« Prev Next »
Some Species Have a Greater Impact Than Others on Community Structure
Dominant species are the most abundant species in a community, exerting a strong influence over the occurrence and distribution of other species. In contrast, keystone species have effects on communities that far exceed their abundance. That is to say, the importance of keystone species would not be predicted based upon their occurrence in an ecosystem. Dominant and keystone species influence the presence and abundance of other organisms through their feeding relationships. Feeding relationships — eating or being eaten — are called trophic interactions.
In addition, some organisms, called foundation species, exert influence on a community not through their trophic interactions, but by causing physical changes in the environment. These organisms alter the environment through their behavior or their large collective biomass. Foundation species may also be dominant species.
Predation can have large effects on prey populations and on community structure. Predators can increase diversity in communities by preying on competitive dominant species or by reducing consumer pressure on foundation species. For example, in rocky intertidal systems of the Pacific Northwestern US, mussels, barnacles, and seaweeds require a hard substrate to grow on, and they compete for space on the rocks. Mussels (dominant species) are superior competitors and can exclude all other species within a few years. However, starfish (keystone species) preferentially consume mussels, and in doing so, free up space for many other organisms to settle and grow, thus increasing biodiversity within this ecosystem. Similarly, kelp forests in Alaska are home to numerous species of fish and invertebrates, but these giant kelps, which are the dominant and foundation species of kelp forest communities, can be completely destroyed by sea urchins grazing. Urchins consume the kelp and create barren areas devoid of life. Urchins however are readily consumed by sea otters (keystone species), and by keeping urchin numbers low, otters assure that the kelp forest community remains intact.
Communities Can Be Structured by "Bottom-up" or "Top-down" Forces
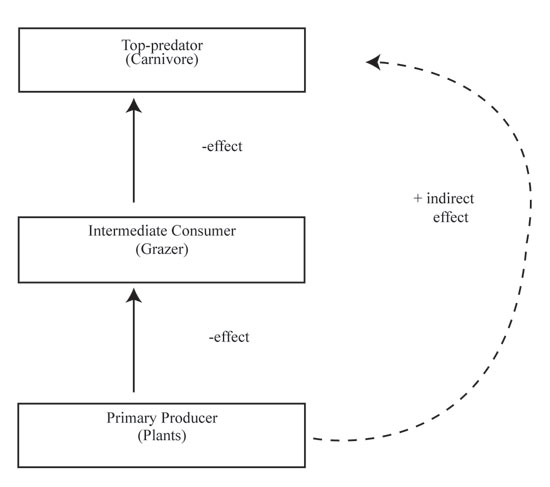
Predation is a top-down force because the effects of predators start at the top of the food chain and cascade downward to lower trophic levels. A trophic cascade occurs when predators indirectly affect the abundance of organisms more than two trophic levels down (Figure 1).
The otter-urchin-kelp interaction is an example of a trophic cascade. In this system, the otter (keystone species) increases the abundance of kelp (foundation species) by consuming urchins, thereby decreasing urchin grazing on kelp. Another example of how top-down forces affect communities via trophic cascades can be found in Yellowstone National Park, USA. A program to reintroduce wolves to Yellowstone has led to an increase in vegetation because wolves (top-predator) consume elk (intermediate consumer), one of the primary grazers in the park. Thus, top-down control (i.e., consumption) of elk by wolves alleviates grazing by elk and increases the abundance of primary producers. Similarly, predation by spiders on grasshoppers decreases grazing on plants in the fields of New England and predation by planktivorous fish on zooplankton increases the abundance of phytoplankton in freshwater lakes.
Human Activities Adversely Affect Communities by Removing Important Species, Especially Predators
These and many similar observations suggest that predators play a key role in determining the presence and abundance of many species aquatic and terrestrial communities. Unfortunately, human activities are causing the populations of many predatory species to decline worldwide. These declines may have significant consequences for communities, and deprive humans of the benefits we receive from these natural communities. In coastal systems, scallops and other bivalves are consumed by stingrays, which in turn are preyed upon by sharks. Overfishing of large shark species (top-predators) has led to an increase in the numbers of rays (intermediate consumers), and greater predation by sting rays has destroyed the scallop fishery along the East Coast of the US. Likewise, in Alaska, sea otter decline has led to an increase in sea urchin abundance and a loss of kelp forest, and this decline has been attributed to greater predation on sea otters by killer whales. Killer whales did not begin eating otters until their preferred prey, sea lions, became less abundant. The decline in sea lion populations likely resulted from overfishing of pollock. Pollock are fish and are sea lions’ primary food source. Thus, overfishing of pollock led a decline in sea lion populations, causing killer whales to seek an alternative food source (otters). The change in predation by killer whales removed an important predator in coastal Alaska and resulted in the loss of kelp forest habitat. These are just two examples of how human activities can have large, unintended consequences on the composition of entire biological communities.
Predators May Affect Communities through Lethal and Non-lethal Processes
Lethal effect (sometimes referred to as a consumptive effect) occurs when predators consume lower trophic levels. Non-lethal effect (also referred to as a non-consumptive effect) occurs when prey react to predators by altering their behavior, morphology, and/or habitat selection. Classic studies of predation, such as those described above, have focused on the lethal or consumptive effects predators have on lower trophic levels. That is to say, predators consume prey, and by reducing prey numbers, have cascading and sometimes large effects upon communities. Recent studies however have shown that predators also affect prey populations through non-lethal or non-consumptive means. In these situations, predators alter prey behavior, morphology, and/or habitat selection. Some prey species may remain in refuges and forgo foraging opportunities to avoid predators, while others may alter their morphology to make themselves less susceptible to predation. Changes in behavior or morphology are often necessary to minimize predation risk, but are costly to prey resulting in decreased growth and fecundity. Examples of non-lethal predator effects are numerous, and have recently been shown to affect community composition in much the same way lethal predator effects do. That is, a trophic cascade may occur not because a predator consumes a prey item, but because the prey species reduces foraging time to minimize risk, which results in a population increase at a lower trophic level. Consider Figure 1. If the intermediate consumer or grazer elects not to forage in response to a top-predator, there will still be an increase in primary producers despite top-predators not actually consuming grazers.
Examples of non-lethal predator effects abound, and these effects have been shown to cause trophic cascades in aquatic and terrestrial communities. In oyster reefs, juvenile oysters (basal trophic level) are consumed by mud crabs (intermediate consumers), but predation on juvenile oysters is alleviated when toad fish (top-predators) are present. Toad fish consume mud crabs (lethal effect) and also cause mud crabs to seek refuge within the reef matrix and stop foraging (non-lethal effect). Both of these effects benefit juvenile oysters by reducing predation on them by mud crabs. In the fields of New England, spiders reduce grasshopper consumption of vegetation by eating grasshoppers, thereby reducing their numbers directly, and by causing the grasshoppers to seek refuge and stop foraging. Indeed the effects of wolves on elk grazing in Yellowstone Park appear to be mediated more by a reluctance of elk to venture into open meadows to forage than by direct predation on elk by wolves. Finally, flies in the family Phoridae are parasitoids of fire ants and many studies have examined their usefulness as biological control of fire ants. These flies decapitate fire ants, but, they also cause fire ant colonies cease foraging and individual ants remain in the nests when these flies are present, which reduces the ants’ impacts in nature.
In these examples, it is clear that predators can have significant effects on the composition of entire communities by consuming lower trophic levels, and by altering the behavior or habitat selection of prey. Understanding how predators affect communities remains a central goal of contemporary ecology as changes in predator population densities or predator behavior may have significant effects on entire ecosystems. Many predator species are in decline globally, and conservation of these important species will likely be essential to insure the long-term stability of freshwater, marine, and terrestrial ecosystems.
References and Recommended Reading
Arnold, W. S. The effects of prey size, predator size, and sediment composition on the rate of predation of the blue crab, Callinectes sapidus Rathbun, on the hard clam, Mercenaria mercenaria (Linne). Journal of Experimental Marine Biology and Ecology 80, 207-219 (1984).
Bertness, M. D., Trussell, G. C. et al. Do alternate stable community states exist in the Gulf of Maine rocky intertidal zone? Ecology 83, 3434-3448 (2002).
Carpenter, S. R., Kitchell, J. F. et al. Cascading trophic interactions and lake productivity. Bioscience 35, 634-639 (1985).
Doering, P. Reduction of attractiveness to the sea star Asterias forbesi (Desor) by the clam Mercenaria mercenaria (Linnaeus). Journal of Experimental Marine Biology and Ecology 60, 47-61 (1982).
Estes, J. A. & J. F. Palmisano. 1974. Sea otters, Their role in structuring nearshore communities. Science 185, 1058-1060.
Ferner, M. C., Smee, D. L. et al. Habitat complexity alters lethal and non-lethal olfactory interactions between predators and prey. Marine Ecology Progress Series 374, 13-22 (2009).
Ferner, M. C. & Weissburg, M. J. Slow-moving predatory gastropods track prey odors in fast and turbulent flow. Journal of Experimental Biology 208, 809-819 (2005).
Finelli, C. M., Pentcheff, N. D. et al. Physical constraints on ecological processes, A field test of odor-mediated foraging. Ecology 81, 784-797 (2000).
Grabowski, J. H. Habitat complexity disrupts predator-prey interactions but not the trophic cascade on oyster reefs. Ecology 85, 995-1004 (2004).
Grabowski, J. H., Hughes, A. R. et al. How habitat setting influences restored oyster reef communities. Ecology 86, 1926-1935 (2005).
Grabowski, J. H. & Kimbro, D. L. Predator-avoidance behavior extends trophic cascades to refuge habitats. Ecology 86, 1312-1319 (2005).
Griffiths, C. & Richardson, C. Chemically induced predator avoidance behaviour in the burrowing bivalve Macoma balthica. Journal of Experimental Marine Biology and Ecology 331, 91-98 (2006).
Hollebone, A. & Hay, M. An invasive crab alters interaction webs in a marine community. Biological Invasions 10, 347-358 (2008).
Irlandi, E. A. & Peterson, C. H. Modification of animal habitat by large plants - mechanisms by which seagrasses influence clam growth. Oecologia 87, 307-318 (1991).
Jackson, J. L., Webster, D. R. et al. Bed roughness effects on boundary-layer turbulence and consequences for odor-tracking behavior of blue crabs (Callinectes sapidus). Limnology and Oceanography 52, 1883 (2007).
Leonard, G. H., Levine, J. M. et al. Flow-driven variation in intertidal community structure in a Maine estuary. Ecology 79, 1395-1411 (2008).
Malmqvist, B. & Sackman, G. Changing risk of predation for a filter-feeding insect along a current velocity gradient. Oecologia 108, 450-458 (1996).
Menge, B. Top-down and bottom-up community regulation in marine rocky intertidal habitats. Journal of Experimental Marine Biology and Ecology 250, 257-289 (2000).
Menge, B. & Sutherland, J. Community regulation,variation in disturbance, competition, and predation in relation to environmental stress and recruitment. American Naturalist 130, 730 (1987).
Menge, B. A. Organization of the New England rocky intertidal community, role of predation, competition, and environmental heterogeneity. Ecological Monographs, 355-393 (1976).
Micheli, F. Effects of predator foraging behavior on patterns of prey mortality in marine soft bottoms. Ecological Monographs 67, 203-224 (1997).
Nakaoka, M. Nonlethal effects of predators on prey populations, predator-mediated change in bivalve growth. Ecology 81, 1031-1045 (2000).
Paine, R. T. Food web complexity and species diversity. American Naturalist 100, 65-75 (1966).
Peterson, C. H. Clam predation by whelks (Busycon spp.) - Experimental tests of the importance of prey size, prey density, and seagrass cover. Marine Biology 66, 159-170 (1982).
Powers, S. P. & Kittinger, J. N. Hydrodynamic mediation of predator-prey interactions, differential patterns of prey susceptibility and predator success explained by variation in water flow. Journal of Experimental Marine Biology and Ecology 273, 171-187 (2002).
Saiz, E., Calbet, A. et al. Effects of small-scale turbulence on copepods, The case of Oithona davisae. Limnology and Oceanography, 1304-1311 (2003).
Schafer, J. F. Hill, W. I. L. et al. Physiological Performance and Stream Microhabitat use by the Centrarchids Lepomis megalotis and Lepomis acrochirus. Environmental Biology of Fishes 54, 303-312 (1999).
Schmitz, O., Beckerman, A. et al. Behaviorally mediated trophic cascades, effects of predation risk on food web interactions. Ecology 78, 1388-1399 (1997).
Schmitz, O., Grabowski, J. et al. From individuals to ecosystem function, toward an integration of evolutionary and ecosystem ecology. Ecology 89, 2436-2445 (2008).
Schmitz, O. J. Direct and indirect effects of predation and predation risk in old-field interaction webs. American Naturalist 151, 327-342 (1998).
Sih, A., Crowley, P. et al. Predation, competition, and prey communities, a review of field experiments. Annual Review of Ecology and Systematics 16, 269-311 (1985).
Sih, A., Englund, G. et al. Emergent impacts of multiple predators on prey. Trends in Ecology & Evolution 13, 350-355 (1998).
Smee, D. & Weissburg M. Clamming up, environmental forces diminish the perceptive ability of bivalve prey. Ecology 87, 1587-1598 (2006a).
Smee, D. & Weissburg M. Hard clams (Mercenaria mercenaria) evaluate predation risk using chemical signals from predators and injured conspecifics. Journal of Chemical Ecology 32, 605-619 (2006b).
Smee, D. L., Ferner, M. C. et al. Alteration of sensory abilities regulates the spatial scale of nonlethal predator effects. Oecologia 156, 399-409 (2008).
Smee, D.L., Ferner, M.C. et al. Hydrodynamic sensory stressors produce nonlinear predation patterns. Ecology 91, 1391- 1400 (2010).
Trussell, G., Ewanchuk, P. et al. The fear of being eaten reduces energy transfer in a simple food chain. Ecology 87, 2979-2984 (2006).
Trussell, G. C., Ewanchuk, P. J. et al. Trait-mediated effects in rocky intertidal food chains, predator risk cues alter prey feeding rates. Ecology 84, 629-640 (2003).
Turner, A. & G. Mittlebach. 1990. Predator avoidance and community structure, interactions among piscivores, planktivores, and plankton. Ecology, 2241-2254.
Turner, A. & Montgomery, S. Spatial and temporal scales of predator avoidance, experiments with fish and snails. Ecology 84, 616-622 (2003).
Van de Meutter, F., De Meester, L. et al. Water turbidity affects predator-prey interactions in a fish-damselfly system. Oecologia 144, 327-336 (2005).
Webster, D. R. & Weissburg, M. J. Chemosensory guidance cues in a turbulent chemical odor plume. Limnology and Oceanography 46, 1034-1047 (2001).
Weissburg, M. J., James, C. P. et al. Fluid mechanics produces conflicting constraints during olfactory navigation of blue crabs, Callinectes sapidus. Journal of Experimental Biology 206, 171-180 (2003).
Weissburg, M. J. & Zimmer-Faust, R. K. Life and death in moving fluids, hydrodynamic effects on chemosensory-mediated predation. Ecology 74, 1428-1443 (1993).
Werner, E. E. & Peacor, S. D. A review of trait-mediated indirect interactions in ecological communities. Ecology 84, 1083-1100 (2003).
Zimmer, R. & Zimmer, C. Dynamic scaling in chemical ecology. Journal of Chemical Ecology 34, 822-836 (2008).































