« Prev Next »
What are vector-borne diseases?

Why study the population dynamics of vector-borne diseases separately?
Population dynamics also has a theoretical component, building on the mathematical foundation of demographic models. This theoretical basis enables the articulation and testing of hypotheses and generation of predictions from models that can represent hosts, vectors, and other aspects of the transmission cycle to describe the dynamics of vector-borne pathogens (May & Anderson 1991). These models can then be linked to experimental or observational data using statistical methods and projected forward in time for forecasting.
Key features of the population dynamics of vector-borne diseases
The theory of population dynamics for vector-borne pathogens
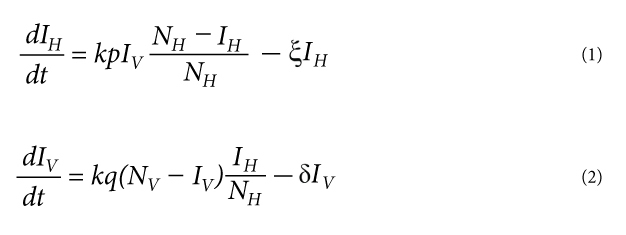
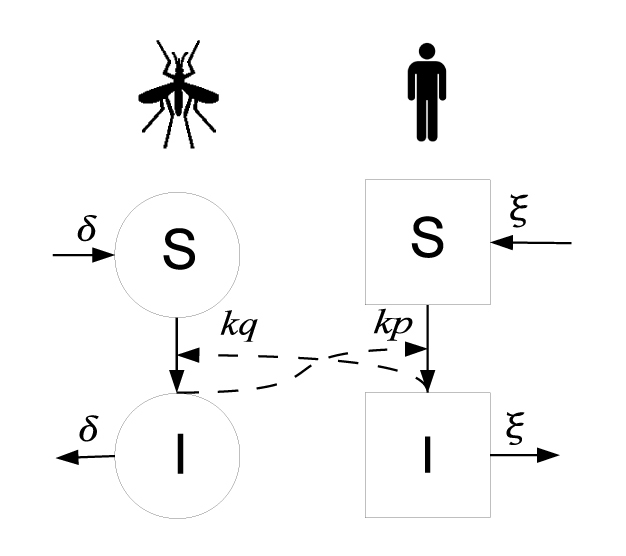
where NV and NH are the total number of vectors and hosts, IV and IH are the number of infected vectors and hosts, respectively, k is the biting rate of the vectors, p is the probability that the bite of infectious vector will lead to the successful infection of a susceptible host, q is the probability that a susceptible vector which bites an infectious host will become infected, ξ is the rate at which infectious hosts recover, while δ is the mortality rate of infected vectors.
While the above two equations only describe the change in the number of infectious hosts and vectors, the change in the number of susceptible hosts and vectors is implicitly followed since host and vector population sizes are kept constant. Therefore, the mortality of infectious vectors immediately increases the number of susceptible vectors (Figure 1), accounting for the turnover in vector populations.
One of the most important characteristics that can be used to describe pathogens is the basic reproductive ratio R0 (Diekmann et al. 1990). It may be defined as the number of secondary infections that a single infectious individual will initiate on average in a population of completely susceptible hosts. It is a threshold quantity in the sense that R0 < 1 means that a single infectious host will typically infect less than one susceptible host during its infectious period (i.e., some infectious hosts infect none and some infect one or more), leading to the eventual extinction of the pathogen from the host population. Such pathogens are unable to invade the susceptible host population. Conversely, hosts infectious with pathogens that have R0 > 1will on average infect more than one susceptible host, leading to the spread of the pathogen in the host population (i.e., an epidemic).
The basic reproductive ratio can be calculated for the Ross-Macdonald model presented above (Eq. 1-2) as follows (Keeling & Rohani 2007). Starting with a single infectious mosquito, one calculates the number of susceptible mosquitoes that become infected indirectly through the infection of susceptible hosts. The average number of hosts that the single infectious mosquito infects in a completely susceptible host population, based on Eq. 1, is kp/δ, which does not depend on the size of the available host population. One of these hosts that become infectious will in turn infect on average (kqNV) / (ξNH )susceptible mosquitoes that bite them. In order to calculate the basic reproductive number for a vector-borne pathogen, one multiplies the number of newly infected hosts with the number of mosquitoes that each infects, assuming that each infected host on average infects the same number of mosquitoes:

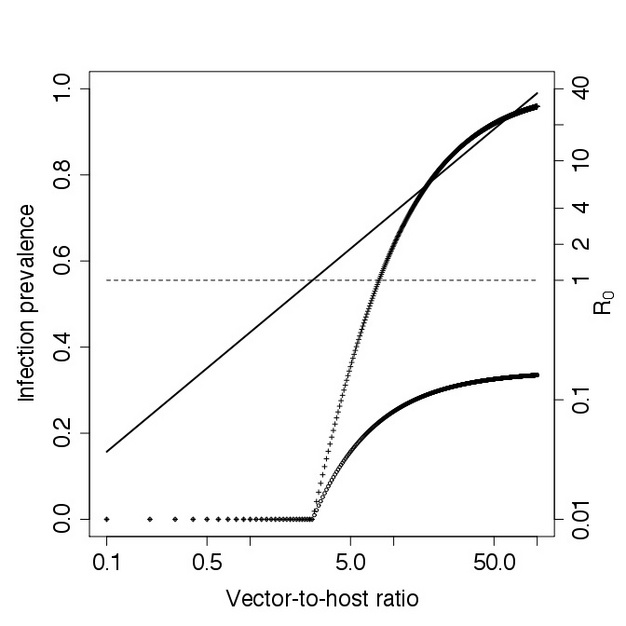
An important feature of the above equation is the biting rate of vectors, k, which factors into the basic reproductive ratio squared, since vectors need to bite once to acquire infection and then again to infect a susceptible host. Consequently, any increase or reduction of the biting rate of infectious vectors will affect the spread of the pathogen disproportionately (Lloyd et al. 2007). This explains the profound success of simple interventions, such as bed-nets, that reduce the biting rate of infectious vectors, in decreasing the burden of vector-borne diseases such as malaria. Another notable feature is the proportion of the total number of vectors and hosts, which is directly proportional to the basic reproductive ratio (Figure 4), implying that reducing the vector population can control vector-borne pathogens. Since the pathogen will not be able to invade and persist unless its basic reproductive ratio exceeds one, vector control may be successful in preventing and halting disease spread even when the vector population is not completely extinct (Focks et al. 2000). For successful control, the vector population has to be reduced only below the entomological threshold:

Another feature of the Ross-Macdonald model is the existence of an endemic equilibrium. Specifically, if R0 > 1, (Eqs. 1-2) have an equilibrium where the number of infectious hosts and vectors remain constant following an initial transient period at the following values:
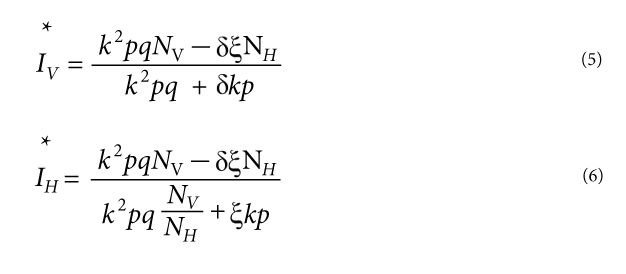
Inspection of these equations shows that decreasing the vector-host ratio by vector control will, in addition to decreasing the basic reproductive ratio of the pathogen, also decrease the equilibrium number of infectious hosts and vectors (Figure 4). In fact, this equilibrium number will approach zero rapidly, and become quite small even before R0 < 1 as the pathogen is unable to persist in the population. Therefore, vector control will simultaneously decrease disease prevalence and protect the population from the re-invasion of the pathogen. An important further point, however, is that if vector control is relaxed after the pathogen has been eliminated from the population, the vector population may grow again above the entomological threshold. At this point, R0 > 1 and the pathogen can successfully re-invade the susceptible host population. This is what happened with dengue viruses in South America. Vector control campaigns in the 1960s eliminated the vector Aedes aegypti in 16 countries (Guzman & Kouri 2003). However, following the eradication of dengue virus, the vector re-established itself from countries in Central America and the United States in the 1970s. The resurgence of the vector subsequently allowed the reintroduction of dengue viruses in Central and South America, starting in the 1980s, so that it is now the most important arboviral disease worldwide (Kyle & Harris 2008).
The simple Ross-Macdonald model demonstrated above is typically not sufficient to describe the biology of the hosts, vectors, and pathogens in adequate detail. Therefore, several extensions and modifications have been employed for specific disease systems. For example, most vector-borne pathogens have to get from the host blood-meal in the midgut to the salivary gland for successful transmission to the next host, during which they sometimes transform and specialize into other cell types (e.g. malaria). The time required for this to happen is called the extrinsic incubation period (EIP), and is of the order of a week for most pathogens. Consequently, mosquitoes have to survive this period in order to be able to transmit the pathogen. Entomopathogenic fungi, such as Metarhizium anisopliae and Beauveria bassiana, increase the daily mortality rate of mosquitoes when applied as a control method, decreasing the proportion of mosquitoes that survive the EIP, leading to reduced human transmission risk (Scholte et al. 2005). Another important extension of the Ross-Macdonald model concerns the population dynamics of hosts and vectors themselves. By relaxing the assumption of a constant host and vector population and incorporating the birth and survival of vectors and hosts, we are able to incorporate cycling and seasonality into these epidemiological models.
Epidemiological implications of interactions between vectors and their environment
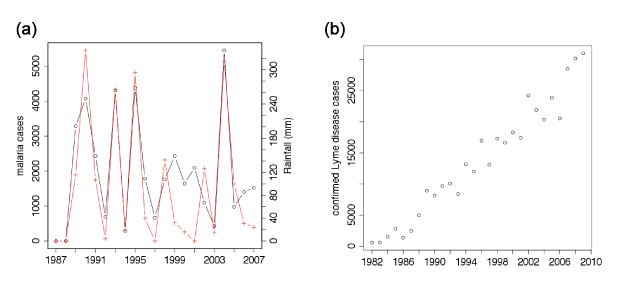
Another consequence of the inherent sensitivity of vectors and pathogens to abiotic conditions is high uncertainty in predicting the fine-scale spatio-temporal dynamics of vector-borne pathogens. For example, the transmission risk of a particular pathogen to humans in a specific area depends on the number of mosquitoes that bite people, the prevalence of the pathogen in those mosquitoes, and the competence of the mosquito species present in transmitting the infection. The number of mosquitoes and their community composition depend both on the prior abiotic conditions and the characteristics of a particular habitat. The prevalence of the pathogen depends on the prior dynamics of the pathogen in the hosts and vectors present in the area. These complex dependencies make predictions of changes in abundance of vector-borne pathogens very difficult.
For some vector-borne diseases, which infect multiple hosts, the ecologies of other species may be important too. In the case of such multi-host pathogens, each host contributes to the overall dynamics of the pathogen, depending on the availability and the competence of each host to the pathogen. For example, Borrelia burgdorferi, the spirochaete that causes Lyme disease in humans, can infect white-footed mice (Peromyscus leucopus), red and grey squirrels (Tamiasciurus hudsonicus and Sciurus carolinensis), white-tailed deer (Odocoileus virginianus), and humans, among others. While white-tailed deer and humans are 'dead-end' hosts that do not contribute to further transmission of the Borrelia pathogen, other hosts can all contribute to the transmission of Borrelia to susceptible ticks to some degree (LoGiudice et al. 2008). In order to eliminate the transmission risk of Lyme disease to people in the area, we have to break the multiple transmission cycles corresponding to each potential host.
In summary, the ecological nature of vector-borne pathogens have profound epidemiological implications, which are inherently difficult to elucidate due to the complex dependency between the population dynamics of vector-borne pathogens and the population dynamics of vectors and hosts and their environment. The epidemiological implications of the ecological nature of vector-borne pathogens make it necessary to understand the population dynamics of vector-borne diseases in order to predict and control these diseases. Despite all the challenges involved, the significant global health burden and economic loss due to vector-borne pathogens in humans, livestock, and crops make this endeavor crucial.References and Recommended Reading
Alonso, D. et al. Epidemic malaria and warmer temperatures in recent decades in an East African highland. Proceedings of the Royal Society of London B doi: 10.1098/rspb.2010.2020 (2010).
Bailey, M. T. J. The Biomathematics of Malaria. London, UK: Charles Griffin & Co. Ltd., 1982.
Center for Disease Control. Division of Vector-borne Infectious Diseases. Reported Cases of Lyme Disease by Year, United States, 1995-2009. Retrieved on March 11, 2011. (link)
Diekmann O. et al. On the definition and the computation of the basic reproduction ratio R0 in models for infectious diseases in heterogeneous populations. Journal of Mathematical Biology 28, 365-382 (1990).
Focks, D. A. et al. Transmission thresholds for dengue in terms of Aedes aegypti pupae per person with discussion of their utility in source reduction efforts. American Journal of Tropical Medicine and Hygiene 62, 11-18 (2000).
Gething, P.W. et al. Climate change and the global malaria recession. Nature 465, 342-345 (2010).
Gilpin, M. E. & McClelland, G. A. H. Systems analysis of the yellow fever mosquito Aedes aegypti. Fortschritte der Zoologie 25, 355-388 (1979).
Guzman, M. G. & Kouri, G. Dengue and dengue hemorrhagic fever in the Americas: lessons and challenges. Journal of Clinical Virology 27, 1-13 (2003).
Keeling, M. J. & Rohani, P. Modeling Infectious Diseases in Humans and Animals. Princeton, NJ: Princeton University Press, 2007.
Kyle, J. L. & Harris, E. Global spread and persistence of dengue. Annual Review of Microbiology 62, 71-92 (2008).
Laneri K. et al. Forcing versus feedback: Epidemic malaria and monsoon rains in Northwest India. PLoS Comput Biol 6, e1000898. doi:10.1371/journal.pcbi.1000898 (2010).
Lloyd, A. L. et al. Stochasticity and heterogeneity in host-vector models. Journal of the Royal Society Interface 4, 851-863 (2007).
LoGiudice, K. et al. Impact of host community composition on Lyme disease risk. Ecology 89, 2841-2849 (2008).
Macdonald, G. The Epidemiology and Control of Malaria. Oxford, UK: Oxford University Press, 1957.
Martens, P. et al. Potential impact of global climate change on malaria risk. Environmental Health Perspectives 103, 458-464 (1995).
May, R. L. & Anderson, R. M. Infectious Diseases of Humans: Dynamics and Control. Oxford, UK: Oxford University Press, 1991.
McKirdy, S. J. & Jones, R. A. C.
Quantification of yield losses caused by barley yellow dwarf virus in wheat and
oats. Plant Disease 86,
769-773 (2002).
Rich, K. M. & Wanyoike, F. An assessment of the regional and national socio-economic impacts of the 2007 Rift Valley fever outbreak in Kenya. American Journal of Tropical Medicine and Hygiene 83, 52-57 (2010).
Ross, R. The Prevention of Malaria. London, UK: John Murray, 1911.
Scholte, E.-J. et al. An entomopathogenic fungus for control of adult African malaria mosquitoes. Science 308, 1641-1642 (2005).
Surveillance for Lyme Disease. United States, 1992-1998. Morbidity and Mortality Weekly Report 49, 1-11 (2000).
Surveillance for Lyme Disease. United States, 1992-2006. Morbidity and Mortality Weekly Report 57, 1-9 (2008).
Tabachnick, W.J. et al. Bluetongue. Entomology and Nematology Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida, Gainesville, (2008). (link)
World Malaria Report 2010. World Health Organization. Retrieved from http://www.who.int/malaria/world_malaria_report_2010/en/index.html on March 11
2011.
World Health Organization. The global burden of disease: 2004 update. Retrieved from http://www.who.int/healthinfo/global_burden_disease/2004_report_update/en/index.html on March 11 2011.































