« Prev Next »
Introduction to Population Growth Limitation
Populations grow at geometric or exponential rates in the presence of unlimited resources. Geometric populations grow through pulsed reproduction (e.g., the annual reproduction of deer, which have a constrained mating and reproduction season). Exponential populations grow continuously, with reproduction occurring at any time, such as among humans. All populations begin exponential growth in favorable environments and at low population densities. Because of this, exponential growth may apply to populations establishing new environments, during transient, favorable conditions, and by populations with low initial population density.
However, geometrical or exponential growth cannot continue indefinitely. In nature, population growth must eventually slow, and population size ceases to increase. As resources are depleted, population growth rate slows and eventually stops: This is known as logistic growth. The population size at which growth stops is generally called the carrying capacity (K), which is the number of individuals of a particular population that the environment can support. At carrying capacity, because population size is approximately constant, birthrates must equal death rates, and population growth is zero.
Populations Cannot Grow Without Limit
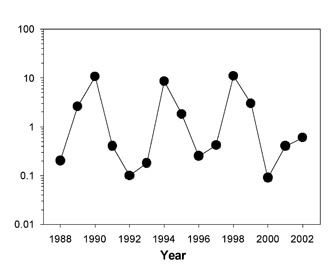
One organism that experiences rapid oscillations in population density in response to growth limiting factors is the lemming. Lemmings are small rodents that live in the high-Arctic tundra of Greenland and in other arctic environments across the world. One species, the collard lemming (Dycrostonyx groenlandicus), is a chubby-looking rodent living in the arctic of North America and Greenland. It is food for a number of vertebrate predators, including the stoat (a short-tailed weasel), the arctic fox, the snowy owl, and the long-tailed skua (a seabird). Because of the simplicity of this system, lemming population dynamics make an excellent case study for examining the factors regulating population growth. Gilg et al. (2003) studied this system in the Karup Valley of northeast Greenland. The lemming population increased and decreased in a regular four-year cycle during the study period, 1988–2002 (Figure 1). The number of lemmings increased to as many as ten per hectare. Gilg et al. (2003) found that the single most important factor limiting lemming population size was the predation pressure affecting those populations. The owl, fox, and skua switched to lemming predation as the lemming numbers increased, preventing rapid population growth. As the lemmings provided the stoat with additional food, their reproductive success increased, allowing an increased stoat population. Stoat population expansion eventually overran lemming population growth, and the lemming population collapsed, soon followed by a collapse in the stoat population, and the cycle repeated itself.
Density Dependant Limitation
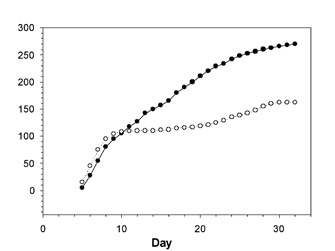
Density-dependant factors may influence the size of the population by changes in reproduction or survival. Wauters & Lens (1995) studied how food availability and density combine to limit red squirrel reproduction rates. The red squirrel (Sciurus vulgaris) is a small rodent inhabiting forests in Europe and Asia. They studied squirrels in both coniferous and deciduous woodlands and investigated how limitations in food resulted in limitations in reproduction as population densities increased. They found that when squirrel densities were high, territoriality relegated some females to poor quality territory, which in turn reduced their reproductive success. When squirrel densities were low, no females occupied the low-quality territory. Thus, it was not all individuals suffering from reduced ability to reproduce (e.g., fecundity) due to the density increase. Instead, a greater proportion of the population was living in poor-quality habitat, while those still living in good habitat continued to have success. This in turn led to a decrease in per capita birth rate, a limitation in population growth as a function of population density.
Density dependant factors may also affect population mortality and migration. Clutton-Brock et al. (2002) found these density-dependant controls in a population of red deer (Cervus elaphus) in the Scottish Highlands. Both juvenile and adult mortality was significantly affected by population density, with juvenile mortality more strongly influenced than adult mortality (Figure 2). Furthermore, they found that these differences were stronger among males than females, so that increasing population density caused a shift in the sex ratio of females to males. This effect was enhanced by decreased male immigration and increased male emigration. Thus, density-dependant controls on population growth not only increased with increasing density, but also differentially affected males and females within the population.
Density Independent Limitation
Factors that decrease population growth can be defined as environmental stress including limitations in food, predation, and other density-dependant factors (Sibley & Hone 2002). However, many sources of environmental stress affect population growth, irrespective of the density of the population. Density-independent factors, such as environmental stressors and catastrophe, are not influenced by population density change. While the previously mentioned density-dependant factors are often biotic, density-independent factors are often abiotic. These density-independent factors include food or nutrient limitation, pollutants in the environment, and climate extremes, including seasonal cycles such as monsoons. In addition, catastrophic factors can also impact population growth, such as fires and hurricanes.
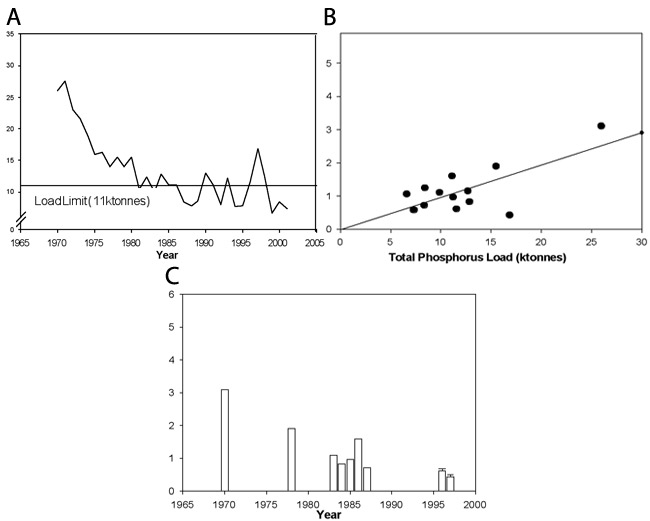
The quality of nutrients (e.g., food quality, amount of particular plant nutrients) in an environment affects the ability of an organism to survive, grow, and reproduce. The lower the quality of the nutrients, the higher the environmental stress. In the freshwater Laurentian Great Lakes, particularly in Lake Erie, the factor limiting algal growth was found to be phosphorus. David Schindler and his colleagues at the Experimental Lakes Area (Ontario, Canada) demonstrated that phosphorus was the growth-limiting factor in temperate North American lakes using whole-lake treatment and controls (Schindler 1974). This work encouraged the passage of the Great Lakes Water Quality Agreement of 1972 (GLWQA 1972) — a reduction in phosphorus load from municipal sources was predicted to lead to a corresponding reduction in the total algal biomass and harmful cyanobacterial (blue-green algae) blooms (McGuken 2000; Figure 3). As annual phosphorus loads decreased in the mid 1980s (Dolan 1993), there was some indication that Lake Erie was improving in terms of decreased total phytoplankton (photosynthetic algae and cyanobacteria) biomass (Makarewicz 1993). Further improvement continued until the mid 1990s, until an introduced species, the zebra mussel, began altering the internal phosphorus dynamics of the lake by mineralization (excretion) of digested algae (Figure 3; Conroy et al. 2005).
Pollutants also contribute to environmental stress, limiting the growth rates of populations. Although each species has specific tolerances for environmental toxins, amphibians in general are particularly susceptible to pollutants in the environment. For example, pesticides and other endocrine disrupting toxins can strongly control the growth of amphibians (Blaustein et al. 2003). These chemicals are used to control agricultural pests but also run into freshwater streams and ponds where amphibians live and breed. They affect the amphibians both with direct increases in mortality and indirect limitation in growth, development, and reduction in fecundity. Rohr et al. (2003) found, among many other examples, that these compounds affect salamander embryo survival in affected ponds, increased deformities, and delayed development and growth, lengthening their vulnerability to predators by remaining small sized for longer periods. These effects limit population growth irrespective of the size of the amphibian population and are not limited to pesticides but also include pH and thermal pollution, herbicides, fungicides, heavy metal contaminations, etc.
Environmental catastrophes such as fires, earthquakes, volcanoes and floods can strongly affect population growth rates via direct mortality and habitat destruction. A large-scale natural catastrophe occurred in 2005 when hurricane Katrina impacted the coastal regions of the Gulf of Mexico in the southern United States. Katrina altered habitat for coastal vegetation by depositing more than 5 cm of sediment over the entire coastal wetland zone. In these areas, substantial improvement in the quality of wetlands for plant growth occurred after many years of wetland loss due to control of the Mississippi River flow (Turner et al. 2006). At the same time, however, almost 100 km2 of wetland was destroyed and converted to open sea, completely eliminating wetland vegetation (Day et al. 2007). More recently the Gulf oil spill in 2010 has again impacted the coastal wetland vegetation. Though human derived, this large-scale environmental disaster will have long-term impacts on the population growth of not only vegetation but all organisms in the wetlands and nearshore regions of the Gulf of Mexico.
References and Recommended Reading
Blaustein, A. R. et al. Ultraviolet radiation, toxic chemicals and amphibian population declines. Diversity and Distributions 9, 123–140 (2003).
Clutton-Brock, T. H. et al. Sex differences in emigration and mortality affect optimal management of deer populations. Nature 415, 633–637 (2002).
Conroy, J. D. et al. Recent increases in Lake Erie plankton biomass: roles of external phosphorus loading and dreissenid mussels. Journal of Great Lakes Research 31 (Supplement 2), 89–110 (2005).
Day, J. D. et al. Restoration of the Mississippi delta: lessons from hurricanes Katrina and Rita. Science 315, 1679–1684 (2007).
Gilg, O. et al. Cyclic dynamics in a simple vertebrate predator-prey community. Science 302, 866–868 (2003).
GLWQA. "Great Lakes water quality agreement, with annexes, texts, and terms of reference between the United States and Canada." In Treaties and Other International Acts, Series 7312. Washington, DC: Government Printing Office, 1972.
Makarewicz, J. C. Phytoplankton biomass and species composition in Lake Erie, 1970 to 1987. Journal of Great Lakes Research 19, 258–274 (1993).
McGucken, W. Lake Erie Rehabilitated: Controlling Cultural Eutrophication, 1960s-1990s. Akron, OH: University of Akron Press, 2000.
Rohr, J. R. et al. Lethal and sublethal effects of atrazine, carbaryl, endosulfan, and octylphenol on the streamside salamander (Ambystoma barbouri). Environmental Toxicology and Chemistry 22, 2385–2392 (2003).
Schindler, D. W. Eutrophication and recovery in experimental lakes: implications for lake management. Science 184, 897–899 (1974).
Sibley, R. M. & Hone J. Population growth rate and its determinants: an overview. Philosophical Transactions of the Royal Society B 357, 1153–1170 (2002).
Turner, R. E. et al. Wetland Sedimentation from Hurricanes Katrina and Rita. Science 314, 449–451 (2006).
Wauters, L. A. & Lens, L. Effects of food availability and density on red squirrel (Sciurus vulgaris) reproduction. Ecology 76, 2460–2469 (1995).































