Abstract
Melanoma, the most lethal form of skin cancer, often has worse outcomes in older patients. We previously demonstrated that an age-related decrease in the secreted extracellular matrix (ECM) protein HAPLN1 has a role in slowing melanoma progression. Here we show that HAPLN1 in the dermal ECM is sufficient to maintain the integrity of melanoma-associated blood vessels, as indicated by increased collagen and VE-cadherin expression. Specifically, we show that HAPLN1 in the ECM increases hyaluronic acid and decreases endothelial cell expression of ICAM1. ICAM1 phosphorylates and internalizes VE-cadherin, a critical determinant of vascular integrity, resulting in permeable blood vessels. We found that blocking ICAM1 reduces tumor size and metastasis in older mice. These results suggest that HAPLN1 alters endothelial ICAM1expression in an indirect, matrix-dependent manner. Targeting ICAM1 could be a potential treatment strategy for older patients with melanoma, emphasizing the role of aging in tumorigenesis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article and its supplementary information and are available upon request. Complete data used to generate Fig. 4a is in Supplementary Table 1.
References
Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 73, 17–48 (2023).
Macdonald, J. B. et al. Malignant melanoma in the elderly: different regional disease and poorer prognosis. J Cancer 2, 538–543 (2011).
Balch, C. M. et al. Age as a prognostic factor in patients with localized melanoma and regional metastases. Ann. Surg. Oncol. 20, 3961–3968 (2013).
The importance of aging in cancer research. Nat. Aging 2, 365–366 (2022).
Fulop, T. et al. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front. Immunol. 8, 1960 (2017).
Hanahan, D. Hallmarks of cancer: new simensions. Cancer Discov 12, 31–46 (2022).
Fane, M. & Weeraratna, A. T. How the ageing microenvironment influences tumour progression. Nat. Rev. Cancer 20, 89–106 (2020).
Ecker, B. L. et al. Age-related changes in HAPLN1 increase lymphatic permeability and affect routes of melanoma metastasis. Cancer Discov. 9, 82–95 (2019).
Kaur, A. et al. Remodeling of the collagen matrix in aging skin promotes melanoma metastasis and affects immune cell motility. Cancer Discov. 9, 64–81 (2019).
Kaur, A. et al. sFRP2 in the aged microenvironment drives melanoma metastasis and therapy resistance. Nature 532, 250–254 (2016).
Cox, T. R. The matrix in cancer. Nat. Rev. Cancer 21, 217–238 (2021).
Phillip, J. M., Aifuwa, I., Walston, J. & Wirtz, D. The mechanobiology of aging. Annu. Rev. Biomed. Eng. 17, 113–141 (2015).
Buckwalter, J. A., Rosenberg, L. C. & Tang, L. H. The effect of link protein on proteoglycan aggregate structure. An electron microscopic study of the molecular architecture and dimensions of proteoglycan aggregates reassembled from the proteoglycan monomers and link proteins of bovine fetal epiphyseal cartilage. J. Biol. Chem. 259, 5361–5363 (1984).
Chen, X. et al. Glycosaminoglycans modulate long-range mechanical communication between cells in collagen networks. Proc. Natl Acad. Sci. USA 119, e2116718119 (2022).
Faries, M. B. et al. Completion dissection or observation for sentinel-node metastasis in melanoma. New Engl. J. Med. 376, 2211–2222 (2017).
Page, A. J. et al. Increasing age is associated with worse prognostic factors and increased distant recurrences despite fewer sentinel lymph node positives in melanoma. Int. J. Surg. Oncol. 2012, 456987 (2012).
Huang, R., Andersen, L. M. K. & Rofstad, E. K. Metastatic pathway and the microvascular and physicochemical microenvironments of human melanoma xenografts. J. Translat. Med. 15, 203 (2017).
Faries, M. B. et al. Lymph node metastasis in melanoma: a debate on the significance of nodal metastases, conditional survival analysis and clinical trials. Clin. Exp. Metastasis 35, 431–442 (2018).
Meeth, K., Wang, J. X., Micevic, G., Damsky, W. & Bosenberg, M. W. The YUMM lines: a series of congenic mouse melanoma cell lines with defined genetic alterations. Pigment Cell Melanoma Res. 29, 590–597 (2016).
Dallas, N. A. et al. Endoglin (CD105): a marker of tumor vasculature and potential target for therapy. Clin. Cancer Res. 14, 1931–1937 (2008).
Fonsatti, E., Altomonte, M., Nicotra, M. R., Natali, P. G. & Maio, M. Endoglin (CD105): a powerful therapeutic target on tumor-associated angiogenetic blood vessels. Oncogene 22, 6557–6563 (2003).
Pandita, A. et al. Intussusceptive angiogenesis in human metastatic malignant melanoma. Am. J. Pathol. 191, 2023–2038 (2021).
Li, B. et al. Fibronectin 1 promotes melanoma proliferation and metastasis by inhibiting apoptosis and regulating EMT. Onco. Targets Ther. 12, 3207–3221 (2019).
Yokoi, K. et al. Capillary-wall collagen as a biophysical marker of nanotherapeutic permeability into the tumor microenvironment. Cancer Res. 74, 4239–4246 (2014).
Franco-Barraza, J., Beacham, D. A., Amatangelo, M. D. & Cukierman, E. Preparation of extracellular matrices produced by cultured and primary fibroblasts.Curr. Protoc. Cell Biol. 71, 10.19.11–10.19.34 (2016).
Giannotta, M., Trani, M. & Dejana, E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev. Cell 26, 441–454 (2013).
Adil, M. S. & Somanath, P. R. Endothelial permeability assays in vitro. Methods Mol. Biol. 2367, 177–191 (2021).
Jiménez, N., Krouwer, V. J. & Post, J. A. A new, rapid and reproducible method to obtain high quality endothelium in vitro. Cytotechnology 65, 1–14 (2013).
Szauter, K. M., Cao, T., Boyd, C. D. & Csiszar, K. Lysyl oxidase in development, aging and pathologies of the skin. Pathol. Biol. (Paris) 53, 448–456 (2005).
Frantz, C., Stewart, K. M. & Weaver, V. M. The extracellular matrix at a glance. J. Cell Sci. 123, 4195–4200 (2010).
Dupont, S. et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011).
Nukuda, A. et al. Stiff substrates increase YAP-signaling-mediated matrix metalloproteinase-7 expression. Oncogenesis 4, e165 (2015).
Melching, L. I. & Roughley, P. J. The role of link protein in mediating the interaction between hyaluronic acid and newly secreted proteoglycan subunits from adult human articular cartilage. J. Biol. Chem. 260, 16279–16285 (1985).
Lee, D. H., Oh, J.-H. & Chung, J. H. Glycosaminoglycan and proteoglycan in skin aging. J. Dermatol. Sci. 83, 174–181 (2016).
Soucy, P. A., Werbin, J., Heinz, W., Hoh, J. H. & Romer, L. H. Microelastic properties of lung cell-derived extracellular matrix. Acta Biomater. 7, 96–105 (2011).
Jayakrishnan, A. & Jameela, S. R. Glutaraldehyde as a fixative in bioprostheses and drug delivery matrices. Biomaterials 17, 471–484 (1996).
Kim, S. S. et al. Tissue engineering of heart valves by recellularization of glutaraldehyde-fixed porcine valves using bone marrow-derived cells. Exp. Mol. Med. 38, 273–283 (2006).
Schnellmann, R., Wei, Z., Choudhury, M. I., Sun, S. & Gerecht, S. Vascular phenotype is compromised in dynamically stiffening hydrogel. FASEB J. 34, 1 (2020).
Schnellmann, R. et al. Stiffening matrix induces age-mediated microvascular phenotype through increased cell contractility and destabilization of adherens junctions. Adv. Sci. (Weinh) 9, e2201483 (2022).
David, M. R. & Jennifer, L. Skin collagen through the lifestages: importance for skin health and beauty. Plast. Aesthet. Res. 8, 2 (2021).
Périn, J. P., Bonnet, F., Thurieau, C. & Jollès, P. Link protein interactions with hyaluronate and proteoglycans. Characterization of two distinct domains in bovine cartilage link proteins. J. Biol. Chem. 262, 13269–13272 (1987).
Mieulet, V. et al. Stiffness increases with myofibroblast content and collagen density in mesenchymal high grade serous ovarian cancer. Sci. Rep. 11, 4219 (2021).
Bui, T. M., Wiesolek, H. L. & Sumagin, R. ICAM-1: a master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J. Leukoc. Biol. 108, 787–799 (2020).
Marlin, S. D. & Springer, T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell 51, 813–819 (1987).
Allingham, M. J., van Buul, J. D. & Burridge, K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J. Immunol. 179, 4053–4064 (2007).
Klemke, M., Weschenfelder, T., Konstandin, M. H. & Samstag, Y. High affinity interaction of integrin α4β1 (VLA-4) and vascular cell adhesion molecule 1 (VCAM-1) enhances migration of human melanoma cells across activated endothelial cell layers. J. Cell. Physiol. 212, 368–374 (2007).
Scott, H. A. et al. Matrix stiffness exerts biphasic control over monocyte-endothelial adhesion via Rho-mediated ICAM-1 clustering. Integr. Biol. (Camb) 8, 869–878 (2016).
Stroka, K. M., Levitan, I. & Aranda-Espinoza, H. OxLDL and substrate stiffness promote neutrophil transmigration by enhanced endothelial cell contractility and ICAM-1. J. Biomech. 45, 1828–1834 (2012).
Chen, W. et al. Matrix stiffness regulates the interactions between endothelial cells and monocytes. Biomaterials 221, 119362 (2019).
Meng, F. et al. Attenuation of lipopolysaccharide-induced lung vascular stiffening by lipoxin reduces lung inflammation. Am. J. Respir. Cell Mol. Biol. 52, 152–161 (2015).
Schaefer, A. & Hordijk, P. L. Cell-stiffness-induced mechanosignaling: a key driver of leukocyte transendothelial migration. J. Cell Sci. 128, 2221–2230 (2015).
Wichert, S. et al. A single-arm, open-label, phase 2 clinical trial evaluating disease response following treatment with BI-505, a human anti-intercellular adhesion molecule-1 monoclonal antibody, in patients with smoldering multiple myeloma. PLoS One 12, e0171205 (2017).
Enlimomab Acute Stroke Trial Investigators. Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology 57, 1428–1434 (2001).
Guo, P. et al. A rationally designed ICAM1 antibody drug conjugate eradicates late-stage and refractory triple-negative breast tumors in vivo. Sci. Adv. 9, eabq7866 (2023).
Xiao, D. et al. Identifying mRNA, microRNA and protein profiles of melanoma exosomes. PLoS One 7, e46874 (2012).
Naba, A. et al. The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol. Cell Proteomics 11, M111.014647 (2012).
Ahmadzadeh, H. et al. Modeling the two-way feedback between contractility and matrix realignment reveals a nonlinear mode of cancer cell invasion. Proc. Natl Acad. Sci. USA 114, E1617–e1626 (2017).
& Wiedmann, L. et al. HAPLN1 is a driver for peritoneal carcinomatosis in pancreatic cancer. Nat. Commun. 14, 2353 (2023).
Mahadevan, D. & Von Hoff, D. D. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol. Cancer Ther. 6, 1186–1197 (2007).
Marino, G. E. & Weeraratna, A. T. A glitch in the matrix: age-dependent changes in the extracellular matrix facilitate common sites of metastasis. Aging Cancer 1, 19–29 (2020).
Attwell, D., Mishra, A., Hall, C. N., O’Farrell, F. M. & Dalkara, T. What is a pericyte? J. Cereb. Blood Flow Metab. 36, 451–455 (2016).
Sun, R., Kong, X., Qiu, X., Huang, C. & Wong, P. P. The emerging roles of pericytes in modulating tumor microenvironment. Front. Cell Dev. Biol. 9, 676342 (2021).
Trevino-Villarreal, J. H., Cotanche, D. A., Sepulveda, R. & Rogers, R. A. Effect of pericytes on melanoma development. J. Clin. Oncol. 30, 83 (2012).
Hubbard, A. K. & Rothlein, R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic. Biol. Med. 28, 1379–1386 (2000).
Chung, H. Y. et al. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res. Rev. 8, 18–30 (2009).
Ferrucci, L. & Fabbri, E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 15, 505–522 (2018).
Zhao, H. et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct. Target Ther. 6, 263 (2021).
Acknowledgements
G.E.M.-B. is supported by NRSA pre-doctoral fellowship 1F31CA261065-01A1 and R01CA232256. We thank the outstanding Core Facilities of the Wistar Institute, supported by P30CA010815 and of the Johns Hopkins Kimmel Cancer Center, P30CA00697356; A.E.C. is supported by R01CA232256 and GT15667. V.W. is supported by T32CA153952. Y.C. is supported by U01CA227550. A.T.W. is supported by grants P01 CA114046, U01CA227550, and R01CA232256, a Bloomberg Distinguished Professorship and the EV McCollum Endowed Chair. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. We thank J. Hayden and F. Keeney at the Wistar Imaging Facility for their training and guidance, especially with the two-photon experiment. This paper is dedicated to the memory of Judith Campisi.
Author information
Authors and Affiliations
Contributions
G.E.M.-B. and A.T.W. conceived the study and designed the experiments. G.E.M.-B. performed the majority of all experiments and analyses. Y.C. aided in experimental design, especially for the Qiagen screen (Fig. 4a) and animal study (Fig. 5), interpretation of data and revisions. A.D. and V.W. also assisted with revisions. Animal experiments were performed by G.E.M.-B., Y.C. and A.E.C., including injections and tissue processing. A.E.C. also aided in the design and interpretation of experiments necessary for the response to reviewers. L.H. aided in preparation of cellular-derived matrices, preparation of spheroids, interpretation of data and reviewer revisions. Skin reconstructs (Fig. 1f) and shRNA used for this study were created and validated by A.K. Y.L. performed and analyzed data from the ECIS experiment in the laboratory of T.S.K.E.-M. (Fig. 2d). S.D. performed experiments necessary for the response to reviewers in the laboratory of L.G. R.S. and S.G. aided in the experimental design of the spheroid assay in fibroblast-modified substrate (Fig. 1e). All authors participated in the reading and editing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
A.T.W. is on the board of ReGAIN Therapeutics, unrelated to the presented work. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Aging thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Increased angiogenesis in the absence of HAPLN1 is not mediated by increased endothelial cell proliferation.
(A) Immunohistochemistry (IHC) of CD31 in primary tumors from young mice treated intradermally with PBS (n = 5) and aged mice treated intradermally with rHAPLN1(n = 6) or PBS (n = 5). Graph represents quantification of stained region normalized to vessel number per area (one-way ANOVA, young +PBS vs. aged +PBS, **p = 0.004, aged +PBS vs. aged +rHAPLN1, *p = 0.02). Data are presented as mean values ± SEM. (B) Immunohistochemistry (IHC) of podoplanin (PDPN), vascular endothelial growth factor receptor-3 (VEGFR3), and lymphatic vessel endothelial hyaluronan receptor-1 (LYVE1) in primary tumors from young mice treated intradermally with PBS (n = 5) and aged mice treated intradermally with rHAPLN1 (n = 6) or PBS (n = 5). (C) Immunofluorescent microscopy of HUVECs cultured on indicated FDMs or control (gelatin coated dish) (n = 3 for all conditions) after 30 min of BRDU incorporation (0.03 mg/mL). BrdU+ (green), nuclei (DAPI stained). (D) Corresponding graph indicates fraction of BrdU+ nuclei compared to all DAPI+ in A (n = 3 for all conditions, one-way ANOVA shows no significance across any conditions). (E) qRT-PCR of 1205Lu human melanoma cells subjected to conditioned media (CM) from young or aged dermal fibroblasts. Graph indicates relative gene expression of HAPLN1 compared to positive control (young fibroblast line) and normalized to 18 s (one way ANOVA, ****p < 0.0001, n = 3). Data are presented as mean values ± SEM. (F) qRT-PCR of WM164 human melanoma cells subjected to CM from young or aged dermal fibroblasts. Graph indicates relative gene expression of HAPLN1 compared to positive control (young fibroblast line) and normalized to 18 s (one way ANOVA, ****p < 0.0001, n = 3). Data are presented as mean values ± SEM.
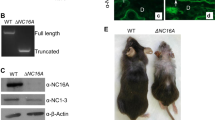
Extended Data Fig. 2 Confirmation of HAPLN1 knockdown in young fibroblast lines and in FDMs.
(A) Western blot for HAPLN1 in young fibroblast lines transduced with indicated shRNA against HAPLN1 (n = 1). (B) Relative quantification of HAPLN1 bands in A normalized to HSP90 loading control. (C) qRT-PCR of HAPLN1 in indicated fibroblast lines. Graph represents relative gene expression normalized to 18 s. (ANOVA, **p = 0.001, ***p = 0.0008). Data are presented as mean values ± SEM. (D) Immunofluorescent microscopy of HUVECs on indicated matrices, with fibronectin (green) used as a readout for successful matrix deposition (red=VE-cadherin, blue=DAPI), Aged +PBS (n = 9), Young +shCTRL (n = 9), Aged+rHAPLN1 (n = 9), Young Fixed (n = 4), Young +shHAPLN1 (n = 9). (E) Mass spectrometry analysis on young vs. aged FDMs showing differentially expressed proteins.
Extended Data Fig. 3 Confirmation of FDM stiffness and spheroid viability staining.
(A) Immunofluorescent microscopy of HUVECs cultured on indicated FDMs (red=YAP, blue=DAPI, purple=double positive nuclei). (B) Quantification of the percent of double-positive nuclei in A based on consistent color and shape thresholding using ImageJ analysis (ANOVA, **p = 0.0012, 0.0013, 0.0029, 0.0032, ***p = 0.0001, ****p < 0.0001, n = 3 FDM experiments per condition). Data are presented as mean values ± SEM. (C) IHC of skin (young, n = 5; aged n = 5) and (D) tumor (aged +PBS n = 5; aged +rHAPLN1 n = 5) in mice using a biotinylated hyaluronic acid Probe. (E) HUVEC spheroids were embedded in the absence of fibroblasts in 1.5 mg/mL (n = 11), 3 mg/mL (n = 18), 5 mg/mL (n = 14), 7 mg/mL (n = 9). Images are spheroids after 24 h in brightfield (left column; centroid=white circle, sprouts=red outline) and fluorescent microscopy (right column) after 20 min of treatment with live/dead viability stain (red=ethidium homodimer/ dead cells, green=calcein-AM/ live cells). (F) Graph is a quantification of the area of red stain for all conditions in E as a proportion of total spheroid area. One-way ANOVA, 1.5 mg/mL vs. 5 mg/mL,1.5 mg/mL vs. 7 mg/mL, all ****p = 0.0001; 3 mg/mL vs. 5 mg/mL (**p = 0.006); 3 mg/mL vs. 7 mg/mL (*p = 0.02); 5 mg/mL vs. 7 mg/mL (not significant). Data are presented as mean values ± SD.
Extended Data Fig. 4 qRT-PCR confirmation of selected targets of HUVEC genetic screen.
qRT-PCR of HUVECs grown on indicated FDMs for 48 h. Graph represents relative gene expression normalized to 18 s (n = 1).
Extended Data Fig. 5 Confirmation of anti-ICAM1 therapy efficacy and identification of distal metastases.
(A) IHC for ICAM1 of primary tumors from control IgG (n = 8) or anti-ICAM1 (n = 7) treated aged mice. (B) Corresponding graph represents quantification of stained region in A (student’s t-test, unpaired, 2-tailed **p = 0.0060). Data are presented as mean values +/- SEM. (C) qRT-PCR for ICAM1 in Yumm1.7 melanoma cells treated for 24 h with either IgG control or neutralizing anti-ICAM1 in vitro compared to untreated HUVECs. Graph represents relative gene expression normalized to 18 s (ordinary one-way ANOVA, ****p = <0.0001, n = 3). (D) qRT-PCR of HUVECs treated in vitro with either IgG (n = 3) or neutralizing anti-ICAM1 (n = 3). Graph represents relative gene expression of ICAM1 normalized to 18 s (student’s t-test, ****p = <0.0001, n = 3 treatments per condition). (E) Representative images of H&E-stained lungs from control IgG or anti-ICAM1 treated aged mice. Arrows indicate macrometastases (>10 cells). (F) Representative confirmation of metastases in control IgG (n = 8) and anti-ICAM1(n = 7) treated aged mouse lungs. Metastases were identified if they stained positive in H&E (top row), MERTK (middle row), and Ki67 (bottom row). (G) Graph represents percentage mice with any number of macrometastases in their lungs based on H&E analysis (n = 8, Control IgG, n = 7, anti-ICAM1). (H) qRT-PCR for CDH5 in HUVECs treated in vitro with either IgG (n = 3) or neutralizing anti-ICAM1 (n = 3). Graph represents relative gene expression normalized to 18 s (student’s t-test, ***p < 0.0001) Data are presented as mean values± SEM. (I) qRT-PCR for ICAM1 in 1205Lu melanoma cells cultured on indicated FDMs compared to HUVECs cultured on young FDM. Graph represented relative gene expression normalized to 18 s (ANOVA, ****p < 0.0001, n = 3). Data are presented as mean values ± SEM.
Supplementary information
Supplementary Information
Supplementary tables 1 and 2
Source data
Source Data All Figs
Statistical source data (graphs) for all figures
Source Data Fig. 4
Unprocessed westerns
Source Data Extended Data Fig. 2
Unprocessed westerns
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Marino-Bravante, G.E., Carey, A.E., Hüser, L. et al. Age-dependent loss of HAPLN1 erodes vascular integrity via indirect upregulation of endothelial ICAM1 in melanoma. Nat Aging 4, 350–363 (2024). https://doi.org/10.1038/s43587-024-00581-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-024-00581-8
This article is cited by
-
Mechanisms of melanoma aggressiveness with age
Nature Aging (2024)