Abstract
Sublethal cell damage can trigger senescence, a complex adaptive program characterized by growth arrest, resistance to apoptosis and a senescence-associated secretory phenotype (SASP). Here, a whole-genome CRISPR knockout screen revealed that proteins in the YAP–TEAD pathway influenced senescent cell viability. Accordingly, treating senescent cells with a drug that inhibited this pathway, verteporfin (VPF), selectively triggered apoptotic cell death largely by derepressing DDIT4, which in turn inhibited mTOR. Reducing mTOR function in senescent cells diminished endoplasmic reticulum (ER) biogenesis, triggering ER stress and apoptosis due to high demands on ER function by the SASP. Importantly, VPF treatment decreased the numbers of senescent cells in the organs of old mice and mice exhibiting doxorubicin-induced senescence. Moreover, VPF treatment reduced immune cell infiltration and pro-fibrotic transforming growth factor-β signaling in aging mouse lungs, improving tissue homeostasis. We present an alternative senolytic strategy that eliminates senescent cells by hindering ER activity required for SASP production.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
RNA-seq fastq files generated for this study are available at the Gene Expression Omnibus under accession number GSE221254 to provide access to all datasets in this study. YAP–TEAD targets were identified by both existing evidence on the literature and curation with MAGIC database. Source data are available with this paper.
References
Gorgoulis, V. et al. Cellular senescence: defining a path forward. Cell 179, 813–827 (2019).
Di Micco, R., Krizhanovsky, V., Baker, D. & d’Adda di Fagagna, F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 22, 75–95 (2021).
Gasek, N. S., Kuchel, G. A., Kirkland, J. L. & Xu, M. Strategies for targeting senescent cells in human disease. Nat Aging 1, 870–879 (2021).
Baar, M. P. et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell 169, 132–147 (2017).
Kirkland, J. L. & Tchkonia, T. Senolytic drugs: from discovery to translation. J. Intern. Med. 288, 518–536 (2020).
Lipina, C. & Hundal, H. S. Is REDD1 a metabolic eminence grise? Trends Endocrinol. Metab. 27, 868–880 (2016).
Doench, J. G. et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR–Cas9. Nat. Biotechnol. 34, 184–191 (2016).
Dupont, S. et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011).
Srikantan, S., Marasa, B. S., Becker, K. G., Gorospe, M. & Abdelmohsen, K. Paradoxical microRNAs: individual gene repressors, global translation enhancers. Cell Cycle 10, 751–759 (2011).
Lee, D. H. et al. LATS-YAP/TAZ controls lineage specification by regulating TGFβ signaling and Hnf4α expression during liver development. Nat. Commun. 7, 11961 (2016).
Chan, M. et al. Novel insights from a multiomics dissection of the Hayflick limit. Elife https://doi.org/10.7554/eLife.70283 (2022).
Munoz-Espin, D. & Serrano, M. Cellular senescence: from physiology to pathology. Nat. Rev. Mol. Cell Biol. 15, 482–496 (2014).
Liu-Chittenden, Y. et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 26, 1300–1305 (2012).
Anerillas, C. et al. A BDNF-TrkB autocrine loop enhances senescent cell viability. Nat. Commun. 13, 6228 (2022).
Munk, R. et al. Acid ceramidase promotes senescent cell survival. Aging 13, 15750–15769 (2021).
Stein, C. et al. YAP1 exerts its transcriptional control via TEAD-mediated activation of enhancers. PLoS Genet. 11, e1005465 (2015).
Zanconato, F. et al. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat. Cell Biol. 17, 1218–1227 (2015).
Pakos-Zebrucka, K. et al. The integrated stress response. EMBO Rep 17, 1374–1395 (2016).
Tabas, I. & Ron, D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 13, 184–190 (2011).
Anerillas, C. et al. Early SRC activation skews cell fate from apoptosis to senescence. Sci. Adv. 8, eabm0756 (2022).
Hetz, C. & Papa, F. R. The unfolded protein response and cell fate control. Mol. Cell 69, 169–181 (2018).
Zhang, D. et al. A non-canonical cGAS-STING-PERK pathway facilitates the translational program critical for senescence and organ fibrosis. Nat. Cell Biol. 24, 766–782 (2022).
Demaria, M. et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 7, 165–176 (2017).
Montalbano, R. et al. Endoplasmic reticulum stress plays a pivotal role in cell death mediated by the pan-deacetylase inhibitor panobinostat in human hepatocellular cancer cells. Transl. Oncol. 6, 143–157 (2013).
Oslowski, C. M. & Urano, F. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol. 490, 71–92 (2011).
Kim, M., Kim, T., Johnson, R. L. & Lim, D. S. Transcriptional co-repressor function of the hippo pathway transducers YAP and TAZ. Cell Rep. 11, 270–282 (2015).
Tirado-Hurtado, I., Fajardo, W. & Pinto, J. A. DNA damage inducible transcript 4 gene: the switch of the metabolism as potential target in cancer. Front. Oncol. 8, 106 (2018).
Lee, Y. et al. Histone deacetylase 4 reverses cellular senescence via DDIT4 in dermal fibroblasts. Aging 14, 4653–4672 (2022).
Foltyn, M. et al. The physiological mTOR complex 1 inhibitor DDIT4 mediates therapy resistance in glioblastoma. Br. J. Cancer 120, 481–487 (2019).
Herranz, N. et al. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat. Cell Biol. 17, 1205–1217 (2015).
Laberge, R. M. et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat. Cell Biol. 17, 1049–1061 (2015).
Carroll, B. et al. Persistent mTORC1 signaling in cell senescence results from defects in amino acid and growth factor sensing. J. Cell Biol. 216, 1949–1957 (2017).
Wu, H. et al. Integration of Hippo signalling and the unfolded protein response to restrain liver overgrowth and tumorigenesis. Nat. Commun. 6, 6239 (2015).
Jacquemyn, J., Cascalho, A. & Goodchild, R. E. The ins and outs of endoplasmic reticulum-controlled lipid biosynthesis. EMBO Rep. 18, 1905–1921 (2017).
Mossmann, D., Park, S. & Hall, M. N. mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat. Rev. Cancer 18, 744–757 (2018).
Brandt, C. et al. Food perception primes hepatic ER homeostasis via melanocortin-dependent control of mTOR activation. Cell 175, 1321–1335 (2018).
Peterson, T. R. et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell 146, 408–420 (2011).
Quinn, W. J. 3rd et al. mTORC1 stimulates phosphatidylcholine synthesis to promote triglyceride secretion. J. Clin. Invest. 127, 4207–4215 (2017).
Soto-Gamez, A. & Demaria, M. Therapeutic interventions for aging: the case of cellular senescence. Drug Discov. Today 22, 786–795 (2017).
Birch, J. & Gil, J. Senescence and the SASP: many therapeutic avenues. Genes Dev. 34, 1565–1576 (2020).
Freund, A., Patil, C. K. & Campisi, J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 30, 1536–1548 (2011).
Dorr, J. R. et al. Synthetic lethal metabolic targeting of cellular senescence in cancer therapy. Nature 501, 421–425 (2013).
Ovadya, Y. et al. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat. Commun. 9, 5435 (2018).
Schafer, M. J. et al. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 8, 14532 (2017).
Angelidis, I. et al. An atlas of the aging lung mapped by single cell transcriptomics and deep tissue proteomics. Nat. Commun. 10, 963 (2019).
Tabula Muris, C. A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature 583, 590–595 (2020).
Goplen, N. P. et al. Tissue-resident CD8+ T cells drive age-associated chronic lung sequelae after viral pneumonia. Sci. Immunol. https://doi.org/10.1126/sciimmunol.abc4557 (2020).
Schneider, J. L. et al. The aging lung: physiology, disease, and immunity. Cell 184, 1990–2019 (2021).
Gillich, A. et al. Capillary cell-type specialization in the alveolus. Nature 586, 785–789 (2020).
Wolf, T. et al. Dynamics in protein translation sustaining T cell preparedness. Nat. Immunol. 21, 927–937 (2020).
Prata, L., Ovsyannikova, I. G., Tchkonia, T. & Kirkland, J. L. Senescent cell clearance by the immune system: emerging therapeutic opportunities. Semin. Immunol. 40, 101275 (2018).
Desdin-Mico, G. et al. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science 368, 1371–1376 (2020).
Kale, A., Sharma, A., Stolzing, A., Desprez, P. Y. & Campisi, J. Role of immune cells in the removal of deleterious senescent cells. Immun Ageing 17, 16 (2020).
Marin, I., Serrano, M. & Pietrocola, F. Recent insights into the crosstalk between senescent cells and CD8 T lymphocytes. NPJ Aging 9, 8 (2023).
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: an expanding universe. Cell 186, 243–278 (2023).
Ogrodnik, M. et al. Cellular senescence drives age-dependent hepatic steatosis. Nat. Commun. 8, 15691 (2017).
Mylonas, K. J. et al. Cellular senescence inhibits renal regeneration after injury in mice, with senolytic treatment promoting repair. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.abb0203 (2021).
Yang, J. et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 21, 341–352 (2020).
Anerillas, C., Altes, G. & Gorospe, M. MAPKs in the early steps of senescence implemEMTation. Front. Cell Dev. Biol. 11, 1083401 (2023).
Chen, H. et al. MacroH2A1 and ATM play opposing roles in paracrine senescence and the senescence-associated secretory phenotype. Mol. Cell 59, 719–731 (2015).
Hernandez-Segura, A., Nehme, J. & Demaria, M. Hallmarks of cellular senescence. Trends Cell Biol. 28, 436–453 (2018).
Wang, M. et al. Verteporfin is a promising anti-tumor agent for cervical carcinoma by targeting endoplasmic reticulum stress pathway. Front. Oncol. 10, 1781 (2020).
Salama, R., Sadaie, M., Hoare, M. & Narita, M. Cellular senescence and its effector programs. Genes Dev. 28, 99–114 (2014).
Narita, M. et al. Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science 332, 966–970 (2011).
Moya, I. M. & Halder, G. Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat. Rev. Mol. Cell Biol. 20, 211–226 (2019).
Strano, S. et al. The transcriptional coactivator Yes-associated protein drives p73 gene-target specificity in response to DNA Damage. Mol. Cell 18, 447–459 (2005).
Furth, N., Aylon, Y. & Oren, M. p53 shades of Hippo. Cell Death Differ. 25, 81–92 (2018).
Sladitschek-Martens, H. L. et al. YAP/TAZ activity in stromal cells prevents ageing by controlling cGAS-STING. Nature 607, 790–798 (2022).
Sturmlechner, I. et al. Senescent cells limit p53 activity via multiple mechanisms to remain viable. Nat. Commun. 13, 3722 (2022).
van Deursen, J. M. Senolytic therapies for healthy longevity. Science 364, 636–637 (2019).
Xu, M. et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 24, 1246–1256 (2018).
Wang, B. et al. An inducible p21-Cre mouse model to monitor and manipulate p21-highly-expressing senescent cells in vivo. Nat Aging 1, 962–973 (2021).
Chaib, S., Tchkonia, T. & Kirkland, J. L. Cellular senescence and senolytics: the path to the clinic. Nat. Med. 28, 1556–1568 (2022).
Yosef, R. et al. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat. Commun. 7, 11190 (2016).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550 (2014).
Kuleshov, M. V. et al. Enrichr: a comprehensive gene-set enrichment analysis web server 2016 update. Nucleic Acids Res. 44, W90–W97 (2016).
Subramanian, A. et al. Gene-set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Roopra, A. MAGIC: A tool for predicting transcription factors and cofactors driving gene sets using ENCODE data. PLoS Comput. Biol. 16, e1007800 (2020).
Acknowledgements
This research was supported in its entirety by the NIA IRP, NIH. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
C.A. and K.M.-M. conceived and designed the study. S.D., K.A., R.d.C. and M.G. supervised the study. C.A., K.M.-M. and M.G. wrote the article. C.A., A.B.H., R.M., M.C.-R., A.G., D.T., J.L.M., G.A., M.R. and C.-Y.C. performed wet-lab and mouse experiments. K.M.-M., K.-W.G.L., Y.P., J.F. and S.D. performed bulk and single-cell sequencing and bioinformatic analyses. All authors reviewed and edited the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Aging thanks Ming Xu, Iván Moya and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 CRISPR screen optimization, validation, and YAP-TEAD inhibition in other senescence models.
a, Cell viability assessment by direct cell counting of senescent WI-38 cells treated with puromycin (1 µg/ml, 48 h) 72 h after being transduced with the Brunello library at the indicated MOIs. The gray bars represent the expected viability if the transduction efficiency was complete while the teal bars represent the viability observed for each of the MOIs after puromycin treatment. b, Cell viability as assessed by direct cell counting of WI-38 cells transfected with the indicated siRNAs and rendered senescent after treatment with etoposide for 6 days (ETIS). c, Analysis of the levels of the indicated mRNAs in proliferating (P) or ETIS WI-38 cells transfected with the indicated siRNAs 24 h before either treatment with etoposide (50 µM) or no treatment, and culture for an additional 6 days. d, Representative western blot analysis (n = 3 independent experiments) of the levels of phosphorylated YAP (S127), YAP, phosphorylated MOB1 (T35), MOB1, and ACTB levels at the indicated conditions. e,f, Analysis of BrdU incorporation (e) and SA-β-Gal staining (f) in the indicated cell types, rendered senescent by etoposide (ETIS), ionizing radiation (IRIS), or replicative exhaustion (RS). Scale bar 100 µm. g, Caspase 3/7 activity measured in RS and IRIS WI-38 cells treated for 72 h with the indicated doses of Verteporfin (VPF). h, i, Cell viability as assessed by direct cell counting (h) and Caspase 3/7 activity measurement (i) for the indicated models of senescence along with proliferating controls, after either no treatment or treatment with VPF for 72 h at the indicated doses. Graphs in (b, c, e, g–i) represent the means and each individual value as a dot ±s.d. n = 3 independent replicates; significance (*P < 0.05, **P < 0.01, ***P < 0.001) was determined using two-tailed Student’s t-test. Unless indicated, statistical tests were performed relative to untreated or proliferating controls.
Extended Data Fig. 2 Extended analysis of YAP-TEAD inhibition.
a, b, Representative western blot analysis (a) and quantification (b) of the levels of YAP and TEAD proteins after immunoprecipitation experiments with the indicated antibodies (IgG or anti-TEAD) in ETIS WI-38 fibroblasts that were either untreated or treated with VPF for 48 h. IgG bands are indicated with arrows placed on the left side of the panel. Inputs are also included. c, Heat map displaying the differential expression of the YAP–TEAD-dependent transcripts (by row Z-Score) in the conditions described in (a). Proliferating untreated cells were included as a baseline control. d, GSEA of the association (enrichment score) with the gene set ‘YAP1_up’ of ETIS WI-38 cells treated with VPF (48 h) compared to untreated senescent cells (-). e, RT-qPCR analysis of the levels of ANKRD1 and TGFB2 mRNAs in ETIS WI-38 cells after treatment for 48 h with the indicated YAP–TEAD inhibitors. Untreated controls were also included for comparison. f, Heat map representing the differential expression (Row Z-Score) among the conditions described in (e) for the indicated transcripts. g, Heat map displaying the differential expression (Row Z-Score) of the indicated transcripts in siCtrl and siTEAD2 ETIS WI-38 cells. h, GSEA of the association with the gene set ‘Hallmark: Epithelial-Mesenchymal Transition’ for the conditions described in (d). i, RT-qPCR analysis of the indicated pro-apoptotic mRNAs for the conditions described in (e). j, Representative Western blot (n = 3 independent experiments) of the levels of ATF6, XBP1s, and loading control ACTB for the conditions described in (d). k, GSEA plot showing the association (enrichment score) of the gene set ‘GOBP: PERK-mediated UPR’ with the conditions described in (d). l, Western blot analysis of the levels of phosphorylated EIF2A (S51) and loading control ACTB in WI-38 cells transfected with siCtrl or siPERK, rendered senescent with etoposide (ETIS) and then either left untreated or treated with 1.5 µM VPF for 48 h. m, n, Cell viability assessment by direct cell counting (m) and RT-qPCR analysis of PERK mRNA levels (n) in the conditions described in (l), but here treated with VPF for 72 h. o, p, Maximal cisternae thickness (o) and disorganization score (p) as measured by TEM in the groups described in (c). Thirty cells were analyzed for each condition. q, RT–qPCR analysis of the indicated transcripts either untreated or treated with 1.5 µM VPF for 8 h. r, Relative binding to the regulatory region of the DDIT4 gene or a negative control (Neg Ctrl) DNA in YAP ChIP samples of ETIS WI-38 cells that were untreated or treated with 1.5 µM VPF (48 h). s, RT-qPCR analysis of the levels of DDIT4 and p53 mRNAs in WI-38 cells transfected with the indicated siRNAs, rendered senescent with etoposide (ETIS) and either left untreated or treated with 1.5 µM VPF for 48 h. Proliferating WI-38 cells transfected with siCtrl were included as controls. Graphs in (b, e, i, m, n, q–s) display the means and the individual values as dots ±SD n = 3 independent replicates; graphs in (o, p) show the means and the individual values as dots ±s.d. of n = 30 different cells. Significance (*P < 0.05, **P < 0.01, ***P < 0.001) was calculated using two-tailed Student’s t-test.
Extended Data Fig. 3 Analysis of mTOR inhibition and VPF treatment on ER stress in senescent cells.
a, Cell viability as assessed by direct cell counting of proliferating (P) or ETIS WI-38 fibroblasts that were either left untreated or treated with 100 nM Torin1 for 72 h. b, Representative micrographs showing the differences in viability in the conditions from (a). Scale bar, 100 µm. c, Western blot analysis of the levels of ATF6, XBP1s, and ACTB in the conditions described in (a), at 48 h instead. d, RT-qPCR analysis of the levels of PUMA, PMAIP1, and TNFRSF10B mRNAs in the conditions described in (c). Untreated P cells were included as baseline controls. e, Direct cell counting after treating as indicated for 72 h (1.5 µM VPF, 100 nM Torin1, or both) in ETIS WI-38 cells. f, RT-qPCR analysis of the indicated transcripts for the treatments described in (e), in this case for 48 h. g, Dot plot representation of the values calculated for the ER-positive relative area per cell (60 cells per condition) in ETIS WI-38 cells either untreated or treated with VPF (1.5 µM) or Torin1 (100 nM) for 48 h. h, Micrographs showing the areas corresponding to the endoplasmic reticulum (ER) in red for the indicated treatments as in (g). Phosphatidylcholine (PtdCho) was simultaneously supplemented at 50 µM where indicated. Scale bar, 100 µm. i, Heat map representation of the differences in SASP mRNA levels represented by row Z-Score for the indicated transcripts when comparing the conditions described in (g). Untreated P WI-38 cells were included as baseline controls. j, Heat map of the row Z-Score calculated for the differences in the secretion of the indicated SASP members among the groups described in (g), including proliferating (P) WI-38 cells as a control for baseline secretion. k, UMAP plot representation of the scRNA-seq data from ETIS WI-38 cells (no VPF treatment) showing the expression score specified in the legends, associated with the indicated gene sets (SASP, a custom gene set of 132 markers; ER stress, GOBP: Response to ER stress; and Oxidative Phosphorylation, Hallmark: Oxidative Phosphorylation). l, Western blot analysis of phosphorylated EIF2A (S51) and ACTB levels in WI-38 cells transfected with the indicated siRNAs, rendered senescent by treatment with etoposide for 6 days, and then either left untreated or treated with 100 nM Torin1 for 48 h. m, Cell viability measurement by direct cell counting of the conditions described in (l), here treated for 72 h. n, o, RT-qPCR analysis (n) and Bioplex analysis of the conditioned media (o) to assess SASP production and secretion in WI-38 cells transfected with siCtrl or siRELA, and rendered senescent with etoposide for 6 days. Proliferating controls transfected with siCtrl siRNA were included. Graphs in (a, d–f, m, n) represent the means and individual values (dots) of n = 3 independent replicates; plot in (g) shows the individual values of 20 different cells from each of the 3 independent replicates analyzed, making a total 60 individual values; Significance (*P < 0.05, **P < 0.01, ***P < 0.001) was calculated using two-tailed Student’s t-test.
Extended Data Fig. 4 Analysis of senescence markers in naturally aged and doxorubicin-treated mice.
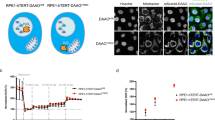
a Representative immunofluorescence images of p21 (red) and p16 (green) in mouse liver and kidney from the groups described in Fig. 4a. Scale bar (white), 200 µm. b, Quantification of the percentage of p16-positive, p21-positive, or p16/p21 double-positive cells in the liver and kidney samples represented in (a). c, RT-qPCR analysis of p16 and p21 mRNA levels (normalized to Actb mRNA) in liver and kidney for the conditions described in Fig. 4a. d, Schematic representation of the treatment regimen carried out to trigger doxorubicin-induced senescence in vivo in mice (10 mg/kg), along with 4 consecutive treatments with DMSO (Vehicle) or VPF (50 mg/kg) from day 6 onward. Samples were collected at day 10 after doxorubicin treatment. e, RT-qPCR analysis of p21 mRNA levels in lung, liver, and kidney from the groups described in (d). Untreated mice were included as baseline controls. f, g, Quantification (f) and representative images (g) of p21 immunofluorescence in the conditions described in (d). Scale bar (white), 200 µm. h, Serum measurement of GDF15 levels for the experimental groups described in (d, left, and Fig. 4a, right). Graphs in (b, c) display the means and the individual values as dots ± s.d. of the included mice (more details in Supplementary Table 6), while graphs in (e, f, h) display the means and the individual values as dots ± s.d. of n = 6 mice per group; significance (*P < 0.05, **P < 0.01, ***P < 0.001) was calculated using one-way ANOVA.
Extended Data Fig. 5 Physiological benefits of senolytic ABT-737 and VPF treatments in naturally aged mice.
a, Pictures of the 24 m.o. mice from the different groups described in Fig. 4a at the end of the experiment. b, UMAP clustering of the scRNA-seq performed in lungs from the indicated groups. c, Feature plot displaying Cdkn2a mRNA expression in the indicated experimental groups. Each plot was obtained by merging the two samples sequenced for each experimental group, shown in (b). d, Plots displaying the signals obtained through flow cytometry analysis of single-cell lung suspensions from the indicated groups. FSC-H axis represents the signals obtained for the forward scatter, while the PE-Cy7-A axis corresponds to CD45 staining with such fluorophore. Cells considered CD45+ are colored in blue, and the percentages are specified on the top right corner of each box. e, Dot plot displaying the association of the indicated cell types and conditions with the top 15 transcripts from Hallmark: Inflammatory Response gene set. The size of the dots represents the percentage of cells expressing such transcript while the intensity of red indicates the relative expression value. f, GSEA plots displaying the association of the indicated cell types from Old DMSO condition (compared to the rest of the experimental groups) with the indicated gene sets (Reactome: Translation; Safford T Lymphocyte Anergy; Reactome: Interleukin 6 Family Signaling; Reactome: Antigen Activates B cell Receptor BCR Leading To Generation Of Second Messengers). g, h, Masson’s trichrome (MTC) staining performed in liver and kidney from the indicated groups, representative images of MTC staining (g) in blue, and quantification of the blue area present at each sample divided by the total area in red (h). Scale bar, 200 µm. i, j, Serum analysis of blood urea nitrogen (i) and AST (j) levels for the indicated experimental groups. Plots in (h–j) represent the means and the individual values as dots ± s.d. of the included mice (more details in Supplementary Table 6); significance (*P < 0.05, **P < 0.01, ***P < 0.001) was calculated using one-way ANOVA.
Supplementary information
Supplementary Table 1
Abundance of the sgRNAs in the different conditions identified by next-generation sequencing of the CRISPR–Cas9 whole-genome screen. Data are plotted in the heat map in Fig. 1f. The data were pre-filtered here to show the significantly less abundant sgRNAs in all t = 14 replicates compared tot = 0.
Supplementary Table
Twofold changes comparing t = 14 to t = 0 in the whole-genome CRISPR–Cas9 screen included in the study for each of the detected sgRNAs, analyzed by Wald test in Deseq2. The different statistical parameters for each comparison between t = 14 and t = 0 are displayed on the rightmost columns. baseMean, average of normalized counts; log2FoldChange, log2fold change; lfcSE, standard error of log2FoldChange; stat, Wald statistic; pvalue, Wald test Pvalue; padj, Benjamini-Hochberg adjusted Pvalue.
Supplementary Table 3
List of transcripts included for the gene set ‘SASP’ used in the scRNA-seq analysis displayed in Fig. 3n.
Supplementary Table 4
Association P values and the −log10(P values) of the indicated gene sets (specified below) for each of the lung scRNA-seq clusters specified in Fig. 4g. The data included in this table are plotted in a heat map in Fig. 4h. The top 200 transcripts (obtained from the log1p average expression values for each condition per cluster) increased in old DMSO at least 2-fold relative to young DMSO and 1.5-fold relative to both old VPF and old ABT-737 were included for the analysis with Enrichr, which calculated the statistical values by itself. The P values obtained for each of the indicated gene sets are specified.
Supplementary Table 5
Percentages of each of the indicated lung cell types in the different conditions analyzed through scRNA-seq. The data included in this table are plotted in Fig. 4i.
Supplementary Table 6
Table showing the mice included in the aging study, with all the samples taken from each for all the specified subsequent analyses.
Supplementary Table 7
List of the primers used for the qPCR analysis of the specified transcripts.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Fig. 2
Uncropped blots.
Source Data Fig. 3
Uncropped blots.
Source Data Extended Data Fig. 1
Uncropped blots.
Source Data Extended Data Fig. 2
Uncropped blots.
Source Data Extended Data Fig. 3
Uncropped blots.
Rights and permissions
About this article
Cite this article
Anerillas, C., Mazan-Mamczarz, K., Herman, A.B. et al. The YAP–TEAD complex promotes senescent cell survival by lowering endoplasmic reticulum stress. Nat Aging 3, 1237–1250 (2023). https://doi.org/10.1038/s43587-023-00480-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-023-00480-4
This article is cited by
-
Sprouty1 is a broad mediator of cellular senescence
Cell Death & Disease (2024)