Abstract
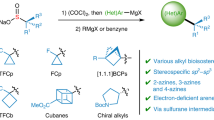
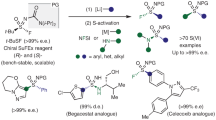
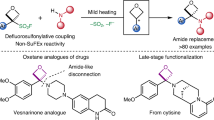
The occurrence of sulfoximines and sulfonimidoyl groups in biologically active molecules within pharmaceuticals and agrochemicals has notably increased in the past decade. This increase has prompted a wave of discovery of methods to install the S(VI) functionality into complex organic molecules. Traditional synthetic methods to form α-substituted sulfonimidoyl motifs rely on S–C bond disconnections and typically require control of the stereogenic S-centre or late-stage modification at sulfur, and comprise multistep routes. Here, we report the development of a stereospecific, modular SNAr approach for the introduction of sulfonimidoyl functional groups into heterocyclic cores. This strategy has been demonstrated across 85 examples, in good to excellent yield, of complex and diverse heterocycles. Sulfoximines, sulfonimidamides and sulfondiimines are all compatible nucleophiles in the SNAr reaction and, hence, the methodology was applied to the synthesis of four sulfoximine-containing pharmaceuticals. Of these synthetic applications, most notably ceralasertib, an ATR inhibitor currently in clinical trials, was synthesized in an eight-step procedure on a gram scale.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The experimental data as well as the characterization data for all the compounds prepared during these studies are provided in the Supplementary Information. Crystallographic data are available from the Cambridge Crystallographic Data Centre with the following codes: 52 (CCDC 2087123), 108 (CCDC 2087121), 112 (CCDC 2087124) and 138 (CCDC 2087122).
References
Ilardi, E. A., Vitaku, E. & Njardarson, J. T. Data-mining for sulfur and fluorine: an evaluation of pharmaceuticals to reveal opportunities for drug design and discovery. J. Med. Chem. 57, 2832–2842 (2014).
Zhao, C., Rakesh, K. P., Ravidar, L., Fang, W.-Y. & Qin, H.-L. Pharmaceutical and medicinal significance of sulfur (S(VI))-containing motifs for drug discovery: a critical review. Eur. J. Med. Chem. 162, 679–734 (2019).
Tang, K.-X. et al. Transition metal-catalyzed C–H bond functionalizations by use of sulfur-containing directing groups. Adv. Synth. Catal. 361, 26–38 (2019).
Wang, J. et al. Conjugated sulfonamides as a class of organic lithium-ion positive electrodes. Nat. Mater. 20, 665–673 (2021).
Scott, K. A. & Njardarson, J. T. Analysis of US FDA-approved drugs containing sulfur atoms. Top. Curr. Chem. 376, 5 (2018).
Mäder, P. & Kattner, L. Sulfoximines as rising stars in modern drug discovery? Current status and perspective on an emerging functional group in medicinal chemistry. J. Med. Chem. 63, 14243–14275 (2020).
Lücking, U. Sulfoximines: a neglected opportunity in medicinal chemistry. Angew. Chem. Int. Ed. 52, 9399–9408 (2013).
Sirvent, J. A. & Lücking, U. Novel pieces for the emerging picture of sulfoximines in drug discovery: synthesis and evaluation of sulfoximine analogues of marketed drugs and advanced clinical candidates. ChemMedChem 12, 487–501 (2017).
Lücking, U. Neglected sulfur(VI) pharmacophores in drug discovery: exploration of novel chemical space by the interplay of drug design and method development. Org. Chem. Front. 6, 1319–1324 (2019).
Devendar, P. & Yang, G. F. Sulfur-containing agrochemicals. Top. Curr. Chem. 375, 82 (2017).
Zasukha, S. V. et al. Sulfonimidamides and imidosulfuric diamides: compounds from an underexplored part of biologically relevant chemical space. Chem. Eur. J. 25, 6928–6940 (2019).
Chinthakindi, P. K. et al. Sulfonimidamides in medicinal and agricultural chemistry. Angew. Chem. Int. Ed. 56, 4100–4109 (2017).
Foote, K. M. et al. Discovery and characterization of AZD6738, a potent inhibitor of ataxia telangiectasia mutated and Rad3 related (ATR) kinase with application as an anticancer agent. J. Med. Chem. 61, 9889–9907 (2018).
Lücking, U. et al. Preparation of modified macrocyclic compounds for the treatment of diseases. Patent WO 2017/060167 A1 (2017)
Lücking, U. et al. Preparation of macrocyclic sulfondiimine compounds as CDK9 inhibitor for treatment of hyperproliferative disorder, viral infection, and cardiovascular diseases. Patent WO 2017/055196 A1 (2017)
Zhu, Y. et al. Discovery and characterization of sulfoxaflor, a novel insecticide targeting sap-feeding pests. J. Agric. Food Chem. 59, 2950–2957 (2011).
Johnson, T., Vairagoundar, R. & Ewin, R. A. Preparation of novel phenicol derivatives useful as antibacterial agents. Patent WO 2014/172443 A1 (2014)
Lücking, U. et al. Changing for the better: discovery of the highly potent and selective CDK9 inhibitor VIP152 suitable for once weekly intravenous dosing for the treatment of cancer. J. Med. Chem. 64, 11651–11674 (2021).
Frings, M., Bolm, C., Blum, A. & Gnamm, C. Sulfoximines from a medicinal chemist’s perspective: physicochemical and in vitro parameters relevant for drug discovery. Eur. J. Med. Chem. 126, 225–245 (2017).
Lücking, U. et al. The lab oddity prevails: discovery of pan-CDK inhibitor (R)-S-cyclopropyl-S-(4-{[4-{[(1R,2R)-2-hydroxy-1-methylpropyl]oxy}-5-(trifluoromethyl)pyrimidin-2-yl]amino}phenyl)sulfoximide (BAY 1000394) for the treatment of cancer. Chem. Med. Chem. 8, 1067–1085 (2013).
Izzo, F. et al. Exploration of novel chemical space: synthesis and in vitro evaluation of N-functionalized tertiary sulfonimidamides. Chem. Eur. J. 24, 9295–9304 (2018).
Clinical Trials Using ATR Kinase Inhibitor AZD6738 (National Cancer Institute, accessed 26 May 2021); https://www.cancer.gov/about-cancer/treatment/clinical-trials/intervention/ceralasertib?redirect=true
Lücking, U. et al. Preparation of novel PTEFB inhibiting macrocyclic compounds. Patent WO 2018/177899 A1 (2018)
Walker, D. P. et al. Sulfoximine-substituted trifluoromethylpyrimidine analogs as inhibitors of proline-rich tyrosine kinase 2 (PYK2) show reduced hERG activity. Bioorg. Med. Chem. Lett. 19, 3253–3258 (2009).
Mc Bride, C. et al. Preparation of sulfonimidamide compounds as inhibitors of interleukin-1 activity. Patent WO 2020/018975 A1 (2020)
Chowdhury, S. et al. Preparation of substituted benzamides as sodium channel inhibitors and therapeutic uses thereof. Patent WO 2017/172802 A1 (2017)
Bartels, B. et al. Preparation of pyridyl-triazabicycles having BACE1 inhibitory activity. Patent WO 2016/012422 A1 (2016)
Katz, J., Roush, W., Seidel, H. M., Shen, D.-M. & Venkatraman, S. Sulfonimidamide compounds and compositions for treating conditions associated with NLRP activity. Patent WO 2020/154499 A1 (2020)
Ouvry, G. et al. Sulfoximines as potent RORγ inverse agonists. Bioorg. Med. Chem. Lett. 28, 1269–1273 (2018).
Loso, M. R. et al. SAR studies directed toward the pyridine moiety of the sap-feeding insecticide sulfoxaflor (Isoclast active). Bioorg. Med. Chem. 24, 378–382 (2016).
Okamura, H. & Bolm, C. Rhodium-catalyzed imination of sulfoxides and sulfides: efficient preparation of N-unsubstituted sulfoximines and sulfilimines. Org. Lett. 6, 1305–1307 (2004).
Correa, A. & Bolm, C. Ligand-free copper-catalyzed N-arylation of nitrogen nucleophiles. Adv. Synth. Catal. 349, 2673–2676 (2007).
Cheng, Y. & Bolm, C. Regioselective syntheses of 1,2-benzothiazines by rhodium-catalyzed annulation reactions. Angew. Chem. Int. Ed. 54, 12349–12352 (2015).
Zenzola, M., Doran, R., Luisi, R. & Bull, J. A. Synthesis of sulfoximine carbamates by rhodium-catalyzed nitrene transfer of carbamates to sulfoxides. J. Org. Chem. 80, 6391–6399 (2015).
Zenzola, M., Doran, R., Degennaro, L., Luisi, R. & Bull, J. A. Transfer of electrophilic NH using convenient sources of ammonia: direct synthesis of NH sulfoximines from sulfoxides. Angew. Chem., Int. Ed. 55, 7203–7207 (2016).
Tota, A. et al. Synthesis of NH-sulfoximines from sulfides by chemoselective one-pot N- and O-transfers. Chem. Commun. 53, 348–351 (2017).
Johnson, C. R. Utilization of sulfoximines and derivatives as reagents for organic synthesis. Acc. Chem. Res. 6, 341–347 (1973).
Sirvent, J. A., Bierer, D., Webster, R. & Lücking, U. Palladium-catalyzed direct α-arylation of p-methoxybenzyl-protected S,S-dimethylsulfoximine. Synthesis 49, 1024–1036 (2017).
Greed, S. et al. Synthesis of highly enantioenriched sulfonimidoyl fluorides and sulfonimidamides by stereospecific sulfur–fluorine exchange (SuFEx) reaction. Chem. Eur. J. 26, 12533–12538 (2020).
Izzo, F., Schaefer, M., Stockman, R. & Lücking, U. A new, practical one-Pot synthesis of unprotected sulfonimidamides by transfer of electrophilic NH to sulfinamides. Chem. Eur. J. 23, 15189–15193 (2017).
Aota, Y., Kano, T. & Maruoka, K. Asymmetric synthesis of chiral sulfoximines through the S-alkylation of sulfinamides. Angew. Chem. Int. Ed. 58, 17661–17665 (2019).
Aota, Y., Kano, T. & Maruoka, K. Asymmetric synthesis of chiral sulfoximines via the S-arylation of sulfinamides. J. Am. Chem. Soc. 141, 19263–19268 (2019).
Gao, B., Li, S., Wu, P., Moses, J. E. & Sharpless, K. B. SuFEx chemistry of thionyl tetrafluoride (SOF4) with organolithium nucleophiles: synthesis of sulfonimidoyl fluorides, sulfoximines, sulfonimidamides, and sulfonimidates. Angew. Chem. Int. Ed. 57, 1939–1943 (2018).
Zheng, Q. et al. SuFEx-enabled, agnostic discovery of covalent inhibitors of human neutrophil elastase. Proc. Natl Acad. Sci. USA 116, 18808–18814 (2019).
Kitamura, S. et al. Sulfur(VI) fluoride exchange (SuFEx)-enabled high-throughput medicinal chemistry. J. Am. Chem. Soc. 142, 10899–10904 (2020).
Davies, T. Q., Hall, A. & Willis, M. C. One-pot, three-component sulfonimidamide synthesis exploiting the sulfinylamine reagent N-sulfinyltritylamine, TrNSO. Angew. Chem. Int. Ed. 56, 14937–14941 (2017).
Zhang, Z.-X., Davies, T. Q. & Willis, M. C. Modular sulfondiimine synthesis using a stable sulfinylamine reagent. J. Am. Chem. Soc. 141, 13022–13027 (2019).
Davies, T. Q. et al. Harnessing sulfinyl nitrenes: a unified one-pot synthesis of sulfoximines and sulfonimidamides. J. Am. Chem. Soc. 142, 15445–15453 (2020).
Pyne, S. G., Dong, Z., Skelton, B. W. & White, A. H. Cyclopropanation reactions of enones with lithiated sulfoximines: application to the asymmetric synthesis of chiral cyclopropanes. J. Org. Chem. 62, 2337–2343 (1997).
Zhang, W., Wang, F. & Hu, J. N-Tosyl-S-difluoromethyl-S-phenylsulfoximine: a new difluoromethylation reagent for S-, N-, and C-nucleophiles. Org. Lett. 11, 2109–2112 (2009).
Shen, X., Liu, Q., Zhang, W. & Hu, J. Stereoselective synthesis of (sulfonimidoyl)cyclopropanes with (R)-PhSO(NTs)CH2Cl and α,β-unsaturated Weinreb amides: tuning the of selectivity between C–Cl and C–S bond cleavage. Eur. J. Org. Chem. 2016, 906–909 (2016).
Cho, G. Y. & Bolm, C. Palladium-catalyzed α-arylation of sulfoximines. Org. Lett. 7, 1351–1354 (2005).
Leftheris, K. et al. Substituted 5-, 6- and 7-membered heterocycles as 11β-HSD1 inhibitors and their preparation, medicaments containing such compounds, and their use. Patent WO 2011/159760 A1 (2011)
Liu, Y., Xia, Q. & Fang, L. Design and synthesis of alkyl substituted pyridino[2,3-D]pyrimidine compounds as PI3Kα/mTOR dual inhibitors with improved pharmacokinetic properties and potent in vivo antitumor activity. Bioorg. Med. Chem. 26, 3992–4000 (2018).
Blades, K. et al. Expedient synthesis of biologically important sulfonylmethyl pyrimidines. Tetrahedron Lett. 55, 3851–3855 (2014).
Haake, M. Ein neues verfahren zur darstellung von S,S-dialkylschwefeldiimiden. Tetrahedron Lett. 11, 4449–4450 (1970).
Park, S. J. et al. N-Cyano sulfoximines: COX inhibition, anticancer activity, cellular toxicity, and mutagenicity. ChemMedChem 8, 217–220 (2013).
Murray, J. M. et al. Potent and highly selective benzimidazole inhibitors of PI3-kinase delta. J. Med. Chem. 55, 7686–7695 (2012).
Kang, D. et al. Structure-based optimization of thiophene[3,2-d]pyrimidine derivatives as potent HIV-1 non-nucleoside reverse transcriptase inhibitors with improved potency against resistance-associated variants. J. Med. Chem. 60, 4424–4443 (2017).
Goundry, W. R. F. et al. Development and scale-up of a route to ATR inhibitor AZD6738. Org. Process Res. Dev. 23, 1333–1342 (2019).
Graham, M. A. et al. Development and scale-up of an improved manufacturing route to the ATR Inhibitor ceralasertib. Org. Process Res. Dev. 25, 43–56 (2021).
Graham, M. A. et al. Development and proof of concept for a large-scale photoredox additive-free Minisci reaction. Org. Process Res. Dev. 25, 57–67 (2021).
Acknowledgements
We gratefully acknowledge the National Institutes of Health (R35-GM142577, J.M.L.) and the Florida Department of Health (Bankhead-Coley #9BC09, J.M.L.) for support of this research. This work has also been supported in part by the Chemical Biology Core Facility at the H. Lee Moffitt Cancer Center & Research Institute, an NCI designated Comprehensive Cancer Center (P30-CA076292). We thank H. Lawrence and S. Yun for NMR and HRMS support, and C. Shan and Q. Tang for assistance with X-ray crystallographic analysis.
Author information
Authors and Affiliations
Contributions
Z.P.S. and T.S. performed the experiments; Z.P.S. and J.M.L. designed the project and wrote the paper; L.W. performed the X-ray crystallographic analysis.
Corresponding author
Ethics declarations
Competing interests
A provisional patent application naming J.M.L., Z.P.S. and T.S. as inventors has been filed by the H. Lee Moffitt Cancer Center & Research Institute, which covers the synthetic methods for the α-(hetero)arylation of sulfonimidoyl functional groups. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Mark Graham, Ulrich Lücking and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Thomas West was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–6, Schemes 1–7, Tables 1–5, procedures and troubleshooting guides.
Supplementary Data 1
Crystallographic data for compound 52.
Supplementary Data 2
Crystallographic data for compound 108.
Supplementary Data 3
Crystallographic data for compound 112.
Supplementary Data 4
Crystallographic data for compound 138.
Rights and permissions
About this article
Cite this article
Shultz, Z.P., Scattolin, T., Wojtas, L. et al. Stereospecific α-(hetero)arylation of sulfoximines and sulfonimidamides. Nat Synth 1, 170–179 (2022). https://doi.org/10.1038/s44160-021-00011-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-021-00011-2