Abstract
This study evaluated the prognostic value of a panel of 29 oncogenes derived from the analysis of The Cancer Genome Atlas (TCGA data) or from the recent literature on bladder tumors on a well-characterized series of muscle-invasive bladder cancer (MIBC) and non-MIBC (NMIBC) samples and tried to identify molecular prognostic markers. Mutations of HRAS, FGFR3, PIK3CA and TERT were found in 2.9%, 27.2%, 14.9% and 76.7% of tumor samples, respectively. Concerning NMIBC, on multivariate analysis, RXRA and FGFR3 levels were associated with recurrence-free survival (RFS) (p = 0.0022 and p = 0.0069) and RXRA level was associated with progression to muscle-invasive disease (p = 0.0068). We identified a 3-gene molecular signature associated with NMIBC prognosis. FGFR3 overexpression was associated with reduced response to Bacillus Calmette–Guerin treatment (p = 0.037). As regards MIBC, on multivariate analysis, ERCC2 overexpression was associated with RFS (p = 0.0011) and E2F3 and EGFR overexpression were associated with overall survival (p = 0.014 and p = 0.035). RT-PCR findings were confirmed by IHC for FGFR3. Genomic alterations in MIBC revealed in TCGA data also concern NMIBC and seem to be associated with prognosis in terms of recurrence and progression. Correcting these alterations by targeted therapies seems a promising pharmacological approach.
Similar content being viewed by others
Introduction
With 430,000 cases diagnosed worldwide, bladder cancer is responsible for 150,000 deaths a year. In France, 11,000 new cases of bladder cancer are diagnosed each year. About 70% of newly diagnosed cases are non-muscle-invasive bladder cancer (NMIBC). These cases have a 60% recurrence rate, and 10% evolve to muscle-invasive tumors. Muscle-invasive bladder cancer (MIBC) represents 30% of cases at diagnosis. Survival greatly differs between NMIBC and MIBC.
Contrary to what was previously thought, focal and recurrent gene amplifications are common in urothelial bladder carcinomas. Recently, genome analysis revealed new genomic alterations that could be used as markers in clinical oncology. Analysis of The Cancer Genome Atlas (TCGA) data revealed 27 amplified regions1. Integrated analysis of the various genetic alterations described in TCGA revealed three main deregulated signaling pathways in bladder tumors and potential therapeutic targets. Deregulations affecting the cell cycle were found in 93% of cases, those affecting histone pathway in 89% of cases, those affecting the PI3K/AKT/mammalian target of rapamycin (mTOR) pathway in 72% of cases, and those involved in chromatin remodeling in 64% of cases1. However, there are few comparative data in the literature regarding these alterations in NMIBC, probably because of the constraints associated with the sampling procedures inherent in NMIBC.
We aimed to identify genes whose abnormalities are related to prognosis, especially NMIBC prognosis. We studied a panel of 29 oncogenes derived from the analysis of TCGA data or from the recent literature on bladder tumors1,2,3,4,5,6,7 on a well-characterized series of NMIBC and MIBC samples and tried to identify molecular prognostic markers.
Results
DNA mutations
NMIBC and MIBC samples showed mutation in HRAS in 4.5% (2/44) and 1.7% (1/59) of cases, respectively; in FGFR3 in 50.0% (22/44) and 10.2% (6/59) of cases, respectively; in PIK3CA in 16.7% (10/60) and 13.5% (9/67) of cases, respectively; and in TERT in 79.5% (35/44) and 74.6% (44/59) of cases, respectively (Table 1 and Supplementary Data 1). DNA mutations were not associated with prognosis in terms of recurrence or progression of NMIBC or in terms of RFS or OS of MIBC (data not shown).
Gene expression
For most genes except PRKCI, FBXW7, E2F3, SOX4, YWHAZ, GDI2, FRS2, ERCC2 and BCL2L1, the expression profile differed between MIBC and NMIBC samples (Supplementary Data 2).
In NMIBC samples, 24/29 (82.8%) genes were significantly deregulated as compared with normal bladder tissue (p < 0.05) (Supplementary Data 2); 4 genes showed significant differences in expression by stage (pTa versus pT1), 4 by grade (low versus high) and 5 by stage and grade (TACC3, FBXW7, SOX4, YWHAZ, Ki67) (Supplementary Data 3).
In MIBC samples, 25/29 genes (86.2%) were significantly deregulated as compared with normal bladder tissue (p < 0.05) (Supplementary Data 2).
Association between gene expression and prognosis of NMIBC
On univariate analysis, only the expression of RXRA, FGFR3 and CCNE1 was associated with RFS (p = 0.0017, p = 0.043 and p = 0.039, respectively) and PFS (p = 0.0043, p = 0.022 and p = 0.022, respectively) (Supplementary Data 4, 5, and 6).
Correlation between PPARG and RXRA mRNA expressions were strong (p < 0.0000001, Spearman’s test); however, PPARG was not associated with prognosis.
Multivariate analyses included covariates associated with RFS or PFS showing significance at p < 0.05 on univariate analysis (i.e., history of NMIBC; RXRA, FGFR3 and CCNE1 status for recurrence and stage, grade, Cis and RXRA, FGFR3 and CCNE1 status for progression to muscle-invasive tumor). On multivariate analysis, history of NMIBC and RXRA and FGFR3 status remained associated with RFS (p = 0.0045, p = 0.0022 and p = 0.0069, respectively) and only RXRA status with PFS (p = 0.0068) (Table 2).
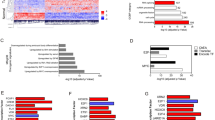
We performed unsupervised hierarchical clustering analyses of 61 NMIBC samples with the 3 genes previously found in NMIBC samples (RXRA, FGFR3, and CCNE1) and found 4 major clusters composed of 15 (Group A), 15 (Group B), 16 (Group C) and 15 (Group D) samples with various mRNA levels of these 3 genes (Fig. 1A,B). The 4 cluster groups significantly differed in terms of PFS (log rank p = 0.010): Kaplan–Meier survival curves showed poor outcome associated with group B and better with group D (Fig. 1C). Multivariate analyses including covariates associated with RFS and PFS with significance at p < 0.05 on univariate analysis and including the 3-gene signature retained history of NMIBC and the 3-gene signature as independent prognostic factors for RFS (p = 0.017 and p = 0.04, respectively) and the 3-gene signature as independent prognostic factor for PFS (p = 0.047) (Table 3). Clinical and histological characteristics of the four groups are presented in Supplemental Data 7. The four groups differed in sex, stage, grade and mutational status of FGFR3.
Supervised classification analysis of the 61 NMIBC tumor samples with the 3-gene signature comprising RXRA, FGFR3 and CCNE1. (A) dendrogram of four tumor groups obtained by hierarchical cluster with the 3-gene signature. (B) mRNA median value [range] and overexpression rates of FGFR3, RXRA and CCNE1 in the four tumor groups. p value calculated by Kruskal Wallis H-test. For each gene, mRNA values ≥ optimal cut-off were considered to represent overexpression and ≤ optimal cut-off, underexpression. (C) Kaplan–Meier curves comparing progression-free survival for tumor groups. p value calculated by log rank test.
Response to Bacillus Calmette–Guerin (BCG) therapy
In total, 21/28 (75%) patients with BCG therapy showed recurrent NMIBC or progression to invasive tumor during follow-up, including 15/28 (53.6%) over the first 2 years. Among the 29 investigated genes, only low expression of FGFR3 was significantly associated with good response to BCG therapy (no or late recurrence) (p = 0.037) (Table 4).
Association between mRNA expression and survival with MIBC
On univariate analysis, RFS and OS prognosis was poor with high expression of EGFR (p = 0.045 and p = 0.010, respectively) (Supplemental Data 8); RFS prognosis was poor with high expression of ERCC2 (p = 0.025) (Supplemental Data 9); and OS prognosis was poor with low expression of E2F3 (p = 0.030) (Supplemental Data 10).
Multivariate analyses included covariates associated with RFS or OS showing significance at p < 0.05 on univariate analysis (i.e. lymph node status and ERCC2 and EGFR status for RFS, and stage, lymph node status and E2F3 and EGFR status for OS). Lymph node status and ERCC2 status remained associated with RFS (p = 0.000014 and p = 0.0011) and lymph node status and E2F3 and EGFR level status with OS (p = 0.00016, p = 0.014 and p = 0.035, respectively) (Table 5).
Protein expression
We chose to assess protein expression of the 2 genes having a significant prognostic impact in NMIBC. The expression of RXRA was nuclear in NMIBC whereas exclusively cytosplasmic in MIBC (Supplemental Data 11). For FGFR3, expression was nuclear in both NMIBC and MIBC. The distribution of the protein expression for NMIBC and MIBC is shown in Supplemental Data 12. There was a statistically significant association between mRNA and protein expression for FGFR3 (p < 0.0001) but not for RXRA (Table 6). Protein expression was not associated with prognosis in terms of recurrence or progression of NMIBC (data not shown).
Discussion
The analysis of TCGA data for bladder tumors has allowed identifying new oncogenes potentially involved in bladder carcinogenesis because they have activating mutations or focal amplifications. The integrated analysis of the different genetic alterations described in the TCGA has revealed deregulated signaling pathways in bladder tumors and potential preferred therapeutic targets1,8. We mainly relied on TCGA data because this study was the gold standard at the time of our study. We aimed to validate some of these TCGA data on a fair-matched cohort of NMIBC and MIBC cases focusing on survival data. Within these 2 populations, the rates of recurrence and/or progression were comparable to those observed typically: half of NMIBC cases show relapse and 35% of pT1 cases progress to muscle-invasive disease. Likewise, more than half of MIBC cases (all stages combined) recur after surgery. In MIBC, we found the same results as in the TCGA data with already known prognostic markers (e.g., EGFR). Regarding NMIBC, we found genes of interest such as FGFR3 but also RXRA and CCNE1. On multivariate analysis, RXRA was associated with RFS but also with PFS. The 3-gene signature highlighted four distinct prognostic groups and was significantly associated with RFS and PFS.
Two studies showed that the transcription factor RXRA regulates the expression of PPARG and that activating mutation of RXRA hyperactivate PPARG and could be responsible for nearly 20% of bladder cancer cases9,10. These data suggest that PPARG, a key gene in bladder cancer development, could be targeted. In this regard, it is noted that association between PPARG and RXRA mRNA expressions were strong (p < 0.0000001 with Spearman’s test). Amplified / overexpressed mutated RXRA-PPARG would modulate the tumor microenvironment and lead to resistance to immunotherapy. Inactivation of PPARG or RXRA would increase sensitivity to immunotherapy11. At this time, to our knowledge, there are no clinical trials involving therapies targeting RXRA or PPARG.
Ward et al. studied the mutations of a panel of 23 genes on a cohort of 956 bladder tumors (NMIBC and MIBC) including TERT (promoter), FGFR3, PIK3CA, ERCC2, ERBB2, HRAS, RXRA, KRAS, FBXW7, ERBB3, BRAF and NRAS12. FGFR3 and HRAS mutations were of better prognosis (p = 0.006 and p = 0.04, respectively) and mutations of RXRA were significantly associated with a higher risk of recurrence (p < 0.05). In our study, we did not find any link between DNA mutations and prognosis. In our cohort, only RXRA overexpression was associated with a higher risk of recurrence.
Telomerase Reverse Transcriptase Gene (TERT) promoter mutations are recognized as one of the most frequent genetic events across all stages in bladder cancer. Mutations in the core promoter region of TERT cause telomerase reactivation in 60–80% of urothelial bladder tumors13. The TERT C228T mutation has been shown to be significantly associated with the prognosis of bladder cancer, particularly with bladder recurrence in NMIBC14, whereas TERT C250 mutation appears to be an independent predictive marker of response to BCG treatment15. However, in our series, neither C228T nor C250 mutation was associated with prognosis of NMIBC or response to BCG-therapy. Rachakonda et al. showed that the association of mutations with patient survival and disease recurrence may be regulated by a common polymorphism rs2853669 acting as a modifier of the effect of the mutations16.
In urothelial carcinomas, the frequency of FGFR3 mutations varies greatly depending on the type of tumors analyzed17. Indeed, the frequency of mutations seems high in pTa and low-grade tumors (about 70%) and much lower in pT1 (30%) or pT2-pT4 and high-grade tumors1,4. In our series, the expression of FGFR3 was stably associated with NMIBC stage, with more frequent overexpression in pTa tumors. mRNA overexpression but not mutations or protein expression were also associated with favorable prognosis. In the literature, immunohistochemical data show protein overexpression also related to good prognosis18. In our series, there was a good correlation between mRNA expression and DNA mutation. We have few clinical trial data on targeted anti-FGFR3 therapies in NMIBC. Some MIBC cases also show mutations in FGFR3 (12%) or FGFR3/TACC3 translocations (5%)19, which suggests a benefit of targeted anti-FGFR3 therapy in these patients. Necchi et al. did not find a prognostic value of FGFR3 expression in MIBC as compared with NMIBC20.
We investigated the response to BCG therapy. Expression of FGFR3 has been found a BCG response marker, which corroborates the data in mice from Langle et al.21. Indeed, in the Langle et al. study, the responder group showed low expression of FGFR3, which we also found. Because FGFR3 is "druggable", anti-FGFR3 treatments could be combined with BCG therapy to improve the response to treatment. Some studies suggest the addition of such treatments to potentiate the effect of BCG22,23, including a phase II trial evaluating dovitinib in BCG-refractory patients with FGFR3 mutated or not24.
FGFR3 is not the only gene with marked overexpression in NMIBC compared to MIBC. Several genes also appear to be involved in the early stage of bladder carcinogenesis, such as RXRA, PPARG and PVRL4. It is noteworthy that PVRL4 encodes the nectin-4 adhesion receptor, a new prognostic biomarker and a therapeutic target in various carcinomas. Anti-nectin-4 antibody–drug-conjugate (ADC) seems to have great potential as a therapeutic agent for metastatic urothelial carcinoma25. In our series, PVRL4 was also strongly expressed in NMIBC suggesting that the evaluation of anti-nectin-4 ADC in earlier steps of the disease through clinical trials could be of interest.
CCNE1 expression is increased in many cancers. As in our study, Rothman et al. showed increased expression of CCNE1 associated with high grade bladder tumors26, which is consistent with its role in cell proliferation. Similarly, in our series, high expression of CCNE1 was associated with poor prognosis in NMIBC.
It is noteworthy that mRNA expression is not always correlated with protein expression, especially for RXRA and CCNE1. Moreover, the protein expression profile appears to be different between NMIBC and MIBC, underlying that NMIBC and MIBC should be considered as two distinct disease entities with different prognostic markers and different putative therapeutic targets.
As regards MIBC, the most attractive gene seems to be EGFR because of its prognostic and theragnostic interest. In our study, EGFR seemed a prognostic factor for RFS and OS. In the TCGA data, a subgroup of tissue representing poor prognosis, called "basal-like", showed abnormal expression of the EGFR pathway18,26.The study by Rebouissou et al. suggests that this well-selected group would be a good candidate for anti-EGFR treatment, provided that patients do not have mutations in the RAS pathway that would limit the response to treatment27. More recently, anti-EGFR targeted therapies have been evaluated in urothelial carcinomas in a trial demonstrating the benefit of cetuximab combined with paclitaxel as second-line treatment for metastatic bladder cancer28.
ERCC2 is involved in DNA repair. It has recently been studied, in the MIBC as a biomarker of response to neo-adjuvant chemotherapy29. Some studies suggest that missenses mutations of ERCC2 increased cisplatin sensitivity30,31,32. A target population that could benefit the most from this treatment could be identified.
E2F3, which was significantly associated with OS in MIBC in our study, belongs to the neuronal subtype8,33 that is characterized by the expression of both neuroendocrine and neuronal genes reflecting a proliferative state.
Taking into account the prognostic impact and the predictive value of these two genes, ERCC2 and E2F3 expression levels may probably allow to guide the indication of chemotherapy in neoadjuvant or metastatic setting.
The strengths of our study lie in the robustness of the material, which is a well-defined cohort of both NMIBC and MIBC with strong clinical follow-up. However, there are some weaknesses notably its retrospective design and incomplete analysis of DNA mutations and protein expression levels since only focusing on target genes.
In conclusion, regarding the prognosis of NMIBC, predicting the risk of progression is difficult and the identification of markers is particularly interesting. Our results on RXRA and FGFR3 and the 3-gene signature, if they are confirmed, could allow for adapting the management by intensifying the surveillance and/or proposing early cystectomy in patients with abnormal expression associated with poor prognosis. It may be an interesting approach to include other omics platforms like metabolomics, proteomics, lipidomics to validate the three genes for prognosis.
Methods
Patients and samples
We analyzed 128 urothelial carcinoma samples from patients who had undergone transurethral bladder resection or radical cystectomy in our hospital between 2002 and 2007. Specimens of normal bladder tissue from 21 patients undergoing surgery unrelated to bladder tumors (transurethral resection of the prostate or prostatic adenomectomy) were used as normal bladder tissue. All patients gave their signed informed consent. This study received approval from an institutional review board (the Research Ethics Committee of Paris Descartes University) and was conducted according to the principles outlined in the Declaration of Helsinki.
Immediately after surgery, tumor samples were frozen in liquid nitrogen and stored at − 80 °C (for RNA extraction) and fixed in formaldehyde. Each tumor was reviewed by 2 pathologists (DD and MS) who were blinded to clinical outcomes. Tumors were re-staged according to the 2009 American Joint Committee on TNM classification of bladder tumors and graded according to the 2004 World Health Organization grading scheme34,35.
Complete clinical, histological and survival data were available for these 128 patients (107 men and 21 women; median age 70 years [range: 31–91]). Pathological staging revealed NMIBC in 61 (25 low-grade pTa, 14 high-grade pTa, 22 high-grade pT1) and high-grade MIBC in 67. For NMIBC patients, the median follow-up was 58 months (range: 3–158 months; mean follow-up, 64 months). For MIBC patients, the median follow-up was 12 months (range: 1–152 months; mean follow-up: 31 months). Clinical, histological and survival characteristics are presented in Table 1.
Gene selection
Recent findings from the genome-wide analysis of TCGA data highlighted new oncogenes potentially involved in bladder carcinogenesis because of activating mutations [PIK3CA (20%), ERCC2 (12%), FGFR3 (12%), ERBB3 (11%), FBXW7 (10%), RXRA (9%), NFE2L2 (8%), FRS2 (8%)] or recurrent amplifications [YWHAZ (22%), E2F3 (20%), SOX4 (20%), PVRL4 (19%), PPARG (17%), PABPC1 (17%), MYC (13%), CCNE1 (12%), BCL2L1 (11%), EGFR (11%), CCND1 (10%), ZNF703 (10%), GDI2 (10%), MDM2 (9%), PIK3CA (8%)] or overexpression [PVRLA4 (26%), PABPC1 (26%), YWHAZ (26%), SOX4 (25%), E2F3 (24%), GDI2 (21%), BCL2L1 (21%), PAIP1 (19%), EGFR (18%), FRS2 (18%), ZNF703 (18%), CCNE1 (17%), PRKCI (14%), PIK3CA (13,5%), ERBB3 (12%), PPARG (12%), CCND1 (11%), ERBB2 (10%), MDM2 (9%), ERCC2 (8,7%)]1. In addition to TCGA data, recent articles have highlighted previously unknown genetic alterations in bladder cancer. MDM4 was described as a prognostic marker of recurrence in NMIBC5. TACC3 and FGFR3 can form a fusion gene, described in some cancers1,4,6. In addition, the role of the RAS family has been extensively studied in oncology. HRAS is mutated in 5% of bladder cancers. Analysis of cell lines of bladder cancer cases revealed that this population could be treated with anti-MEK and -mTOR therapy7. In the same way, the MYC proto-oncogene is potentially "drugable"; it has been found a prognostic factor of NMIBC recurrence2.
Finally, we selected 29 genes (Supplemental Data 13) for analysis. We chose one endogenous RNA control gene to RT-PCR analysis, namely TBP (GenBank accession No. NM_003194), which encodes the TATA box-binding protein.
DNA mutation analysis
We analyzed HRAS, FGFR3, PIK3CA and TERT which have well-described mutational spots (codons 12–13 and 61 for HRAS; exon 7 (codons 248–249) and exon 10 (codons 372–373-375–382) for FGFR3; exons 9 and 20 for PIK3CA; C228T and C250T mutations for TERT). The assessment involved a screening with high-resolution melt (HRM) analysis followed by Sanger sequencing of samples with a mutated profile on HRM to validate the HRM data and to determine the nomenclature of mutations found.
Real-time quantitative RT-PCR
The theoretical basis, primers and PCR consumables, RNA extraction, cDNA synthesis, and PCR reaction conditions have been previously described in detail36. Each sample was normalized to TBP level. Ntarget values for samples were normalized such that the median of the 21 normal-bladder Ntarget values was 1. For each investigated gene, mRNA values ≥ 3 were considered to represent overexpression and ≤ 0.33 under-expression. We previously used the same cut-off value for altered tumor gene expression36. We also used the optimal cut-off calculated by the area under the receiver operating characteristic curve for prognostic studies.
Analysis of protein expression
Representative blocks of paraffin-embedded tumor samples were available. For each tumor, 2 observers, including at least 1 expert pathologist, selected the tumor block. The area of interest was defined as the one with the highest stage and grade tumor area. A table is then arranged to guide the reading of the Tissu-Micro-Array (TMA). The removal of the tissue in the area of interest is done using a trocar of the Tissu-Micro-Arrayer machine (Alphelys) and is placed on a blank paraffin block according to the plane defined beforehand on paper. Positive control tissues are also deposited and will be used when reading the TMA. Finally, the spots are fixed by depositing the block thus made on a hot plate (37–40° C). Briefly, serial 3-µm tissue sections were made using a paraffin microtome (Microtome Micron AM 360) and then were deparaffinized, rehydrated and pretreated in appropriate buffer for antigen retrieval by using a Leica automat. Tissue slides were then incubated at 48 °C with a primary antibody, anti-RXRA (ab191176, Abcam) or anti-FGFR3 (sc-13121, Santa Cruz); (both 1:100), then appropriate secondary antibodies.
We used a semi-quantitative analysis of protein expression with the following scores: 0 (no positive cells), 1 + (few positive cells), 2 + (numerous positive cells) and 3 + (a lot of positive cells). All quantification was performed with blinding to patient status by an expert pathologist (DD).
Statistical analysis
The clinicopathological features of NMIBC and MIBC cases were tested for their association with tumor recurrence and survival by using chi-square test for categorical variables. The associations between clinical and histological variables and mRNA levels were tested by the non-parametric Mann–Whitney U and Kruskal–Wallis H tests. Unsupervised hierarchical cluster analyses involved use of the WARD algorithm to identify homogenous tumor groups in terms of molecular data. Survival curves were derived by the Kaplan–Meier method, with the log-rank test used to compare survival between groups. Cox proportional-hazards regression was used to estimate hazard ratios (HRs) and their 95% confidence intervals (95% CIs) for covariates associated with RFS, PFS or OS showing significance at p < 0.05 on univariate analysis. Differences between two populations were considered statistically significant at confidence levels > 95% (p < 0.05).
Data availability
Materials and data are available.
Abbreviations
- BCG:
-
Bacillus Calmette–Guerin
- cDNA:
-
Complementary deoxyribonucleic acid
- IHC:
-
Immunohistochemistry
- NMIBC:
-
Non-muscle-invasive bladder cancer
- MIBC:
-
Muscle-invasive bladder cancer
- mRNA:
-
Messenger ribonucleic acid
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- RFS:
-
Recurrence-free survival
- RT-PCR:
-
Reverse-transcriptase polymerase chain reaction
- TCGA:
-
The Cancer Genome Atlas
- TNM:
-
Tumor-node metastasis
References
Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 507, 315–322 (2014).
Massari, F. et al. Prognostic value of beta-tubulin-3 and c-Myc in muscle invasive urothelial carcinoma of the bladder. PLoS ONE 10, e0127908 (2015).
Fu, Y.-P. et al. The 19q12 bladder cancer GWAS signal: association with cyclin E function and aggressive disease. Cancer Res. 74, 5808–5818 (2014).
Pignot, G., le Goux, C. & Bieche, I. Recent advances in bladder urothelial carcinogenesis. Bull. Cancer (Paris) 102, 1020–1035 (2015).
Salvi, S. et al. Copy number analysis of 24 oncogenes: MDM4 identified as a putative marker for low recurrence risk in non muscle invasive bladder cancer. Int. J. Mol. Sci. 15, 12458–12468 (2014).
Williams, S. V., Hurst, C. D. & Knowles, M. A. Oncogenic FGFR3 gene fusions in bladder cancer. Hum. Mol. Genet. 22, 795–803 (2013).
Kiessling, M. K. et al. Mutant HRAS as novel target for MEK and mTOR inhibitors. Oncotarget 6, 42183–42196 (2015).
Robertson, A. G. et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell 171, 540-556.e25 (2017).
Halstead, A. M. et al. Bladder-cancer-associated mutations in RXRA activate peroxisome proliferator-activated receptors to drive urothelial proliferation. eLife 6, e30862 (2017).
Goldstein, J. T. et al. Genomic activation of PPARG reveals a candidate therapeutic axis in bladder cancer. Cancer Res. 77, 6987–6998 (2017).
Korpal, M. et al. Evasion of immunosurveillance by genomic alterations of PPARγ/RXRα in bladder cancer. Nat. Commun. 8, 1–14 (2017).
Ward, D. G. et al. Targeted deep sequencing of urothelial bladder cancers and associated urinary DNA: a 23-gene panel with utility for non-invasive diagnosis and risk stratification. BJU Int. https://doi.org/10.1111/bju.14808 (2019).
Günes, C. et al. Implications of TERT promoter mutations and telomerase activity in urothelial carcinogenesis. Nat. Rev. Urol. 15, 386–393 (2018).
Hayashi, Y. et al. TERT C228T mutation in non-malignant bladder urothelium is associated with intravesical recurrence for patients with non-muscle invasive bladder cancer. Mol. Oncol. https://doi.org/10.1002/1878-0261.12746 (2020).
Batista, R. et al. TERT promoter mutation as a potential predictive biomarker in BCG-treated bladder cancer patients. Int. J. Mol. Sci. 21, 947 (2020).
Rachakonda, P. S. et al. TERT promoter mutations in bladder cancer affect patient survival and disease recurrence through modification by a common polymorphism. Proc. Natl. Acad. Sci. 110, 17426–17431 (2013).
Knowles, M. A. Role of FGFR3 in urothelial cell carcinoma: biomarker and potential therapeutic target. World J. Urol. 25, 581–593 (2007).
Poyet, C. et al. Positive fibroblast growth factor receptor 3 immunoreactivity is associated with low-grade non-invasive urothelial bladder cancer. Oncol. Lett. 10, 2753–2760 (2015).
Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 511, 543–550 (2014).
Necchi, A. et al. Prognostic effect of FGFR mutations or gene fusions in patients with metastatic urothelial carcinoma receiving first-line platinum-based chemotherapy: results from a large, single-institution cohort. Eur. Urol. Focus 5, 853–856 (2019).
Langle, Y. V. et al. FGFR3 down-regulation is involved in bacillus Calmette–Guérin induced bladder tumor growth inhibition. J. Urol. 195, 188–197 (2016).
Vandeveer, A. J. et al. Systemic immunotherapy of non-muscle invasive mouse bladder cancer with avelumab, an anti-PD-L1 immune checkpoint inhibitor. Cancer Immunol. Res. https://doi.org/10.1158/2326-6066.CIR-15-0176 (2016).
Donin, N. M. et al. Immunotherapy in the treatment of urothelial carcinoma. J. Urol. https://doi.org/10.1016/j.juro.2016.02.3005 (2016).
Hahn, N. M. et al. A phase II trial of dovitinib in BCG-unresponsive urothelial carcinoma with FGFR3 mutations or overexpression: hoosier cancer research network trial HCRN 12-157. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 23, 3003–3011 (2017).
Rosenberg, J. et al. EV-101: a phase I study of single-agent enfortumab vedotin in patients with nectin-4-positive solid tumors, including metastatic urothelial carcinoma. J. Clin. Oncol. 38, 1041–1049 (2020).
Rothman, N. et al. A multi-stage genome-wide association study of bladder cancer identifies multiple susceptibility loci. Nat. Genet. 42, 978–984 (2010).
Rebouissou, S. et al. EGFR as a potential therapeutic target for a subset of muscle-invasive bladder cancers presenting a basal-like phenotype. Sci. Transl. Med. 6, 244ra91 (2014).
Wong, Y.-N. et al. Phase II trial of cetuximab with or without paclitaxel in patients with advanced urothelial tract carcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 30, 3545–3551 (2012).
Liu, D. et al. Clinical validation of chemotherapy response biomarker ERCC2 in muscle-invasive urothelial bladder carcinoma. JAMA Oncol. 2, 1094–1096 (2016).
Tse, J., Ghandour, R., Singla, N. & Lotan, Y. Molecular predictors of complete response following neoadjuvant chemotherapy in urothelial carcinoma of the bladder and upper tracts. Int. J. Mol. Sci. 20, 793 (2019).
Li, Q. et al. ERCC2 helicase domain mutations confer nucleotide excision repair deficiency and drive cisplatin sensitivity in muscle-invasive bladder cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 25, 977–988 (2019).
Pietzak, E. J. et al. Genomic differences between ‘primary’ and ‘secondary’ muscle-invasive bladder cancer as a basis for disparate outcomes to cisplatin-based neoadjuvant chemotherapy. Eur. Urol. 75, 231–239 (2019).
Sjödahl, G., Eriksson, P., Liedberg, F. & Höglund, M. Molecular classification of urothelial carcinoma: global mRNA classification versus tumour-cell phenotype classification. J. Pathol. 242, 113–125 (2017).
Sobin, L., Gospodarowicz, M. & Wittekind, C. TNM classification of malignant tumors. In UICC International Union Against Cancer 262–5 (2006).
Molinié, V. Classification of bladder tumors in 2006. Prog. Urol. FMC 16, 7–10 (2006).
Pignot, G. et al. Large-scale real-time reverse transcription-PCR approach of angiogenic pathways in human transitional cell carcinoma of the bladder: identification of VEGFA as a major independent prognostic marker. Eur. Urol. 56, 678–688 (2009).
Author information
Authors and Affiliations
Contributions
L.G.C. (data collection, statistical analysis, manuscript writing), S.V., S.A., B.D.N., Z.M., P.M., S.M., A.Y. (data collection), B.I., D.D., P.G. (data collection, manuscript reviewing).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Le Goux, C., Vacher, S., Schnitzler, A. et al. Assessment of prognostic implication of a panel of oncogenes in bladder cancer and identification of a 3-gene signature associated with recurrence and progression risk in non-muscle-invasive bladder cancer. Sci Rep 10, 16641 (2020). https://doi.org/10.1038/s41598-020-73642-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-73642-8
This article is cited by
-
BICC1 drives pancreatic cancer progression by inducing VEGF-independent angiogenesis
Signal Transduction and Targeted Therapy (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.