of RhoGD12 negatively correlated with the presence of metastases at follow-up, which is consistent with work showing that RhoGD12 is a metastases suppressor in bladder cancer (Gildea et al, 2002; Theodorescu et al, 2004). Previous work has reported that expression of Src and RhoGD12 confers improved prognosis in bladder cancer (Fanning et al, 1992; Theodorescu et al, 2004; Blaveri et al, 2005; Sanchez-Carbayo et al, 2006; Thomas et al, 2011). It has also been reported active Src (Y530) results in elevated levels of RhoGD12 (Wu et al, 2009). Given that, when Src is dephosphorylated at Y530, it is not active yet, and this would suggest a relationship between inactive Src and RhoGD12. This study does demonstrate that expression of RhoGD12 is associated with less likelihood of metastases, but also shows a positive relationship between RhoGD12 and membrane c-Src. As c-Src is active when located in the membrane, we have therefore shown that it is active c-Src that has a relationship with RhoGD12.
This current study further reinforces that expression of Cav-1 confers poor prognosis. This study reports that RhoGD12 confers improved prognosis, but has a positive correlation with active c-Src. It has also been demonstrated that expression of dephosphorylated Src (Y527), autophosphorylated Src (Y416) and the downstream marker FAK Y861 confer improved cancer-specific survival. Furthermore, expression of Src Y416 is an independent factor associated with increased cancer-specific survival, suggesting that expression of another SFK member other than Src itself confers improved prognosis.
Change history
28 March 2012
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Advanced Bladder Cancer (ABC) Meta-analysis Collaboration ( 2005 ) Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration . Eur Urol 48 (2) : 202 – 205
Blaveri E, Simko JP, Korkola JE, Brewer JL, Baehner F, Mehta K, Devries S, Koppie T, Pejavar S, Carroll P, Waldman FM ( 2005 ) Bladder cancer outcome and subtype classification by gene expression . Clin Cancer Res 11 (11) : 4044 – 4055
Bolen JB, Veillette A, Schwartz AM, DeSeau V, Rosen N ( 1987 ) Activation of pp60c-src protein kinase activity in human colon carcinoma . Proc Natl Acad Sci USA 84 (8) : 2251 – 2255
Calalb MB, Polte TR, Hanks SK ( 1995 ) Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases . Mol Cell Biol 15 (2) : 954 – 963
Calalb MB, Zhang X, Polte TR, Hanks SK ( 1996 ) Focal adhesion kinase tyrosine-861 is a major site of phosphorylation by Src . Biochem Biophys Res Commun 228 (3) : 662 – 668
Campbell EJ, McDuff E, Tatarov O, Tovey S, Brunton V, Cooke TG, Edwards J ( 2008 ) Phosphorylated c-Src in the nucleus is associated with improved patient outcome in ER-positive breast cancer . Br J Cancer 99 (11) : 1769 – 1774
Cao H, Courchesne WE, Mastick CC ( 2002 ) A phosphotyrosine-dependent protein interaction screen reveals a role for phosphorylation of caveolin-1 on tyrosine 14: recruitment of C-terminal Src kinase . J Biol Chem 277 (11) : 8771 – 8774
Cho HJ, Baek KE, Park SM, Kim IK, Choi YL, Cho HJ, Nam IK, Hwang EM, Park JY, Han JY, Kang SS, Kim DC, Lee WS, Lee MN, Oh GT, Kim JW, Lee CW, Yoo J ( 2009 ) RhoGDI2 expression is associated with tumor growth and malignant progression of gastric cancer . Clin Cancer Res 15 (8) : 2612 – 2619
Cooper JA, Howell B ( 1993 ) The when and how of Src regulation . Cell 73 (6) : 1051 – 1054
Dalbagni G, Genega E, Hashibe M, Zhang ZF, Russo P, Herr H, Reuter V ( 2001 ) Cystectomy for bladder cancer: a contemporary series . J Urol 165 (4) : 1111 – 1116
Davidson B, Nesland JM, Goldberg I, Kopolovic J, Gotlieb WH, Bryne M, Ben-Baruch G, Berner A, Reich R ( 2001 ) Caveolin-1 expression in advanced-stage ovarian carcinoma – a clinicopathologic study . Gynecol Oncol 81 (2) : 166 – 171
Elsberger B, Tan BA, Mitchell TJ, Brown SB, Mallon EA, Tovey SM, Cooke TG, Brunton VG, Edwards J ( 2009 ) Is expression or activation of Src kinase associated with cancer-specific survival in ER-, PR- and HER2-negative breast cancer patients? Am J Pathol 175 (4) : 1389 – 1397
Engen JR, Wales TE, Hochrein JM, Meyn III MA, Banu OS, Bahar I, Smithgall TE ( 2008 ) Structure and dynamic regulation of Src-family kinases . Cell Mol Life Sci 65 (19) : 3058 – 3073
Epstein JI, Amin MB, Reuter VR, Mostofi FK ( 1998 ) The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee . Am J Surg Pathol 22 (12) : 1435 – 1448
Fanning P, Bulovas K, Saini KS, Libertino JA, Joyce AD, Summerhayes IC ( 1992 ) Elevated expression of pp60c-src in low grade human bladder carcinoma . Cancer Res 52 (6) : 1457 – 1462
Fine SW, Lisanti MP, Galbiati F, Li M ( 2001 ) Elevated expression of caveolin-1 in adenocarcinoma of the colon . Am J Clin Pathol 115 (5) : 719 – 724
Fong A, Garcia E, Gwynn L, Lisanti MP, Fazzari MJ, Li M ( 2003 ) Expression of caveolin-1 and caveolin-2 in urothelial carcinoma of the urinary bladder correlates with tumor grade and squamous differentiation . Am J Clin Pathol 120 (1) : 93 – 100
Frame MC ( 2002 ) Src in cancer: deregulation and consequences for cell behaviour . Biochim Biophys Acta 1602 (2) : 114 – 130
Galbiati F, Volonte D, Engelman JA, Watanabe G, Burk R, Pestell RG, Lisanti MP ( 1998 ) Targeted downregulation of caveolin-1 is sufficient to drive cell transformation and hyperactivate the p42/44 MAP kinase cascade . EMBO J 17 (22) : 6633 – 6648
Galbiati F, Volonte D, Liu J, Capozza F, Frank PG, Zhu L, Pestell RG, Lisanti MP ( 2001 ) Caveolin-1 expression negatively regulates cell cycle progression by inducing G(0)/G(1) arrest via a p53/p21(WAF1/Cip1)-dependent mechanism . Mol Biol Cell 12 (8) : 2229 – 2244
Gildea JJ, Seraj MJ, Oxford G, Harding MA, Hampton GM, Moskaluk CA, Frierson HF, Conaway MR, Theodorescu D ( 2002 ) RhoGDI2 is an invasion and metastasis suppressor gene in human cancer . Cancer Res 62 (22) : 6418 – 6423
Glenney Jr JR, Zokas L ( 1989 ) Novel tyrosine kinase substrates from Rous sarcoma virus-transformed cells are present in the membrane skeleton . J Cell Biol 108 (6) : 2401 – 2408
Grande-Garcia A, Echarri A, de RJ, Alderson NB, Waterman-Storer CM, Valdivielso JM, del Pozo MA ( 2007 ) Caveolin-1 regulates cell polarization and directional migration through Src kinase and Rho GTPases . J Cell Biol 177 (4) : 683 – 694
Ho CC, Huang PH, Huang HY, Chen YH, Yang PC, Hsu SM ( 2002 ) Up-regulated caveolin-1 accentuates the metastasis capability of lung adenocarcinoma by inducing filopodia formation . Am J Pathol 161 (5) : 1647 – 1656
Ito Y, Yoshida H, Nakano K, Kobayashi K, Yokozawa T, Hirai K, Matsuzuka F, Matsuura N, Kakudo K, Kuma K, Miyauchi A ( 2002 ) Caveolin-1 overexpression is an early event in the progression of papillary carcinoma of the thyroid . Br J Cancer 86 (6) : 912 – 916
Kirkegaard T, Edwards J, Tovey S, McGlynn LM, Krishna SN, Mukherjee R, Tam L, Munro AF, Dunne B, Bartlett JM ( 2006 ) Observer variation in immunohistochemical analysis of protein expression, time for a change? Histopathology 48 (7) : 787 – 794
Kopetz S, Shah AN, Gallick GE ( 2007 ) Src continues aging: current and future clinical directions . Clin Cancer Res 13 (24) : 7232 – 7236
Kunze E, Von BF, Werner C, Wendt M, Schlott T ( 2006 ) Transitional cell carcinomas and nonurothelial carcinomas of the urinary bladder differ in the promoter methylation status of the caveolin-1, hDAB2IP and p53 genes, but not in the global methylation of Alu elements . Int J Mol Med 17 (1) : 3 – 13
Lee H, Volonte D, Galbiati F, Iyengar P, Lublin DM, Bregman DB, Wilson MT, Campos-Gonzalez R, Bouzahzah B, Pestell RG, Scherer PE, Lisanti MP ( 2000 ) Constitutive and growth factor-regulated phosphorylation of caveolin-1 occurs at the same site (Tyr-14) in vivo: identification of a c-Src/Cav-1/Grb7 signaling cassette . Mol Endocrinol 14 (11) : 1750 – 1775
Li S, Couet J, Lisanti MP ( 1996 ) Src tyrosine kinases, Galpha subunits, and H-Ras share a common membrane-anchored scaffolding protein, caveolin. Caveolin binding negatively regulates the auto-activation of Src tyrosine kinases . J Biol Chem 271 (46) : 29182 – 29190
Liu P, Rudick M, Anderson RG ( 2002 ) Multiple functions of caveolin-1 . J Biol Chem 277 (44) : 41295 – 41298
Luo FR, Barrett YC, Yang Z, Camuso A, McGlinchey K, Wen ML, Smykla R, Fager K, Wild R, Palme H, Galbraith S, Blackwood-Chirchir A, Lee FY ( 2008 ) Identification and validation of phospho-SRC, a novel and potential pharmacodynamic biomarker for dasatinib (SPRYCEL), a multi-targeted kinase inhibitor . Cancer Chemother Pharmacol 62 (6) : 1065 – 1074
Masaki T, Okada M, Shiratori Y, Rengifo W, Matsumoto K, Maeda S, Kato N, Kanai F, Komatsu Y, Nishioka M, Omata M ( 1998 ) pp60c-src activation in hepatocellular carcinoma of humans and LEC rats . Hepatology 27 (5) : 1257 – 1264
Moon HG, Jeong SH, Ju YT, Jeong CY, Lee JS, Lee YJ, Hong SC, Choi SK, Ha WS, Park ST, Jung EJ ( 2010 ) Up-regulation of RhoGDI2 in human breast cancer and its prognostic implications . Cancer Res Treat 42 (3) : 151 – 156
Nam S, Kim D, Cheng JQ, Zhang S, Lee JH, Buettner R, Mirosevich J, Lee FY, Jove R ( 2005 ) Action of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cells . Cancer Res 65 (20) : 9185 – 9189
Niu H, Li H, Xu C, He P ( 2010 ) Expression profile of RhoGDI2 in lung cancers and role of RhoGDI2 in lung cancer metastasis . Oncol Rep 24 (2) : 465 – 471
Rajjayabun PH, Garg S, Durkan GC, Charlton R, Robinson MC, Mellon JK ( 2001 ) Caveolin-1 expression is associated with high-grade bladder cancer . Urology 58 (5) : 811 – 814
Sanchez-Carbayo M, Socci ND, Lozano J, Saint F, Cordon-Cardo C ( 2006 ) Defining molecular profiles of poor outcome in patients with invasive bladder cancer using oligonucleotide microarrays . J Clin Oncol 24 (5) : 778 – 789
Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT ( 1994 ) Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src . Mol Cell Biol 14 (3) : 1680 – 1688
Slack NH, Bross ID, Prout Jr GR ( 1977 ) Five-year follow-up results of a collaborative study of therapies for carcinoma of the bladder . J Surg Oncol 9 (4) : 393 – 405
Smith Jr JA, Crawford ED, Paradelo JC, Blumenstein B, Herschman BR, Grossman HB, Christie DW ( 1997 ) Treatment of advanced bladder cancer with combined preoperative irradiation and radical cystectomy vs radical cystectomy alone: a phase III intergroup study . J Urol 157 (3) : 805 – 807
Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, Skinner E, Bochner B, Thangathurai D, Mikhail M, Raghavan D, Skinner DG ( 2001 ) Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients . J Clin Oncol 19 (3) : 666 – 675
Stein JP, Skinner DG ( 2006 ) Radical cystectomy for invasive bladder cancer: long-term results of a standard procedure . World J Urol 24 (3) : 296 – 304
Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, Newling DW, Kurth K ( 2006 ) Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials . Eur Urol 49 (3) : 466 – 465
Tapper J, Kettunen E, El-Rifai W, Seppala M, Andersson LC, Knuutila S ( 2001 ) Changes in gene expression during progression of ovarian carcinoma . Cancer Genet Cytogenet 128 (1) : 1 – 6
Tatarov O, Mitchell TJ, Seywright M, Leung HY, Brunton VG, Edwards J ( 2009 ) SRC family kinase activity is up-regulated in hormone-refractory prostate cancer . Clin Cancer Res 15 (10) : 3540 – 3549
Theodorescu D, Sapinoso LM, Conaway MR, Oxford G, Hampton GM, Frierson Jr HF ( 2004 ) Reduced expression of metastasis suppressor RhoGDI2 is associated with decreased survival for patients with bladder cancer . Clin Cancer Res 10 (11) : 3800 – 3806
Thomas S, Overdevest JB, Nitz MD, Williams PD, Owens CR, Sanchez-Carbayo M, Frierson HF, Schwartz MA, Theodorescu D ( 2011 ) Src and caveolin-1 reciprocally regulate metastasis via a common downstream signaling pathway in bladder cancer . Cancer Res 71 (3) : 832 – 841
Volonte D, Zhang K, Lisanti MP, Galbiati F ( 2002 ) Expression of caveolin-1 induces premature cellular senescence in primary cultures of murine fibroblasts . Mol Biol Cell 13 (7) : 2502 – 2517
Wu Y, Moissoglu K, Wang H, Wang X, Frierson HF, Schwartz MA, Theodorescu D ( 2009 ) Src phosphorylation of RhoGDI2 regulates its metastasis suppressor function . Proc Natl Acad Sci USA 106 (14) : 5807 – 5812
Zhang Y, Zhang B ( 2006 ) D4-GDI, a Rho GTPase regulator, promotes breast cancer cell invasiveness . Cancer Res 66 (11) : 5592 – 5598
Acknowledgements
This work was supported by Think Pink.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Qayyum, T., Fyffe, G., Duncan, M. et al. The interrelationships between Src, Cav-1 and RhoGD12 in transitional cell carcinoma of the bladder. Br J Cancer 106, 1187–1195 (2012). https://doi.org/10.1038/bjc.2012.52
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2012.52
Keywords
This article is cited by
-
BSA-bounded p-cresyl sulfate potentiates the malignancy of bladder carcinoma by triggering cell migration and EMT through the ROS/Src/FAK signaling pathway
Cell Biology and Toxicology (2020)
-
Temporally sequenced anticancer drugs overcome adaptive resistance by targeting a vulnerable chemotherapy-induced phenotypic transition
Nature Communications (2015)
-
Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity
Nature Reviews Cancer (2015)
-
Expression and prognostic significance of Src family members in renal clear cell carcinoma
British Journal of Cancer (2012)