« Prev Next »

Plants and herbivores are locked in an evolutionary arms race in which the survival of each depends on their ability to counter the adaptations of the other. Plant defenses against herbivores can be classified into several categories, including mechanical (spines/thorns, armor coatings, etc.), chemical (a vast assortment of organic and inorganic compounds), visual (crypsis and mimicry), behavioral (phenology), and associational (defensive mutualist animals or other defended plants). Chemical defenses are ubiquitous, and explain many plant-herbivore interactions. Most studies of plant defense chemicals have focused upon organic compounds (secondary compounds), many of which have been identified, and whose roles in plant defense are well known. But some plants, called hyperaccumulators (Brooks et al. 1977), contain large amounts of certain chemical elements (frequently metals or metallic compounds) that also play important defensive roles.
Hyperaccumulator Plants

Most hyperaccumulators (about 75%, Reeves & Baker 2000) accumulate Ni, and hyperaccumulators have been reported from every continent except Antarctica. Most hyperaccumulators are found on serpentine (ultramafic) soils (Proctor 1999). Serpentine soils contain large amounts of Fe and Mg, relatively small amounts of Si and Ca, and sometimes large amounts of other metals (Ni, Co, Cu, Zn, etc.) and are often more shallow and stony than other soils (Kruckeberg 1984). As a result, serpentine soils can be challenging substrates for plant growth (Figure 1) and there are a number of cases in which populations have adapted to serpentine soil conditions, sometimes resulting in speciation (Alexander et al. 2007, Brady et al. 2005). Some plant taxa, including most hyperaccumulator species, are endemic to serpentine soils (Reeves & Baker 2000).
Elemental Defense

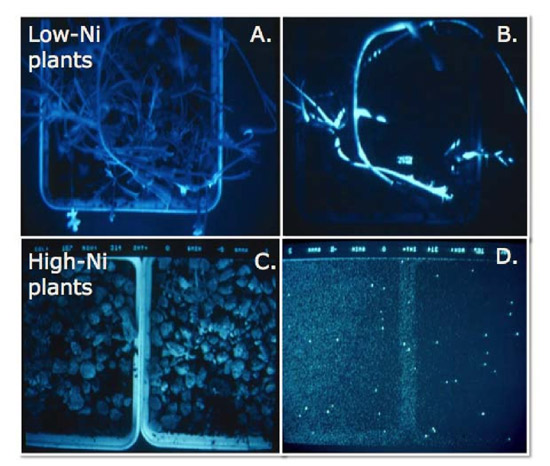
A number of laboratory studies have shown that hyperaccumulator plants can be toxic to generalist insect herbivores (see review by Boyd 2007). Several studies have also shown that metallic elemental defenses can be effective against plant pathogens. For example, Boyd et al. (1994) used the California Ni hyperaccumulator S. polygaloides grown on high- or low-Ni soils and a strain of a bacterial pathogen that had been genetically engineered to emit light. Plants were inoculated with the pathogen and allowed to incubate for 72 hours: when checked for light emission, only low-Ni plants still contained living (light-emitting) pathogens (Figure 3). Attempts to recover the pathogen from the inoculated plants were only successful for low-Ni plants, showing that the bacteria were unable to survive in high-Ni plants. Other hyperaccumulated elements that have been shown effective against plant pathogens include Se (Hanson et al. 2003) and Zn (Ghaderian et al. 2000).
Martens & Boyd (1994) suggested that elevated metal concentration in plants be considered a new category of plant chemical defense: elemental defense. Boyd (1998) noted several differences between elemental metal and synthetic, organic plant defenses. First, elemental defenses are acquired from the soil and are not synthesized by plants. Second, elements cannot be chemically degraded, preventing the evolution of this herbivore defense mechanism. Finally, elemental defenses may be less metabolically expensive than organic plant defenses. Metals are obtained from the soil and often complexed with relatively small organic molecules (Verbruggen et al. 2009). The cost implication is that plants with elemental defenses may be able to decrease their energetic input into generation of organic defenses. This hypothesis has been termed the trade-off hypothesis by Boyd (1998). There is little evidence regarding this hypothesis, but Tolrà et al. (2001) showed that Zn hyperaccumulating Thlaspi caerulescens contained lower glucosinolate concentrations than non-hyperaccumulating T. caerulescens.
Most research on the ecological consequences of plant elemental composition has targeted hyperaccumulators. As extreme examples of elemental accumulation, these species are a good starting point for testing hypotheses regarding the function of accumulated elements. However, the qualitative and quantitative limits of elemental defenses are poorly understood. For example, there are few studies of plant defense by hyperaccumulated Cu, Co or Pb (Boyd 2007). In addition, the role of Zn in plant defense is actively debated: some studies support elemental defense by Zn (Pollard & Baker 1997, Behmer et al. 2005) but others do not (Huitson & Macnair 2003, Noret et al. 2007).
Elemental Defense and Joint Effects of Chemical Defenses
Plants often possess multiple defenses against herbivores and these may combine their effects in certain ways. Two positive effects between chemicals, additivity and synergy, make each more effective together than separately. Synergy, defined as a joint effect greater than that expected due to additivity, is especially interesting because synergy essentially magnifies the benefits of each defense. The idea that organic defenses increase the defensive effect of metals through additivity or synergy comprises the joint effects hypothesis of Boyd (2007). Boyd (2004) initially speculated that elemental defenses might interact with organic chemical defenses, and initial tests by Jhee et al. (2006) showed additive effects between Ni and several organic defense chemicals. Joint effects may also explain the evolution of co-accumulation, if there are joint effects between different elements that are co-accumulated by plants. On the other hand, it is possible that co-accumulation evolved because each element is effective against a different natural adversary (Boyd 2007).
Synergistic effects are potentially important for two reasons. First, they allow elemental defenses to contribute to plant fitness at concentrations lower than those expected from the defensive capabilities of a single metallic element. This can extend the defensive effects of metals to more plant species than otherwise expected, and thus broaden the applicability of elemental defenses to plants in general. Second, joint effects can help explain how metal accumulation and hyperaccumulation have evolved. This scenario involves a second hypothesis, the defensive enhancement hypothesis, which suggests that element accumulation and hyperaccumulation evolved in a stepwise fashion driven by increased defensive benefits of higher levels of an element (Boyd 2007). In the defensive enhancement hypothesis, a plant able to take up and tolerate elevated levels of a metal derives a defensive benefit from that ability at a relatively low metal concentration. This benefit could be due to a direct effect of the metal, or through joint effects between that metal and an organic defense. A joint effect would reduce the threshold metal concentration at which that metal first makes a contribution to plant defense, so that relatively low levels of metal uptake could be defensively beneficial. After an initial defensive effect is achieved, further elemental accumulation could evolve in a population to result in even better protection.
Circumvention of Elemental Defenses by Natural Enemies
One factor that complicates studies of the elemental defense hypothesis is that all plant defenses can be circumvented by at least some natural enemies, so that even well defended plants can be successfully attacked in some cases. In the case of elemental defenses, circumvention may occur through three possible pathways (Boyd 2009): the attack of relatively undefended tissues, the ability of generalist herbivores to dilute the defended tissues they ingest by also consuming low-element plants, and the physiological adaptations that allow enemies to tolerate elemental defenses. The first pathway (undefended tissues) depends on variability of element concentration among tissues and/or cells of the plant, and on the feeding mode used by the herbivore (Jhee et al. 2005). The last pathway (tolerance) is of particular interest because tolerant enemies may themselves contain relatively elevated element concentrations. This is the case for elemental defense by Ni, in which herbivorous insects that specialize on Ni hyperaccumulators have been identified by several studies spanning three continents (Boyd 2009). One of the best studied of these high-Ni insects, defined as containing at least 500 μg Ni/g dry mass (Boyd 2009), is Melanotrichus boydi (Figure 4). This mirid bug specializes by feeding on the California Ni-hyperaccumulator plant, S. polygaloides (Wall & Boyd 2006) and contains about 750 μg Ni/g. But much higher Ni concentrations are found in some insects: the highest mean body Ni concentration reported to date is 3,500 μg/g for a grasshopper that is likely a specialist on a South African Ni-hyperaccumulator (Boyd et al. 2007).

The concept of elemental defense may extend to high-element insects (Boyd 1998). For example, Vickerman and Trumble (2003) showed that consumption of high-Se herbivores negatively affected the predacious bug Podisus maculiventris. There is only one report of elemental defense for a high-Ni insect: Boyd & Wall (2001) found that survival of the crab spider Misumena vatia was significantly reduced when fed Melanotrichus boydi, suggesting toxicity of the high-Ni insect prey. An experiment using entomophagous pathogens (two nematodes and a fungus) reported that high-Ni Melanotrichus boydi survived no better than a low-Ni insect (Lygus hesperus) when challenged by any of the three pathogens (Boyd 2002). More experimental investigation in this area is certainly warranted.
Elemental Defenses in Other Habitats
Research on elemental defenses has focused on hyperaccumulator plants, but elemental defenses may also occur in other organisms. For example, Capon et al. (1993) reported very high concentrations of Cd and Zn in an Antarctic marine sponge (Tedania charcoti), and demonstrated that these concentrations were capable of antibacterial effects. In another case, high levels of Cd, Cu, Ni or Zn were reported in some samples of Antarctic sea spiders (Pycnogonida) and the authors suggested that the high Ni levels in some samples (200 μg/g on a dry mass basis) may have defensive effects (Jöst & Zauke 2008). Although studies of elemental defenses are still in their infancy, growing awareness of their potential importance provides a new dimension to our understanding of chemical ecology.
References and Recommended Reading
Alexander, E. B., Coleman, R. G. et al. Serpentine Geoecology of Western North America: Geology, Soils, and Vegetation. New York, NY: Oxford University Press, 2007.
Babaoglu M., Gezgin, S. et al. Gypsophila spaerocephala Fenzl ex Tchihat.: a boron hyperaccumulator plant species that may phytoremediate soils with toxic B levels. Turkish Journal of Botany 28, 273-278 (2004).
Behmer, S. T., Lloyd, C. M. et al. Metal hyperaccumulation in plants: mechanisms of defence against herbivores. Functional Ecology 19, 55-66 (2005).
Boyd, R. S. Hyperaccumulation as a plant defensive strategy. In Plants that Hyperaccumulate Heavy Metals: Their Role in Phytoremediation, Microbiology, Archaeology, Mineral Exploration and Phytomining, ed. Brooks, R.R. (Oxford: CAB International, 1998) 181-201.
Boyd, R. S. Does elevated body Ni concentration protect insects against pathogens? A test using Melanotrichus boydi (Heteroptera: Miridae). American Midland Naturalist 147, 225-236 (2002).
Boyd, R. S. Ecology of metal hyperaccumulation. New Phytologist 162,563-567 (2004).
Boyd, R. S. The defense hypothesis of elemental hyperaccumulation: status, challenges and new directions. Plant and Soil 293, 153-176 (2007).
Boyd, R. S. High-nickel insects and nickel hyperaccumulator plants: A review. Insect Science 16, 19-31 (2009).
Boyd, R. S., Davis, M. A. et al. K. Host-herbivore studies of Stenoscepa sp. (Orthoptera: Pyrgomorphidae), a high-Ni herbivore of the South African Ni hyperaccumulator Berkheya coddii (Asteraceae). Insect Science 14, 133-143 (2007).
Boyd, R. S. & Jhee, E. M. A test of elemental defence against slugs by Ni in hyperaccumulator and non-hyperaccumulator Streptanthus species. Chemoecology 15, 179-185 (2005).
Boyd, R. S. & Martens, S. N. "The raison d'être for metal hyperaccumulation by plants." In The Vegetation of Ultramafic (Serpentine) Soils, ed. Baker, A.J.M., Proctor J. et al. (Andover: Intercept Limited, 1992) 279-289.
Boyd, R. S., Shaw, J. et al. Nickel hyperaccumulation defends Streptanthus polygaloides (Brassicaceae) against pathogens. American Journal of Botany 81, 294-300 (1994).
Boyd, R. S. & Wall, M. A. Responses of generalist predators fed high-Ni Melanotrichus boydi (Heteroptera: Miridae): Elemental defense against the third trophic level. American Midland Naturalist 146,186-198 (2001).
Brady, K. U., Kruckeberg, A. R. et al. Evolutionary ecology of plant adaptation to serpentine soils. Annual Review of Ecology, Evolution and Systematics 36, 243-266 (2005).
Brooks, R. R. Serpentine and its Vegetation: A Multidisciplinary Approach. Portland, OR: Dioscorides Press, 1987.
Brooks, R. R., Lee, J. et al. Detection of nickeliferous rocks by analysis of herbarium specimens of indicator plants. Journal of Geochemical Exploration 7, 49-77 (1977).
Capon, R. J., Elsbury, K. et al. Extraordinary levels of cadmium and zinc in a marine sponge, Tedania charcoti Topsent: inorganic defense agents. Experientia 49, 263-264 (1993).
Ghaderian, S. M., Lyon, A. J. E. et al. Seedling mortality of metal hyperaccumulator plants resulting from damping-off by Pythium spp. New Phytologist 146, 219-224 (2000).
Hanson, B., Garifullina, G. F. et al. Selenium accumulation protects Brassica juncea from invertebrate herbivory and fungal infection. New Phytologist 159, 461-469 (2003).
Hanson, B., Lindblom, S. D. et al. Selenium protects plants from phloem-feeding aphids due to both deterrence and toxicity. New Phytologist 162, 655-662 (2004).
Huitson, S. & Macnair, M. R. Does zinc protect the zinc hyperaccumulator Arabidopsis halleri from herbivory by snails? New Phytologist 159, 453-459 (2003).
Jansen, S., Broadley, M. R. et al. Aluminium hyperaccumulation in angiosperms: A review of its phylogenetic significance. Botanical Review 68, 235-269 (2002).
Jhee, E. M., Boyd, R. S. et al. Effectiveness of metal-metal and metal-organic compound combinations against Plutella xylostella (Lepidoptera: Plutellidae): Implications for plant elemental defense. Journal of Chemical Ecology 32, 239-259 (2006).
Jhee, E. M., Boyd, R. S. et al. Nickel hyperaccumulation as an elemental defence of Streptanthus polygaloides (Brassicaceae): influence of herbivore feeding mode. New Phytologist 168, 331-344 (2005).
Jöst, C. & Zauke, G.P. Trace metal concentrations in Antarctic sea spiders (Pycnogonida: Pantopoda). Marine Pollution Bulletin 56, 1396-1399 (2008).
Kruckeberg, A. R. California Serpentines: Flora, Vegetation, Geology, Soils, and Management Problems. Berkeley, CA: University of California Press, 1984.
Ma, L. Q., Komar, K. M. et al. A fern that hyperaccumulates arsenic. Nature 209, 579 (2001).
Martens, S. N. & Boyd, R. S. The ecological significance of nickel hyperaccumulation: a plant chemical defense. Oecologia 98, 379-384 (1994).
Noret, N., Meerts, P. et al. Do metal-rich plants deter herbivores? A field test of the defence hypothesis. Oecologia 151, 92-100 (2007).
Pollard, A. J. & Baker, A. J. M. Deterrence of herbivory by zinc hyperaccumulation in Thlaspi caerulescens (Brassicaceae). New Phytologist 135, 655-658 (1997).
Proctor, J. Toxins, nutrient shortages and droughts: the serpentine challenge. Trends in Ecology & Evolution 14, 334-335 (1999).
Reeves, R. D. & Baker, A. J. M. "Metal-accumulating plants." In Phytoremediation of Toxic Metals: Using Plants to Clean Up the Environment, eds. Raskin, I. & Ensley, B.D. (New York: John Wiley and Sons, 2000): 193-229.
Rodríguez, N., Menéndez, N. et al. Internal iron biomineralization in Imperata cylindrica, a perennial grass: chemical composition, speciation and plant localization. New Phytologist 165, 781-789 (2005).
Tolrà, R. O., Poschenrieder, C. et al. Influence of zinc hyperaccumulation on glucosinolates in Thlaspi caerulescens. New Phytologist 151, 621-626 (2001).
Verbruggen, N., Hermans, C. et al. Molecular mechanisms of metal hyperaccumulation in plants. New Phytologist 181, 759-776 (2009).
Vickerman, D. B. & Trumble, J. T. Biotransfer of selenium: effects on an insect predator, Podisus maculiventris. Ecotoxicology 12, 497-504 (2003).
Wall, M. A. & Boyd, R. S. Melanotrichus boydi (Hemiptera: Miridae) is a specialist on the nickel hyperaccumulator Streptanthus polygaloides (Brassicaceae). Southwestern Naturalist 51, 481-489 (2006).






























