« Prev Next »

If you thought you left balancing chemical reactions (stoichiometry) behind in freshman chemistry class, think again. Biological Stoichiometry is the study of the balance of energy and multiple chemical elements in living systems (Elser et al. 2000). This approach uses the same concepts and principles as chemical stoichiometry, but applies them to many phenomena at all levels of biology, from genes and molecules to whole organisms and even to ecosystems and the biosphere. Biological Stoichiometry is not a new concept; it emerged from Ecological Stoichiometry (the study of the balance of energy and multiple chemical elements in ecological systems) (Sterner & Elser 2002), which itself has deep intellectual roots in the work of the famous scientists Justus Leibig, AJ Lotka, and AC Redfield. While the approach is general and can be applied to any set of elements, most work to date has focused on the major elements carbon (C), nitrogen (N), and phosphorus (P).
Let's imagine an example involving carbon and nitrogen and a locust eating grass. In this situation, the locust has 5 atoms of carbon for every atom of nitrogen but the grass has 33 atoms of carbon for every atom of nitrogen. The locust grows by converting the grass (C:N = 33) to its own biomass (C:N = 5), but, since animals maintain relatively constant C:element ratios in their biomass, the carbon in the food is greatly in excess relative to nitrogen. So the locust has to process a lot of excess carbon to obtain the nitrogen it needs to produce each gram of its own biomass. What should it do with the excess carbon to convert the food from a C:N of 33 to its own tissues with a C:N of 5? It can excrete it or respire it away so that the C:N ratio narrows down to 5. In fact, it does both. In addition, herbivores often, but not always, retain all the nitrogen they can, since nitrogen is deficient in their food relative to their own body requirements. (See Figure 1.)

The result, in this case, of maintaining a constant body C:N ratio while increasing animal mass using high C:N: food, is low conversion efficiency for C and high conversion efficiency for N.
Stoichiometric Patterns in Different Trophic Levels
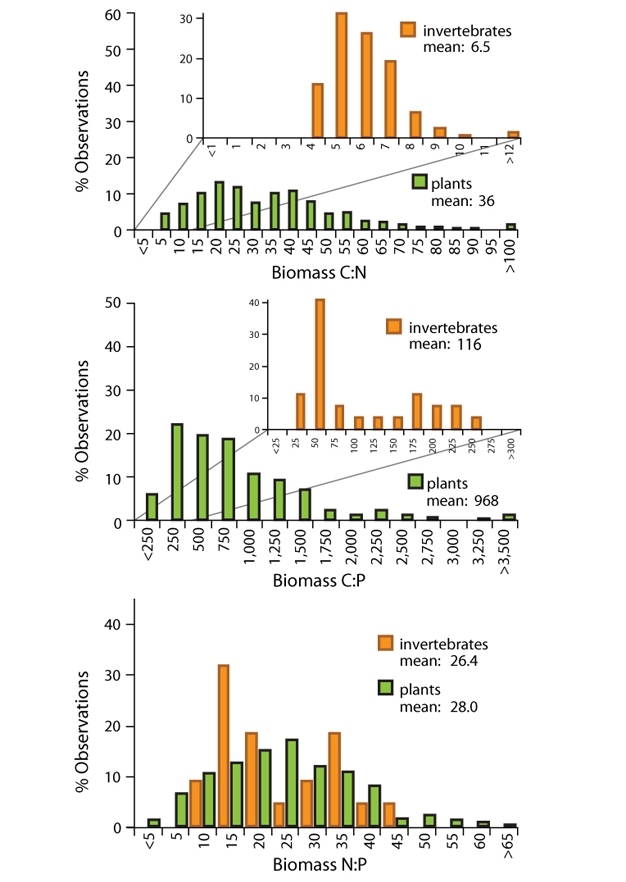
Among living organisms, there are some major patterns of stoichiometry. Looking first at autotrophs, the food web base, we find that there is large variability in their elemental composition. Photosynthetic organisms (autotrophs), such as plants, algae, and cyanobacteria, appear to be quite flexible in their growth rate and chemical composition and are able to adjust their ratios depending on availability of nutrients in the environment. Thus, plants exhibit "weak stoichiometric homeostasis," meaning that their biomass C:N:P ratios tend to track those of environmental supplies. For example, when grown in a range of nutrient concentrations, one species of algae can exhibit nearly as much variation in its N:P ratio as is found when comparing average values for autotrophs from algae to trees. Abiotic factors such as light, temperature, and water availability can also influence autotroph stoichiometry. If autotrophs are exposed to more sunlight, they can increase photosynthesis and growth rate, leading to greater carbon input via photosynthesis, and hence increased biomass with the same amount of nutrients available, which results in an increased C:nutrient ratio.
As we move up the food chain, the story changes. Heterotrophs, compared to autotrophs, tend to be more nutrient-rich (low C:nutrient ratios) and generally exhibit strong stoichiometric homeostasis; that is, they maintain a given elemental composition even when consuming food with a broad variety of C:N:P ratios. Any given human, for example, has a ratio of carbon to nitrogen to phosphorus roughly around 18:2:1. So the mantra ‘you are what you eat' should be ‘you are what you eat except for what you excrete, egest, or metabolize.' (See figure 2.)
Moving Through a Food Web: Compensating for a Stoichiometric Imbalance
Consumers possess various physiological mechanisms to help compensate for stoichiometric imbalance. For example, when consuming an excess of carbohydrates, herbivores can decrease the efficiency with which they assimilate C-rich compounds during digestion by altering production of digestive enzymes and excrete the excess C, store excess C as lipids, or increase metabolic rate and thus respire excess carbon in the form of CO2. However, as with most traits, there are tradeoffs or costs for these physiological compensatory mechanisms. A large stoichiometric imbalance between the primary producer and consumer generally results in decreased growth, reproduction, and survivorship for the consumer.
Thinking Bigger: Nutrient Cycling in Food Webs and Ecosystems
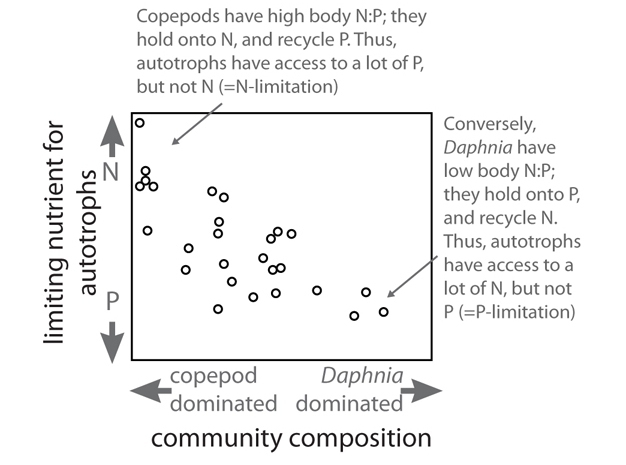
Stoichiometric relationships can also affect the relative rates at which N and P are recycled in ecosystems, with important feedback effects. For example, some species of zooplankton (such as the crustacean Daphnia) have low body N:P ratio in their biomass while others (such as copepods) have high N:P ratio. Theoretical models, lab and field experiments, and whole-ecosystem studies have shown that these differences result in differential recycling of N and P. Low-N:P species tend to retain P and recycle N in excretion and thus have high recycling N:P ratio, while high-N:P species retain N and recycle P in excretion and have low recycling N:P ratio. The excreted material, however, generally decomposes quickly and releases the excess nutrient back into the environment where plants can take it up again. In some situations these differences can affect the overall nutrient limitation regime experienced by the autotrophs, which show signs of P limitation when low N:P consumers are dominant and excrete N while retaining P, but are N-limited when high N:P consumers predominate while excreting P and retaining N. In some situations this differential nutrient recycling can affect whole-ecosystem nutrient fluxes because free recycling of N by consumers can reduce N-fixation by cyanobacteria. (See Figure 3.)
Phosphorus, RNA, and the Growth Rate Hypothesis
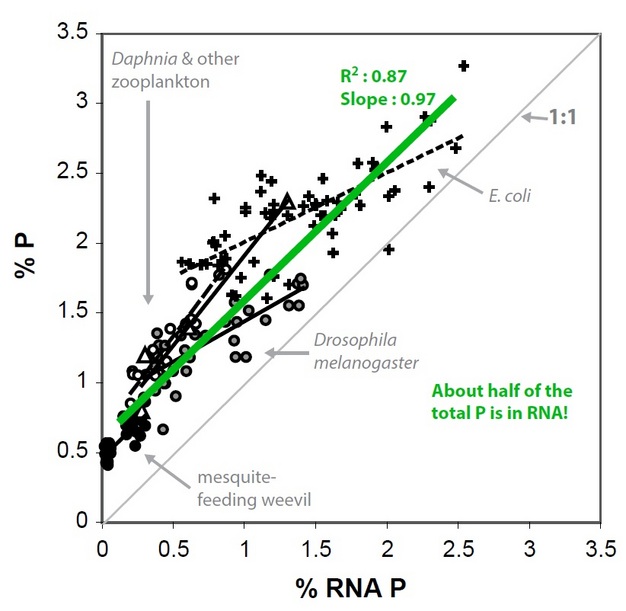
So, why do C:N:P ratios vary in individual organisms? Much of the answer arises from the chemical composition of biomolecules - and the proportional allocation of those molecules - in a given organism. An interesting pattern that emerged in the biological stoichiometry literature is that rapidly growing organisms (e.g., Daphnia) have a higher P content (low C:P and low N:P) than slower growing organisms (e.g., calanoid copepods, another group of zooplankton). A variety of studies have shown that inter- and intraspecific variation in organismal P content for these small invertebrates, as well as other biota such as bacteria, can be explained by an increase in P-rich ribosomal RNA (rRNA; 9% P by mass). Ribosomes are the protein manufacturing machinery in all living cells and are needed in great abundance for rapid cellular proliferation. Thus, high levels of rRNA (and the availability of P-rich food to elevate production of rRNA) is a necessity for rapid growth. The proposal that variation in C:N:P stoichiometry is driven by growth-dependent variation in allocation to P-rich RNA is known as the "Growth Rate Hypothesis." (See Figure 4.)
Other Determinants of Biomass Stoichiometry
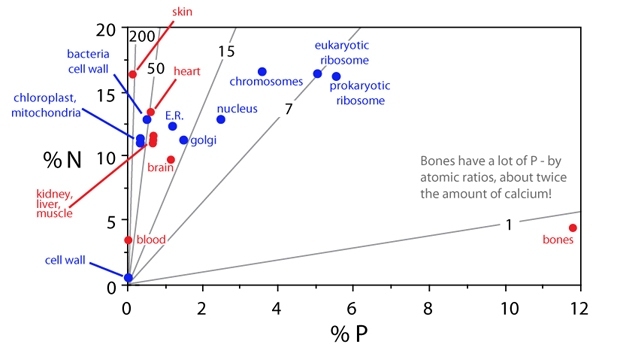
All living organisms on Earth have a similar elemental composition profile. This elemental composition does not represent a random sample of the chemical elements available on Earth. Thinking back to physical chemistry, we know that the physical properties of elements such as C, N, and P dictate the chemical effects of which they are capable. Moving to organic chemistry to consider biomolecules, we know that their elemental composition is directly linked to biological function. Determining which biomolecules, cellular structures, or cellular processes differ strongly in their demands with respect to specific elements may identify particular biological functions associated with different contents of these elements. For example, plant cell walls are nutrient (e.g., N and P)-poor and carbon-rich; it is the carbon-rich architecture that provides their rigid structure. Chloroplasts and mitochondria, organelles involved in cellular energetics, are N-rich but relatively P-poor, because N is used repeatedly in the enzymes and structural proteins necessary to process energy. Thus, chloroplasts and mitochondria have high N:P ratios. In contrast, ribosomes contain both protein and ribosomal RNA, resulting in a low N:P ratio for this organelle.
Making the leap from plants, microbes, and invertebrates to vertebrates, a new pool of phosphorus arises: bones, which are 12% phosphorus on average. Bones have more P than calcium (by mass) and there is a strong association between low body C:P and N:P (high P) and increased bone allocation in vertebrates. This high allocation of P to bones can have significant impacts on ecosystem nutrient cycling. For example, armored catfish can have an unusually high body P content (~5% P), primarily due to their large mass of bones and scales (which are derived from bones) (Vanni et al. 2002). As a consequence, armored catfish have very low rates of P recycling (and high rates of C and N recycling) as they sequester and allocate this element to bony structures. Thus, this pool of phosphate going into vertebrate bones has major consequences for organisms and their ecosystem. (See Figure 5.)
Expanding the Application of Stoichiometric Theory: Could Increased Dietary P Stimulate Tumor Proliferation?
Some of the first studies evaluating these possibilities have been performed and provide some support. Compared to paired normal samples, human tumors of colon and lung were shown to have 2-3 times higher contents of nucleic acids and of P. However, tumors of liver and kidney did not show such differences, suggesting that tumor evolution in different organs might proceed along different pathways, some favoring transformed cells with rapid proliferation and others favoring cells with lower mortality rates. Interestingly, experimental studies of lung and skin cancer in mice in which dietary P content has been manipulated have shown a significant positive association of P intake with tumor number, size, and proliferation rate. Overall, the application of stoichiometric theory to cancer biology is very preliminary but provides an example of how the relatively new theory of biological stoichiometry might provide novel insights in a variety of new areas (Elser et al. 2007).
Summary
References and Recommended Reading
Cease, A. J. et al. Heavy Livestock Grazing Promotes Locust Outbreaks by Lowering Plant Nitrogen Content. Science 335:467-469 (2012).
Elser, J. J. & Hamilton, A. Stoichiometry and the new biology: The future is now. PLoS Biology 5, 1403-1405 (2007).
Elser, J. J. et al. Zooplankton-mediated transitions between N-and P-limited algal growth. Limnology and Oceanography 33, 1-14 (1988).
Elser, J. J. et al. Organism size, life history, and N:P stoichiometry. BioScience 674-684 (1996).
Elser, J. J. et al. Biological stoichiometry from genes to ecosystems. Ecology Letters 3, 540-550 (2000a).
Elser, J. J. et al. Nutritional constraints in terrestrial and freshwater food webs. Nature 408, 578-580 (2000b).
Elser, J. J. et al. Growth rate-stoichiometry couplings in diverse biota. Ecology Letters 6, 936-943 (2003).
Elser, J. J. et al. Biological stoichiometry in human cancer. PLoS ONE 2, 1985-1993 (2007).
Sterner, R. & Elser, J. J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere. Princeton, NJ: Princeton University Press, 2002.
Vanni, M. J. et al. Stoichiometry of nutrient recycling by vertebrates in a tropical stream: Linking species identity and ecosystem processes. Ecology Letters 5, 285-293 (2002).






























