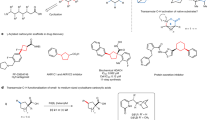
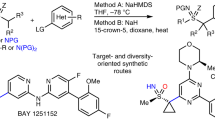
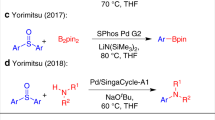
Abstract
In recent years, a variety of cycloalkyl groups with quaternary carbons, in particular cyclopropyl and cyclobutyl trifluoromethyl groups, have emerged as promising bioisosteres in drug-like molecules. The modular installation of such bioisosteres remains challenging to synthetic chemists. Alkyl sulfinate reagents have been developed as radical precursors to prepare functionalized heterocycles with the desired alkyl bioisosteres. However, the innate (radical) reactivity of this transformation poses reactivity and regioselectivity challenges for the functionalization of any aromatic or heteroaromatic scaffold. Here we showcase the ability of alkyl sulfinates to engage in sulfurane-mediated C(sp3)–C(sp2) cross-coupling, thereby allowing for programmable and stereospecific installation of these alkyl bioisosteres. The ability of this method to simplify retrosynthetic analysis is exemplified by the improved synthesis of multiple medicinally relevant scaffolds. Experimental studies and theoretical calculations for the mechanism of this sulfur chemistry reveal a ligand-coupling trend under alkyl Grignard activation via the sulfurane intermediate, stabilized by solvation of tetrahydrofuran.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Experimental data, as well as characterization data for all compounds prepared in the course of these studies, are provided in the Supplementary Information. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2086919 (40), 2086920 (41), 2172148 (74) and 2172149 (116) (see X-ray crystallographic data in the Supplementary Information). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Talele, T. T. Opportunities for tapping into three-dimensional chemical space through a quaternary carbon. J. Med. Chem. 63, 13291–13315 (2020).
Bauer, M. R. et al. Put a ring on it: application of small aliphatic rings in medicinal chemistry. RSC Med. Chem. 12, 448–471 (2021).
Mykhailiuk, P. K. Saturated bioisoteres of benzene: where to go next? Org. Biomol. Chem. 17, 2839–2849 (2019).
Li, J. J. Medicinal Chemistry for Practitioners Ch. 4 (Wiley, 2020)
Meanwell, N. A. Fluorine and fluorinated motifs in the design and application of bioisosteres for drug design. J. Med. Chem. 61, 5822–5880 (2018).
Gillis, E. P., Eastman, K. J., Hill, M. D., Donnelly, D. J. & Meanwell, N. A. Applications of fluorine in medicinal chemistry. J. Med. Chem. 58, 8315–8359 (2015).
Barnes-Seeman, D. et al. Metabolically stable tert-butyl replacement. ACS Med. Chem. Lett. 4, 514–516 (2013).
Westphal, M. V., Wolfstädter, B. T., Plancher, J. M., Gatfield, J. & Carreira, E. M. Evaluation of tert-butyl isosteres: case studies of physicochemical and pharmacokinetic properties, efficacies and activities. ChemMedChem 10, 461–469 (2015).
Bezencon, O. et al. Discovery of a potent, selective T-type calcium channel blocker as a drug candidate for the treatment of generalized epilepsies. J. Med. Chem. 60, 9769–9789 (2017).
Phelan, J. P. et al. Redox-neutral photocatalytic cyclopropanation via radical/polar crossover. J. Am. Chem. Soc. 140, 8037–8047 (2018).
Kautzky, J. A., Wang, T., Evans, R. W. & MacMillan, D. W. C. Decarboxylative trifluoromethylation of aliphatic carboxylic acids. J. Am. Chem. Soc. 140, 6522–6526 (2018).
Cyr, P., Flynn-Robitaille, J., Boissarie, P. & Marinier, A. Mild and diazo-free synthesis of trifluoromethyl-cyclopropanes using sulfonium ylides. Org. Lett. 21, 2265–2268 (2019).
Mercadante, M. A. et al. 1,3-γ-Silyl-elimination in electron-deficient cationic systems. Chem. Sci. 5, 3983–3994 (2014).
Zhao, Q.-H. et al. An efficient substrate-induced method for the synthesis of CF3-substituted cyclopropanes by metal-free reaction of trifluoromethyl styrylisoxazoles with nitromethane. Synthesis 53, 1597–1604 (2020).
Bugera, M. et al. Deoxofluorination of aliphatic carboxylic acids: a route to trifluoromethyl-substituted derivatives. J. Org. Chem. 84, 16105–16115 (2019).
Liu, S., Huang, Y., Qing, F.-L. & Xu, X.-H. Transition-metal-free decarboxylation of 3,3,3-trifluoro-2,2-dimethylpropanoic acid for the preparation of C(CF3)Me2-containing heteroarenes. Org. Lett. 20, 5497–5501 (2018).
Tanbouza, N., Carreras, V. & Ollevier, T. Photochemical cyclopropenation of alkynes with diazirines as carbene precursors in continuous flow. Org. Lett. 23, 5420–5424 (2021).
Denton, J. R., Sukumaran, D. & Davies, H. M. L. Enantioselective synthesis of trifluoromethyl-substituted cyclopropanes. Org. Lett. 9, 2625–2628 (2007).
Zhang, X. et al. Fluoroalkyl N-triftosylhydrazones as easily decomposable diazo surrogates for asymmetric [2 + 1] cycloaddition: synthesis of chiral fluoroalkyl cyclopropenes and cyclopropanes. ACS Catal. 11, 8527–8537 (2021).
Kelly, C. B. et al. Synthesis of perfluoroalkyl-substituted vinylcyclopropanes by way of enhanced neighboring group participation. Eur. J. Org. Chem. 2015, 4071–4076 (2015).
Huang, W.-S. et al. General catalytic enantioselective access to monohalomethyl and trifluoromethyl cyclopropanes. Chem. Eur. J. 24, 10339–10343 (2018).
Fuchibe, K., Oki, R., Hatta, H. & Ichikawa, J. Single C−F bond activation of the CF3 group with a Lewis acid: CF3-cyclopropanes as versatile 4,4-difluorohomoallylating agents. Chem. Eur. J. 24, 17932–17935 (2018).
Smith, J. M., Dixon, J. A., deGruyter, J. N. & Baran, P. S. Alkyl sulfinates: radical precursors enabling drug discovery. J. Med. Chem. 62, 2256–2264 (2019).
Kuttruff, C. A., Haile, M., Kraml, J. & Tautermann, C. S. Late-stage functionalization of drug-like molecules using diversinates. ChemMedChem 13, 983–987 (2018).
Michaudel, Q., Ishihara, Y. & Baran, P. S. Academia-industry symbiosis in organic chemistry. Acc. Chem. Res. 48, 712–721 (2015).
Fujiwara, Y. et al. Practical and innate carbon–hydrogen functionalization of heterocycles. Nature 492, 95–99 (2012).
Yan, M., Lo, J. C., Edwards, J. T. & Baran, P. S. Radicals: reactive intermediates with translational potential. J. Am. Chem. Soc. 138, 12692–12714 (2016).
Smith, J. M., Harwood, S. J. & Baran, P. S. Radical retrosynthesis. Acc. Chem. Res. 51, 1807–1817 (2018).
Proctor, R. S. J. & Phipps, R. J. Recent advances in minisci-type reactions. Angew. Chem. Int. Ed. 58, 13666–13699 (2019).
Gianatassio, R. et al. Simple sulfinate synthesis enables C-H trifluoromethyl-cyclopropanation. Angew. Chem. Int. Ed. 53, 9851–9855 (2014).
Oae, S. & Furukawa, N. Heteroaromatic sulfoxides and sulfones: ligand exchange and coupling in sulfuranes and ipso-substitutions. Adv. Heterocycl. Chem. 48, 1–63 (1990).
Oae, S. & Uchida, Y. Ligand-coupling reactions of hypervalent species. Acc. Chem. Res. 24, 202–208 (1991).
Oae, S. Ligand coupling reactions of hypervalent species. Pure Appl. Chem. 68, 805–812 (1996).
Zhou, M., Tsien, J. & Qin, T. Unsymmetrical heterocycle cross-couplings enabled by sulfur(IV) reagents. Synlett 31, 1962–1966 (2020).
Oae, S., Kawai, T. & Furukawa, N. Ligand coupling through σ-sulfurane complete retention of configuration of 1-phenylethyl group in the reaction of 1-phenylethyl 2-pyridyl sulfoxide with Grignard reagent. Tetrahedron Lett. 25, 69–72 (1984).
Oae, S., Kawai, T., Furukawa, N. & Iwasaki, F. Ligand coupling within σ-sulphurane intermediates formed in the reaction of benzyl 2-pyridyl and related sulphoxides with Grignard reagents. J. Chem. Soc. Perkin Trans. 2, 405–411 (1987).
Wakabayashi, S., Ishida, M., Takeda, T. & Oae, S. Sensitive nature of ligand coupling and pseudorotation to electronic effect of substituent ligand coupling in the reactions of benzylic aryl sulfoxides with benzylic Grignard reagents. Tetrahedron Lett. 29, 4441–4444 (1988).
Oae, S., Kawai, T. & Furukawa, N. Ligand exchange and ligand coupling via the σ-sulfurane intermediate in the reaction of alkyl 2-pyridyl sulfoxide with Grignard reagents: convenient preparation of 2,2′-bipyridines. Phosphorus Sulfur Relat. Elem. 34, 123–132 (1987).
Furukawa, N., Shibutani, T. & Fujihara, H. Preparation of pyridyl grignard reagents and cross coupling reactions with sulfoxides bearing azaheterocycles. Tetrahedron Lett. 28, 5845–5848 (1987).
Oae, S., Takeda, T. & Wakabayashi, S. Ligand coupling through σ-sulfurane—complete retention of geometric configuration of allylic and vinylic groups in the reactions of allylic and vinylic sulfoxides with Grignard reagents. Tetrahedron Lett. 29, 4445–4448 (1988).
Oae, S., Kawai, T. & Furukawa, N. A convenient preparation of bipyridines through ligand coupling reaction with α-sulfurane formed by treatment of methyl 2-pyridyl sulfoxide with Grignard reagents. Tetrahedron Lett. 25, 2549–2552 (1984).
LaRochelle, R. W. & Trost, B. M. Reactions of organolithiums with arylsulfonium salts. J. Am. Chem. Soc. 93, 6077–6086 (1971).
Trost, B. M. & Arndt, H. C. σ-Sulfurane chemistry. Effect of substituents on the coupling reactions. J. Am. Chem. Soc. 95, 5288–5298 (1973).
Oae, S., Takeda, T., Kawai, T. & Furukawa, N. Ligand coupling and pseudorotation in the reaction of alkyl 2-pyridyl sulfoxide with Grignard reagents. Phosphorus Sulfur Relat. Elem. 34, 133–137 (1987).
Oae, S., Takeda, T., Uenishi, J. & Wakabayashi, S. Ligand coupling reactions of 2-pyridyl, 4-pyridyl and 2-pyrimidyl sulfoxides with Grignard reagents. Phosphorus Sulfur Relat. Elem. 115, 179–182 (1996).
Oae, S., Inabushi, Y. & Yoshihara, M. Occurrence of ligand coupling in the reaction of the 2‐thienyl sulfoxides with organometallic compounds. Heteroatom. Chem. 4, 185–188 (1993).
Dean, W. M., Šiaučiulis, M., Storr, T., Lewis, W. & Stockman, R. A. Versatile C(sp2)-C(sp3) ligand couplings of sulfoxides for the enantioselective synthesis of diarylalkanes. Angew. Chem. Int. Ed. 55, 10013–10016 (2016).
Šiaučiulis, M., Ahlsten, N., Pulis, A. P. & Procter, D. J. Transition-metal-free cross-coupling of benzothiophenes and styrenes in a stereoselective synthesis of substituted (E, Z)-1,3-dienes. Angew. Chem. Int. Ed. 58, 8779–8783 (2019).
Duong, V. K., Horan, A. M. & McGarrigle, E. M. Synthesis of pyridylsulfonium salts and their application in the formation of functionalized bipyridines. Org. Lett. 22, 8451–8457 (2020).
Morofuji, T., Yoshida, T., Tsutsumi, R., Yamanaka, M. & Kano, N. Arylation of aryllithiums with S-arylphenothiazinium ions for biaryl synthesis. Chem. Commun. 56, 13995–13998 (2020).
Yoshida, T., Honda, Y., Morofuji, T. & Kano, N. N-methylphenothiazine S-oxide enabled oxidative C(sp2)-C(sp2) coupling of boronic acids with organolithiums via phenothiaziniums. Org. Lett. 23, 9664–9668 (2021).
Yoshida, T., Honda, Y., Morofuji, T. & Kano, N. Transition-metal-free O-arylation of alcohols and phenols with S-arylphenothiaziniums. J. Org. Chem. 87, 7565–7573 (2022).
Chen, D.-L. et al. Desulfurization of diaryl(heteroaryl) sulfoxides with benzyne. Org. Lett. 21, 3986–3989 (2019).
Ritts, C. B. & Hoye, T. R. Sulfurane [S(IV)]-mediated fusion of benzynes leads to helical dibenzofurans. J. Am. Chem. Soc. 143, 13501–13506 (2021).
Melzig, J., Rauhut, C. B., Naredi-Rainer, N. & Knochel, P. Difunctionalisation of arenes and heteroarenes by directed metallation and sulfoxide-magnesium exchange. Chem. Eur. J. 17, 5362–5372 (2011).
Zhou, M., Tsien, J. & Qin, T. Sulfur (IV)-mediated heterocycle cross-couplings. Angew. Chem. Int. Ed. 59, 7372–7376 (2020).
Kawai, T. et al. Occurrence of ligand coupling in the reactions of various sulfoxide with Grignard regents. Phosphorus Sulfur Relat. Elem. 34, 139–148 (1987).
Morgan, K. F., Doran, R., Croft, R. A., Hollingsworth, I. A. & Bull, J. A. 2-Sulfinyl oxetanes: synthesis, stability and reactivity. Synlett. 27, 106–110 (2016).
Shi, W.-Q. et al. Synthesis of CMe2CF3‑containing heteroarenes via tandem 1,1-dimethyltrifluoroethylation and cyclization of isonitriles. J. Org. Chem. 83, 15236–15244 (2018).
Johnston, L. J., Scaiano, J. C. & Ingold, K. U. Kinetics of cyclopropyl radical reactions. 1. Absolute rate constants for some addition and abstraction reactions. J. Am. Chem. Soc. 106, 4877–4881 (1984).
Sun, A. C., McClain, E. J., Beatty, J. W. & Stephenson, C. R. J. Visible light-mediated decarboxylative alkylation of pharmaceutically relevant heterocycles. Org. Lett. 20, 3487–3490 (2018).
Kirihara, M., Kambayashi, T. & Momose, T. Allylic fluorides via the cleavage of tertiary cyclopropyl silyl ethers with diethylaminosulfur trifluoride. Chem. Commun. 1996, 1103–1104 (1996).
Meyer, O. G. J., Fröhlich, R. & Haufe, G. Asymmetric cyclopropanation of vinyl fluorides: access to enantiopure monofluorinated cyclopropane carboxylates. Synthesis 10, 1479–1490 (2000).
Arlt, A. et al. Novel heteroaryl-triazole compounds as pesticides. PCT patent WO2021013720A1 (2018).
Fang, Z., Cordes, D. B., Slawin, A. M. Z. & O’Hagan, D. Fluorine containing cyclopropanes: synthesis of aryl substituted all-cis 1,2,3- trifluorocyclopropanes, a facially polar motif. Chem. Commun. 55, 10539–10542 (2019).
Conte, A. et al. Anthranilamide/2-amino-heteroarene carboxamide derivatives. PCT patent WO2007090752A1 (2007).
Lapointe, T. et al. Substituted indazole compounds as RORγT inhibitors and uses thereof. PCT patent WO2017/075182A1 (2017).
Liang, S., Hofman, K., Friedrich, M. & Manolikakes, G. Recent advances in the synthesis and direct application of sulfinate salts. Eur. J. Org. Chem. 2020, 4664–4676 (2020).
Reddy, R. J. & Kumari, A. H. Synthesis and applications of sodium sulfinates (RSO2Na): a powerful building block for the synthesis of organosulfur compounds. RSC Adv. 11, 9130–9221 (2021).
Sarver, P. J., Bissonnette, N. B. & MacMillan, D. W. C. Decatungstate-catalyzed C(sp3)-H sulfinylation: rapid access to diverse organosulfur functionality. J. Am. Chem. Soc. 143, 9737–9743 (2021).
Johnson, M. G., Gribble, M. W. Jr., Houze, J. B. & Paras, N. A. Convenient route to secondary sulfinates: application to the stereospecific synthesis of α‑C‑chiral sulfonamides. Org. Lett. 16, 6248–6251 (2014).
O’ Neill, M. J. & Cornella, J. Retaining alkyl nucleophile regiofidelity in transition-metal mediated cross-couplings to aryl electrophiles. Synthesis 50, 3974–3996 (2018).
Li, L., Wang, C. Y., Huang, R. & Biscoe, M. R. Stereoretentive Pd-catalysed Stille cross-coupling reactions of secondary alkyl azastannatranes and aryl halides. Nat. Chem. 5, 607–612 (2013).
Khim, Y. H. & Oae, S. The mechanism of the alkaline decomposition of triarylsulfonium bromide with phenyllithium. Bull. Chem. Soc. Jpn. 42, 1968–1971 (1969).
Moc, J., Dorigo, A. & Morokuma, K. Transition structures for H2 elimination from XH4 hypervalent species (X = S, Se and Te). Ab initio MO study. Chem. Phys. Lett. 204, 65–72 (1993).
Krasovskiy, A. & Knochel, P. A LiCl-Mediated Br/Mg exchange reaction for the preparation of functionalized aryl- and heteroarylmagnesium compounds from organic bromides. Angew. Chem. Int. Ed. 43, 3333–3336 (2004).
Krasovskiy, A., Krasovskaya, V. & Knochel, P. Mixed Mg/Li amides of the type R2NMgCl·LiCl as highly efficient bases for the regioselective generation of functionalized aryl and heteroaryl magnesium compounds. Angew. Chem. Int. Ed. 45, 2958–2961 (2006).
Jaric, M., Haag, B. A., Unsinn, A., Karaghiosoff, K. & Knochel, P. Highly selective metalations of pyridines and related heterocycles using new frustrated Lewis pairs or tmp-zinc and tmp-magnesium bases with BF3·OEt2. Angew. Chem. Int. Ed. 49, 5451–5455 (2010).
Huang, P. Q. et al. Spirocyclic compounds. PCT patent WO2016/161160A1 (2016).
Schirok, H. et al. Substituted 2-aminopyrazolyl-[1,2,4]triazolo [1,5a]pyridine and use. PCT WO2020/215094A1 (2020).
Caravatti, G., Fairhurst, R. A., Furet, P., Guagnano, V. & Imbach, P. Organic compounds. US patent 20090163469A1 (2009).
Bell, I. M. et al. Heterocyclic CGRP receptor antagonists. PCT patent WO2016/022644A1 (2016).
Aversa, R. J. et al. Compounds and compositions as RAF kinas inhibitors. PCT patent WO2016/038582A1 (2016).
Xi, N., Wang, L. & Wang, T. Substituted pyridine compounds and methods of use. PCT patent WO2015/034729A1 (2015).
Kang, C.-Q., Cheng, Y.-Q., Guo, H.-Q., Qiu, X.-P. & Gao, L.-X. The natural alkaloid isoanabasine: synthesis from 2,3′-bipyridine, efficient resolution with BINOL, and assignment of absolute configuration by Mosher’s method. Tetrahedron Asymmetry 16, 2141–2147 (2005).
Schäfer, P., Palacin, T., Sidera, M. & Fletcher, S. P. Asymmetric Suzuki–Miyaura coupling of heterocycles via rhodium-catalysed allylic arylation of racemates. Nat. Commun. 8, 15762 (2017).
van Dijk, K. et al. Mechanistic investigation of Rh(I)-catalysed asymmetric Suzuki–Miyaura coupling with racemic allyl halides. Nat. Catal. 4, 284–292 (2021).
Peltzer, R. M., Eisenstein, O., Nova, A. & Cascella, M. How solvent dynamics controls the Schlenk equilibrium of Grignard reagents: a computational study of CH3MgCl in tetrahydrofuran. J. Phys. Chem. B 121, 4226–4237 (2017).
Peltzer, R. M., Gauss, J., Eisenstein, O. & Cascella, M. The Grignard reaction – unraveling a chemical puzzle. J. Am. Chem. Soc. 142, 2984–2994 (2020).
Ess, D. H. Quasiclassical direct dynamics trajectory simulations of organometallic reactions. Acc. Chem. Res. 54, 4410–4422 (2021).
Acknowledgements
Financial support for this work was provided by the Welch Foundation (I-2010-20190330 to T.Q. and A-2102-20220331 to O.G.), the National Institutes of Health (R01GM141088 to T.Q. and R35GM137797 to O.G.), the Camille and Henry Dreyfus Foundation (to O.G.), the American Chemistry Society Petroleum Research Fund (62223-DNI1 to T.Q.) and a UT Southwestern Eugene McDermott Scholarship (to T.Q.). We thank F. Lin (UTSW) for assistance with NMR spectroscopy, H. Baniasadi (UTSW) for HRMS and V. Lynch (UT-Austin) for X-ray crystallographic analysis. We thank the Chen, Tambar, Ready, De Brabander, Smith and Falck groups at UTSW for generous access to equipment and helpful discussions. We are grateful to E. Wappes, K. McClymont and C. Hethcox (Merck and Co., Inc.) for feedback on this manuscript.
Author information
Authors and Affiliations
Contributions
M.Z., J.T. and T.Q. performed experiments. R.D. and O.G. performed DFT theoretical studies. J.M.E.H., B.K.P., R.R.M. and T.Q. designed and supervised the project; M.Z., J.T., R.D., J.M.E.H., B.K.P., R.R.M., O.G. and T.Q. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Georg Manolikakes, Raju Reddy and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
General experimental, optimization studies, general procedures, reaction guidelines and troubleshooting, experimental procedures and characterization data of starting materials, experimental procedures and characterization data of substrates, control experiments, capture of sulfur part by-product, control experiments on stereochemistry fidelity, VT 19F-NMR experiment, ReactIR experiment, computer investigation, X-ray crystallographic data, references and NMR spectra.
Supplementary Data 1
Crystallographic data for compound 40; CCDC no. 2086919.
Supplementary Data 2
Crystallographic data for compound 41; CCDC no. 2086920.
Supplementary Data 3
Crystallographic data for compound 74; CCDC no. 2172148.
Supplementary Data 4
Crystallographic data for compound 116; CCDC no. 2172149.
Supplementary Data 5
Coordinates and energies of calculated structures.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, M., Tsien, J., Dykstra, R. et al. Alkyl sulfinates as cross-coupling partners for programmable and stereospecific installation of C(sp3) bioisosteres. Nat. Chem. 15, 550–559 (2023). https://doi.org/10.1038/s41557-023-01150-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01150-z