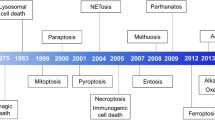
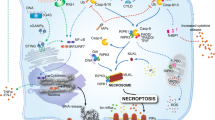
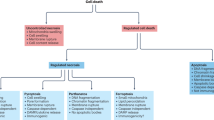
Abstract
Regulated cell death mediated by dedicated molecular machines, known as programmed cell death, plays important roles in health and disease. Apoptosis, necroptosis and pyroptosis are three such programmed cell death modalities. The caspase family of cysteine proteases serve as key regulators of programmed cell death. During apoptosis, a cascade of caspase activation mediates signal transduction and cellular destruction, whereas pyroptosis occurs when activated caspases cleave gasdermins, which can then form pores in the plasma membrane. Necroptosis, a form of caspase-independent programmed necrosis mediated by RIPK3 and MLKL, is inhibited by caspase-8-mediated cleavage of RIPK1. Disruption of cellular homeostatic mechanisms that are essential for cell survival, such as normal ionic and redox balance and lysosomal flux, can also induce cell death without invoking programmed cell death mechanisms. Excitotoxicity, ferroptosis and lysosomal cell death are examples of such cell death modes. In this Review, we provide an overview of the major cell death mechanisms, highlighting the latest insights into their complex regulation and execution, and their relevance to human diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Yuan, J., Lipinski, M. & Degterev, A. Diversity in the mechanisms of neuronal cell death. Neuron 40, 401–413 (2003).
Lockshin, R. A. & Williams, C. M. Programmed cell death–I. Cytology of degeneration in the intersegmental muscles of the pernyi silkmoth. J. Insect Physiol. 11, 123–133 (1965).
Lockshin, R. A. Programmed cell death 50 (and beyond). Cell Death Differ. 23, 10–17 (2016).
Choi, D. W. Glutamate neurotoxicity and diseases of the nervous system. Neuron 1, 623–634 (1988).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Stockwell, B. R. Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell 185, 2401–2421 (2022).
Aits, S. & Jaattela, M. Lysosomal cell death at a glance. J. Cell Sci. 126, 1905–1912 (2013).
Overholtzer, M. et al. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell 131, 966–979 (2007).
Frisch, S. M. & Francis, H. Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol. 124, 619–626 (1994).
Vaux, D. L., Cory, S. & Adams, J. M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 335, 440–442 (1988).
Kelekar, A. & Thompson, C. B. Bcl-2-family proteins: the role of the BH3 domain in apoptosis. Trends Cell Biol. 8, 324–330 (1998).
Cory, S. & Adams, J. M. The Bcl2 family: regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2, 647–656 (2002).
Green, D. R. The mitochondrial pathway of apoptosis part II: the BCL-2 protein family. Cold Spring Harb. Perspect. Biol. 14, a041046 (2022).
Motoyama, N. et al. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science 267, 1506–1510 (1995).
Rinkenberger, J. L., Horning, S., Klocke, B., Roth, K. & Korsmeyer, S. J. Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes Dev. 14, 23–27 (2000).
Veis, D. J., Sorenson, C. M., Shutter, J. R. & Korsmeyer, S. J. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell 75, 229–240 (1993).
Zou, H., Henzel, W. J., Liu, X., Lutschg, A. & Wang, X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell 90, 405–413 (1997).
Yuan, S. et al. The holo-apoptosome: activation of procaspase-9 and interactions with caspase-3. Structure 19, 1084–1096 (2011).
Li, Y. et al. Mechanistic insights into caspase-9 activation by the structure of the apoptosome holoenzyme. Proc. Natl Acad. Sci. USA 114, 1542–1547 (2017).
Julien, O. & Wells, J. A. Caspases and their substrates. Cell Death Differ. 24, 1380–1389 (2017).
Lakhani, S. A. et al. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science 311, 847–851 (2006).
Lindsten, T. et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol. Cell 6, 1389–1399 (2000).
Lindsten, T. & Thompson, C. B. Cell death in the absence of Bax and Bak. Cell Death Differ. 13, 1272–1276 (2006).
Krammer, P. H. CD95’s deadly mission in the immune system. Nature 407, 789–795 (2000).
Nagata, S. Apoptosis by death factor. Cell 88, 355–365 (1997).
Fisher, G. H. et al. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell 81, 935–946 (1995).
Rieux-Laucat, F. et al. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science 268, 1347–1349 (1995).
Takahashi, T. et al. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell 76, 969–976 (1994).
Watanabe-Fukunaga, R., Brannan, C. I., Copeland, N. G., Jenkins, N. A. & Nagata, S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 356, 314–317 (1992).
Martin, D. A. et al. Defective CD95/APO-1/Fas signal complex formation in the human autoimmune lymphoproliferative syndrome, type Ia. Proc. Natl Acad. Sci. USA 96, 4552–4557 (1999).
Micheau, O. & Tschopp, J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114, 181–190 (2003).
Hsu, H., Huang, J., Shu, H. B., Baichwal, V. & Goeddel, D. V. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity 4, 387–396 (1996).
Bertrand, M. J. et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol. Cell 30, 689–700 (2008).
Mahoney, D. J. et al. Both cIAP1 and cIAP2 regulate TNFα-mediated NF-κB activation. Proc. Natl Acad. Sci. USA 105, 11778–11783 (2008).
Varfolomeev, E. et al. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor α (TNFα)-induced NF-κB activation. J. Biol. Chem. 283, 24295–24299 (2008).
Haas, T. L. et al. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol. Cell 36, 831–844 (2009).
Draber, P. et al. LUBAC-recruited CYLD and A20 regulate gene activation and cell death by exerting opposing effects on linear ubiquitin in signaling complexes. Cell Rep. 13, 2258–2272 (2015).
Tokunaga, F. et al. Involvement of linear polyubiquitylation of NEMO in NF-κB activation. Nat. Cell Biol. 11, 123–132 (2009).
Wang, C. et al. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412, 346–351 (2001).
Ea, C. K., Deng, L., Xia, Z. P., Pineda, G. & Chen, Z. J. Activation of IKK by TNFα requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol. Cell 22, 245–257 (2006).
Geng, J. et al. Regulation of RIPK1 activation by TAK1-mediated phosphorylation dictates apoptosis and necroptosis. Nat. Commun. 8, 359 (2017).
Daniel, S. et al. A20 protects endothelial cells from TNF-, Fas-, and NK-mediated cell death by inhibiting caspase 8 activation. Blood 104, 2376–2384 (2004).
Wertz, I. E. et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signalling. Nature 430, 694–699 (2004).
He, K. L. & Ting, A. T. A20 inhibits tumor necrosis factor (TNF) alpha-induced apoptosis by disrupting recruitment of TRADD and RIP to the TNF receptor 1 complex in Jurkat T cells. Mol. Cell Biol. 22, 6034–6045 (2002).
Micheau, O., Lens, S., Gaide, O., Alevizopoulos, K. & Tschopp, J. NF-κB signals induce the expression of c-FLIP. Mol. Cell Biol. 21, 5299–5305 (2001).
Kataoka, T. The caspase-8 modulator c-FLIP. Crit. Rev. Immunol. 25, 31–58 (2005).
Degterev, A. et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 4, 313–321 (2008).
Ofengeim, D. et al. Activation of necroptosis in multiple sclerosis. Cell Rep. 10, 1836–1849 (2015).
Newton, K. et al. RIPK1 inhibits ZBP1-driven necroptosis during development. Nature 540, 129–133 (2016).
Zhang, X. et al. Ubiquitination of RIPK1 suppresses programmed cell death by regulating RIPK1 kinase activation during embryogenesis. Nat. Commun. 10, 4158 (2019).
Xu, D. et al. TBK1 suppresses RIPK1-driven apoptosis and inflammation during development and in aging. Cell 174, 1477–1491.e19 (2018).
Dondelinger, Y. et al. NF-κB-independent role of IKKα/IKKβ in preventing RIPK1 kinase-dependent apoptotic and necroptotic cell death during TNF signaling. Mol. Cell 60, 63–76 (2015).
Gerlach, B. et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature 471, 591–596 (2011).
Wang, L., Du, F. & Wang, X. TNF-α induces two distinct caspase-8 activation pathways. Cell 133, 693–703 (2008).
Jaco, I. et al. MK2 phosphorylates RIPK1 to prevent TNF-induced cell death. Mol. Cell 66, 698–710.e5 (2017).
Degterev, A. et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 1, 112–119 (2005). Refs 48 and 57 provided the first evidence for the existence of necroptosis and the role of RIPK1 in mediating necroptosis by isolating Nec1, which was the first small-molecule RIPK1 inhibitor.
Shan, B., Pan, H., Najafov, A. & Yuan, J. Necroptosis in development and diseases. Genes Dev. 32, 327–340 (2018).
Cho, Y. S. et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137, 1112–1123 (2009).
He, S. et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-α. Cell 137, 1100–1111 (2009).
Zhang, D. W. et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325, 332–336 (2009).
Wu, J. et al. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res. 23, 994–1006 (2013).
Degterev, A., Maki, J. L. & Yuan, J. Activity and specificity of necrostatin-1, small-molecule inhibitor of RIP1 kinase. Cell Death Differ. 20, 366 (2013).
Peltzer, N., Darding, M. & Walczak, H. Holding RIPK1 on the ubiquitin leash in TNFR1 signaling. Trends Cell Biol. 26, 445–461 (2016).
Li, X. et al. Ubiquitination of RIPK1 regulates its activation mediated by TNFR1 and TLRs signaling in distinct manners. Nat. Commun. 11, 6364 (2020).
Ito, Y. et al. RIPK1 mediates axonal degeneration by promoting inflammation and necroptosis in ALS. Science 353, 603–608 (2016).
Dziedzic, S. A. et al. ABIN-1 regulates RIPK1 activation by linking Met1 ubiquitylation with Lys63 deubiquitylation in TNF-RSC. Nat. Cell Biol. 20, 58–68 (2018).
Vlantis, K. et al. NEMO prevents RIP kinase 1-mediated epithelial cell death and chronic intestinal inflammation by NF-κB-dependent and -independent functions. Immunity 44, 553–567 (2016).
Meng, H. et al. Death-domain dimerization-mediated activation of RIPK1 controls necroptosis and RIPK1-dependent apoptosis. Proc. Natl Acad. Sci. USA 115, E2001–E2009 (2018).
Lin, Y., Devin, A., Rodriguez, Y. & Liu, Z. G. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 13, 2514–2526 (1999).
Tao, P. et al. A dominant autoinflammatory disease caused by non-cleavable variants of RIPK1. Nature 577, 109–114 (2020).
Lalaoui, N. et al. Mutations that prevent caspase cleavage of RIPK1 cause autoinflammatory disease. Nature 577, 103–108 (2020).
Li, J. et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell 150, 339–350 (2012).
Wu, X. et al. The structure of a minimum amyloid fibril core formed by necroptosis-mediating RHIM of human RIPK3. Proc. Natl Acad. Sci. USA 118, e2022933118 (2021).
Chen, W. et al. Diverse sequence determinants control human and mouse receptor interacting protein 3 (RIP3) and mixed lineage kinase domain-like (MLKL) interaction in necroptotic signaling. J. Biol. Chem. 288, 16247–16261 (2013).
Sun, L. et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148, 213–227 (2012). Refs 59, 60, 61 and 76 provided evidence for the roles of RIPK3 and MLKL in mediating necroptosis.
Murphy, J. M. et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity 39, 443–453 (2013).
Hildebrand, J. M. et al. Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. Proc. Natl Acad. Sci. USA 111, 15072–15077 (2014).
Polykratis, A. et al. Cutting edge: RIPK1 kinase inactive mice are viable and protected from TNF-induced necroptosis in vivo. J. Immunol. 193, 1539–1543 (2014).
Berger, S. B. et al. Cutting edge: RIP1 kinase activity is dispensable for normal development but is a key regulator of inflammation in SHARPIN-deficient mice. J. Immunol. 192, 5476–5480 (2014).
Laurien, L. et al. Autophosphorylation at serine 166 regulates RIP kinase 1-mediated cell death and inflammation. Nat. Commun. 11, 1747 (2020).
Blanchett, S., Dondelinger, Y., Barbarulo, A., Bertrand, M. J. M. & Seddon, B. Phosphorylation of RIPK1 serine 25 mediates IKK dependent control of extrinsic cell death in T cells. Front. Immunol. 13, 1067164 (2022).
Dondelinger, Y. et al. Serine 25 phosphorylation inhibits RIPK1 kinase-dependent cell death in models of infection and inflammation. Nat. Commun. 10, 1729 (2019).
Zelic, M. et al. RIP kinase 1-dependent endothelial necroptosis underlies systemic inflammatory response syndrome. J. Clin. Invest. 128, 2064–2075 (2018).
Zhang, T. et al. Metabolic orchestration of cell death by AMPK-mediated phosphorylation of RIPK1. Science 380, 1372–1380 (2023).
Sun, W. et al. Small molecule activators of TAK1 promotes its activity-dependent ubiquitination and TRAIL-mediated tumor cell death. Proc. Natl Acad. Sci. USA 120, e2308079120 (2023).
Kang, K., Park, C. & Chan, F. K. Necroptosis at a glance. J. Cell Sci. 135, jcs260091 (2022).
Balachandran, S. & Mocarski, E. S. Viral Z-RNA triggers ZBP1-dependent cell death. Curr. Opin. Virol. 51, 134–140 (2021).
He, S., Liang, Y., Shao, F. & Wang, X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc. Natl Acad. Sci. USA 108, 20054–20059 (2011).
Riebeling, T., Kunzendorf, U. & Krautwald, S. The role of RHIM in necroptosis. Biochem. Soc. Trans. 50, 1197–1205 (2022).
Jiao, H. et al. Z-nucleic-acid sensing triggers ZBP1-dependent necroptosis and inflammation. Nature 580, 391–395 (2020).
Ingram, J. P. et al. ZBP1/DAI drives RIPK3-mediated cell death induced by IFNs in the absence of RIPK1. J. Immunol. 203, 1348–1355 (2019).
Zhang, T. et al. Prolonged hypoxia alleviates prolyl hydroxylation-mediated suppression of RIPK1 to promote necroptosis and inflammation. Nat. Cell Biol. 25, 950–962 (2023).
Hardie, D. G. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 8, 774–785 (2007).
Ohh, M. et al. Ubiquitination of hypoxia-inducible factor requires direct binding to the β-domain of the von Hippel-Lindau protein. Nat. Cell Biol. 2, 423–427 (2000).
Naito, M. G. et al. Sequential activation of necroptosis and apoptosis cooperates to mediate vascular and neural pathology in stroke. Proc. Natl Acad. Sci. USA 117, 4959–4970 (2020).
Linkermann, A. et al. Necroptosis in immunity and ischemia-reperfusion injury. Am. J. Transpl. 13, 2797–2804 (2013).
Jouan-Lanhouet, S. et al. Necroptosis, in vivo detection in experimental disease models. Semin. Cell Dev. Biol. 35, 2–13 (2014).
Degterev, A., Boyce, M. & Yuan, J. A decade of caspases. Oncogene 22, 8543–8567 (2003).
Yuan, J., Najafov, A. & Py, B. F. Roles of caspases in necrotic cell death. Cell 167, 1693–1704 (2016).
Newton, K., Dixit, V. M. & Kayagaki, N. Dying cells fan the flames of inflammation. Science 374, 1076–1080 (2021).
Rathinam, V. A. K., Zhao, Y. & Shao, F. Innate immunity to intracellular LPS. Nat. Immunol. 20, 527–533 (2019).
Wang, S. et al. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 92, 501–509 (1998).
Kang, S. J. et al. Dual role of caspase-11 in mediating activation of caspase-1 and caspase-3 under pathological conditions. J. Cell Biol. 149, 613–622 (2000).
de Zoete, M. R., Palm, N. W., Zhu, S. & Flavell, R. A. Inflammasomes. Cold Spring Harb. Perspect. Biol. 6, a016287 (2014).
Broz, P., Pelegrin, P. & Shao, F. The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol. 20, 143–157 (2020).
Kayagaki, N. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
Ding, J. et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116 (2016).
Liu, X. et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158 (2016). Refs 107, 108, 109 and 110 demonstrated the role of caspase-1 and caspase-11 in the cleavage of GSDMD to promote necroptosis and the structural basis of GSDMD-NT pore formation.
Xia, S. et al. Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature 593, 607–611 (2021).
He, W. T. et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 25, 1285–1298 (2015).
Moonen, S. et al. Pyroptosis in Alzheimer’s disease: cell type-specific activation in microglia, astrocytes and neurons. Acta Neuropathol. 145, 175–195 (2023).
LaRock, D. L. et al. Group A Streptococcus induces GSDMA-dependent pyroptosis in keratinocytes. Nature 605, 527–531 (2022).
Hansen, J. M. et al. Pathogenic ubiquitination of GSDMB inhibits NK cell bactericidal functions. Cell 184, 3178–3191.e18 (2021).
Johnson, A. G. et al. Bacterial gasdermins reveal an ancient mechanism of cell death. Science 375, 221–225 (2022).
Sangiuliano, B., Perez, N. M., Moreira, D. F. & Belizario, J. E. Cell death-associated molecular-pattern molecules: inflammatory signaling and control. Mediators Inflamm. 2014, 821043 (2014).
Nagata, S. Apoptosis and clearance of apoptotic cells. Annu. Rev. Immunol. 36, 489–517 (2018).
deCathelineau, A. M. & Henson, P. M. The final step in programmed cell death: phagocytes carry apoptotic cells to the grave. Essays Biochem. 39, 105–117 (2003).
Nagata, S. & Segawa, K. Sensing and clearance of apoptotic cells. Curr. Opin. Immunol. 68, 1–8 (2021).
Segawa, K. et al. Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science 344, 1164–1168 (2014).
Suzuki, J., Denning, D. P., Imanishi, E., Horvitz, H. R. & Nagata, S. Xk-related protein 8 and CED-8 promote phosphatidylserine exposure in apoptotic cells. Science 341, 403–406 (2013). Refs 121 and 122 described the mechanism by which caspase mediates the cleavage of phospholipid flippase to promote phosphatidylserine exposure on apoptotic cells in efferocytosis.
Lu, J. et al. Efficient engulfment of necroptotic and pyroptotic cells by nonprofessional and professional phagocytes. Cell Discov. 5, 39 (2019).
Zargarian, S. et al. Phosphatidylserine externalization, “necroptotic bodies” release, and phagocytosis during necroptosis. PLoS Biol. 15, e2002711 (2017).
Kayagaki, N. et al. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature 591, 131–136 (2021).
Degen, M. et al. Structural basis of NINJ1-mediated plasma membrane rupture in cell death. Nature 618, 1065–1071 (2023). Refs 125 and 126 report on the role of NINJ1 in mediating membrane disrupture after cell death and the structure of NINJ1.
Kayagaki, N. et al. Inhibiting membrane rupture with NINJ1 antibodies limits tissue injury. Nature 618, 1072–1077 (2023).
Le, H. et al. Disruption of ninjurin1 leads to repetitive and anxiety-like behaviors in mice. Mol. Neurobiol. 54, 7353–7368 (2017).
Liu, K., Wang, Y. & Li, H. The role of ninjurin1 and its impact beyond the nervous system. Dev. Neurosci. 42, 159–169 (2020).
Tomita, Y. et al. Ninjurin 1 mediates peripheral nerve regeneration through Schwann cell maturation of NG2-positive cells. Biochem. Biophys. Res. Commun. 519, 462–468 (2019).
Ifergan, I. et al. Role of Ninjurin-1 in the migration of myeloid cells to central nervous system inflammatory lesions. Ann. Neurol. 70, 751–763 (2011).
Lee, H. J., Ahn, B. J., Shin, M. W., Choi, J. H. & Kim, K. W. Ninjurin1: a potential adhesion molecule and its role in inflammation and tissue remodeling. Mol. Cell 29, 223–227 (2010).
Lee, H. K., Lee, H., Luo, L. & Lee, J. K. Induction of nerve injury-induced protein 1 (ninjurin 1) in myeloid cells in rat brain after transient focal cerebral ischemia. Exp. Neurobiol. 25, 64–74 (2016).
Ahn, B. J. et al. Ninjurin1 enhances the basal motility and transendothelial migration of immune cells by inducing protrusive membrane dynamics. J. Biol. Chem. 289, 21926–21936 (2014).
Dondelinger, Y., Hulpiau, P., Saeys, Y., Bertrand, M. J. M. & Vandenabeele, P. An evolutionary perspective on the necroptotic pathway. Trends Cell Biol. 26, 721–732 (2016).
Kuida, K. et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1β converting enzyme. Science 267, 2000–2003 (1995).
Kelliher, M. A. et al. The death domain kinase RIP mediates the TNF-induced NF-κB signal. Immunity 8, 297–303 (1998).
Newton, K. et al. RIPK3 deficiency or catalytically inactive RIPK1 provides greater benefit than MLKL deficiency in mouse models of inflammation and tissue injury. Cell Death Differ. 23, 1565–1576 (2016).
Kaiser, W. J. et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471, 368–372 (2011).
Oberst, A. et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 471, 363–367 (2011).
Liu, Y., Li, X., Zhou, X., Wang, J. & Ao, X. FADD as a key molecular player in cancer progression. Mol. Med. 28, 132 (2022).
Najafov, A., Chen, H. & Yuan, J. Necroptosis and cancer. Trends Cancer 3, 294–301 (2017).
Koo, G. B. et al. Methylation-dependent loss of RIP3 expression in cancer represses programmed necrosis in response to chemotherapeutics. Cell Res. 25, 707–725 (2015).
Najafov, A. et al. BRAF and AXL oncogenes drive RIPK3 expression loss in cancer. PLoS Biol. 16, e2005756 (2018).
Schweichel, J. U. & Merker, H. J. The morphology of various types of cell death in prenatal tissues. Teratology 7, 253–266 (1973).
Anding, A. L. & Baehrecke, E. H. Autophagy in cell life and cell death. Curr. Top. Dev. Biol. 114, 67–91 (2015).
Levine, B. & Yuan, J. Autophagy in cell death: an innocent convict? J. Clin. Invest. 115, 2679–2688 (2005).
Tsukada, M. & Ohsumi, Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 333, 169–174 (1993).
White, E. Autophagic cell death unraveled: pharmacological inhibition of apoptosis and autophagy enables necrosis. Autophagy 4, 399–401 (2008).
Fleming, A. et al. The different autophagy degradation pathways and neurodegeneration. Neuron 110, 935–966 (2022).
Menzies, F. M. et al. Autophagy and neurodegeneration: pathogenic mechanisms and therapeutic opportunities. Neuron 93, 1015–1034 (2017).
de Duve, C. et al. Commentary. Lysosomotropic agents. Biochem. Pharmacol. 23, 2495–2531 (1974).
Xie, Z. et al. Cathepsin B in programmed cell death machinery: mechanisms of execution and regulatory pathways. Cell Death Dis. 14, 255 (2023).
Vitner, E. B. et al. RIPK3 as a potential therapeutic target for Gaucher’s disease. Nat. Med. 20, 204–208 (2014).
Liu, S. et al. Lysosomal damage after spinal cord injury causes accumulation of RIPK1 and RIPK3 proteins and potentiation of necroptosis. Cell Death Dis. 9, 476 (2018).
Pan, C. et al. Lipofuscin causes atypical necroptosis through lysosomal membrane permeabilization. Proc. Natl Acad. Sci. USA 118, e2100122118 (2021).
Ryckman, A. E., Brockhausen, I. & Walia, J. S. Metabolism of glycosphingolipids and their role in the pathophysiology of lysosomal storage disorders. Int. J. Mol. Sci. 21, 6881 (2020).
Yanez, M. J. et al. Finding pathogenic commonalities between Niemann-Pick type C and other lysosomal storage disorders: opportunities for shared therapeutic interventions. Biochim. Biophys. Acta Mol. Basis Dis. 1866, 165875 (2020).
White, E. Entosis: it’s a cell-eat-cell world. Cell 131, 840–842 (2007).
Zeng, C., Zeng, B., Dong, C., Liu, J. & Xing, F. Rho-ROCK signaling mediates entotic cell death in tumor. Cell Death Discov. 6, 4 (2020).
Florey, O., Kim, S. E., Sandoval, C. P., Haynes, C. M. & Overholtzer, M. Autophagy machinery mediates macroendocytic processing and entotic cell death by targeting single membranes. Nat. Cell Biol. 13, 1335–1343 (2011).
Rich, K. A., Burkett, C. & Webster, P. Cytoplasmic bacteria can be targets for autophagy. Cell Microbiol. 5, 455–468 (2003).
Nakagawa, I. et al. Autophagy defends cells against invading group A Streptococcus. Science 306, 1037–1040 (2004).
Ogawa, M. et al. Escape of intracellular Shigella from autophagy. Science 307, 727–731 (2005).
Hamann, J. C. et al. Entosis is induced by glucose starvation. Cell Rep. 20, 201–210 (2017).
Bozkurt, E. et al. TRAIL signaling promotes entosis in colorectal cancer. J. Cell Biol. 220, e202010030 (2021).
Yang, W. S. & Stockwell, B. R. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 15, 234–245 (2008).
Trachootham, D., Lu, W., Ogasawara, M. A., Nilsa, R. D. & Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal. 10, 1343–1374 (2008).
Sato, M. et al. The ferroptosis inducer erastin irreversibly inhibits system xc- and synergizes with cisplatin to increase cisplatin’s cytotoxicity in cancer cells. Sci. Rep. 8, 968 (2018).
Friedmann Angeli, J. P. et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 16, 1180–1191 (2014).
Zhang, H., Morgan, T. E. & Forman, H. J. Age-related alteration in HNE elimination enzymes. Arch. Biochem. Biophys. 699, 108749 (2021).
Vazdar, K., Skulj, S., Bakaric, D., Margetic, D. & Vazdar, M. Chemistry and reactivity of 4-hydroxy-2-nonenal (HNE) in model biological systems. Mini Rev. Med. Chem. 21, 1394–1405 (2021).
Gentile, F. et al. DNA damage by lipid peroxidation products: implications in cancer, inflammation and autoimmunity. AIMS Genet. 4, 103–137 (2017).
Ayala, A., Munoz, M. F. & Arguelles, S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 360438 (2014).
Zheng, H., Jiang, L., Tsuduki, T., Conrad, M. & Toyokuni, S. Embryonal erythropoiesis and aging exploit ferroptosis. Redox Biol. 48, 102175 (2021).
Coyle, J. T. & Puttfarcken, P. Oxidative stress, glutamate, and neurodegenerative disorders. Science 262, 689–695 (1993).
Dmitriev, L. F. & Titov, V. N. Lipid peroxidation in relation to ageing and the role of endogenous aldehydes in diabetes and other age-related diseases. Ageing Res. Rev. 9, 200–210 (2010).
Luczaj, W., Gegotek, A. & Skrzydlewska, E. Antioxidants and HNE in redox homeostasis. Free Radic. Biol. Med. 111, 87–101 (2017).
Lynch, D. R. & Johnson, J. Omaveloxolone: potential new agent for Friedreich ataxia. Neurodegener. Dis. Manag. 11, 91–98 (2021).
Doll, S. et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575, 693–698 (2019).
Bersuker, K. et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575, 688–692 (2019).
Mishima, E. et al. A non-canonical vitamin K cycle is a potent ferroptosis suppressor. Nature 608, 778–783 (2022).
Liang, D. et al. Ferroptosis surveillance independent of GPX4 and differentially regulated by sex hormones. Cell 186, 2748–2764.e22 (2023). Refs 6, 181, 182 and 183 describe the roles of xCT, GPX4, FSP1 and sex hormone-mediated control of cellular lipid peroxidation and illustrate how inactivation of cellular defence mechanisms against lipid peroxidation can lead to ferroptosis.
Matak, P. et al. Disrupted iron homeostasis causes dopaminergic neurodegeneration in mice. Proc. Natl Acad. Sci. USA 113, 3428–3435 (2016).
Cozzi, A. et al. Oxidative stress and cell death in cells expressing L-ferritin variants causing neuroferritinopathy. Neurobiol. Dis. 37, 77–85 (2010).
Ryan, S. K. et al. Microglia ferroptosis is regulated by SEC24B and contributes to neurodegeneration. Nat. Neurosci. 26, 12–26 (2023).
Chalfie, M. & Wolinsky, E. The identification and suppression of inherited neurodegeneration in Caenorhabditis elegans. Nature 345, 410–416 (1990).
Driscoll, M. & Chalfie, M. The mec-4 gene is a member of a family of Caenorhabditis elegans genes that can mutate to induce neuronal degeneration. Nature 349, 588–593 (1991).
Syntichaki, P., Xu, K., Driscoll, M. & Tavernarakis, N. Specific aspartyl and calpain proteases are required for neurodegeneration in C. elegans. Nature 419, 939–944 (2002).
Bianchi, L. et al. The neurotoxic MEC-4(d) DEG/ENaC sodium channel conducts calcium: implications for necrosis initiation. Nat. Neurosci. 7, 1337–1344 (2004).
Hardingham, G. E., Fukunaga, Y. & Bading, H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 5, 405–414 (2002).
Hernandez, D. E. et al. Axonal degeneration induced by glutamate excitotoxicity is mediated by necroptosis. J. Cell Sci. 131, jcs214684 (2018).
D’Orsi, B. et al. Bax regulates neuronal Ca2+ homeostasis. J. Neurosci. 35, 1706–1722 (2015).
Mahmoud, S., Gharagozloo, M., Simard, C. & Gris, D. Astrocytes maintain glutamate homeostasis in the CNS by controlling the balance between glutamate uptake and release. Cells 8, 184 (2019).
Dawson, T. M. & Dawson, V. L. Nitric oxide signaling in neurodegeneration and cell death. Adv. Pharmacol. 82, 57–83 (2018).
Wang, Y. & Golledge, J. Neuronal nitric oxide synthase and sympathetic nerve activity in neurovascular and metabolic systems. Curr. Neurovasc. Res. 10, 81–89 (2013).
Steinert, J. R., Chernova, T. & Forsythe, I. D. Nitric oxide signaling in brain function, dysfunction, and dementia. Neuroscientist 16, 435–452 (2010).
Ghatak, S., Nakamura, T. & Lipton, S. A. Aberrant protein S-nitrosylation contributes to hyperexcitability-induced synaptic damage in Alzheimer’s disease: mechanistic insights and potential therapies. Front. Neural Circuits 17, 1099467 (2023).
Vakifahmetoglu, H., Olsson, M. & Zhivotovsky, B. Death through a tragedy: mitotic catastrophe. Cell Death Differ. 15, 1153–1162 (2008).
Castedo, M. et al. Cell death by mitotic catastrophe: a molecular definition. Oncogene 23, 2825–2837 (2004).
Castedo, M. et al. Mitotic catastrophe constitutes a special case of apoptosis whose suppression entails aneuploidy. Oncogene 23, 4362–4370 (2004).
Brinkmann, K., Ng, A. P., de Graaf, C. A. & Strasser, A. What can we learn from mice lacking pro-survival BCL-2 proteins to advance BH3 mimetic drugs for cancer therapy? Cell Death Differ. 29, 1079–1093 (2022).
Smith, W. M. & Reed, D. R. Targeting apoptosis in ALL. Curr. Hematol. Malig. Rep. 17, 53–60 (2022).
Mifflin, L., Ofengeim, D. & Yuan, J. Receptor-interacting protein kinase 1 (RIPK1) as a therapeutic target. Nat. Rev. Drug Discov. 19, 553–571 (2020).
Ofengeim, D. et al. RIPK1 mediates a disease-associated microglial response in Alzheimer’s disease. Proc. Natl Acad. Sci. USA 114, E8788–E8797 (2017).
Zelic, M. et al. RIPK1 activation mediates neuroinflammation and disease progression in multiple sclerosis. Cell Rep. 35, 109112 (2021).
Li, W. et al. Nuclear RIPK1 promotes chromatin remodeling to mediate inflammatory response. Cell Res. 32, 621–637 (2022).
Li, W. & Yuan, J. Targeting RIPK1 kinase for modulating inflammation in human diseases. Front. Immunol. 14, 1159743 (2023).
Rathinam, V. A. & Fitzgerald, K. A. Inflammasome complexes: emerging mechanisms and effector functions. Cell 165, 792–800 (2016).
Zhan, X., Li, Q., Xu, G., Xiao, X. & Bai, Z. The mechanism of NLRP3 inflammasome activation and its pharmacological inhibitors. Front. Immunol. 13, 1109938 (2022).
Sweeney, P. et al. Protein misfolding in neurodegenerative diseases: implications and strategies. Transl. Neurodegener. 6, 6 (2017).
Xu, D. et al. Modulating TRADD to restore cellular homeostasis and inhibit apoptosis. Nature 587, 133–138 (2020).
Sies, H. & Jones, D. P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 21, 363–383 (2020).
Markesbery, W. R., Kryscio, R. J., Lovell, M. A. & Morrow, J. D. Lipid peroxidation is an early event in the brain in amnestic mild cognitive impairment. Ann. Neurol. 58, 730–735 (2005).
Pratico, D., Uryu, K., Leight, S., Trojanoswki, J. Q. & Lee, V. M. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J. Neurosci. 21, 4183–4187 (2001).
Yuan, J. & Horvitz, H. R. A first insight into the molecular mechanisms of apoptosis. Cell 116, S53–S56 (2004).
Thornberry, N. A. et al. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J. Biol. Chem. 272, 17907–17911 (1997).
Cerretti, D. P. et al. Molecular cloning of the interleukin-1β converting enzyme. Science 256, 97–100 (1992).
Thornberry, N. A. et al. A novel heterodimeric cysteine protease is required for interleukin-1β processing in monocytes. Nature 356, 768–774 (1992).
Horvitz, H. R., Shaham, S. & Hengartner, M. O. The genetics of programmed cell death in the nematode Caenorhabditis elegans. Cold Spring Harb. Symp. Quant. Biol. 59, 377–385 (1994).
Miura, M., Zhu, H., Rotello, R., Hartwieg, E. A. & Yuan, J. Induction of apoptosis in fibroblasts by IL-1β-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell 75, 653–660 (1993).
Yuan, J., Shaham, S., Ledoux, S., Ellis, H. M. & Horvitz, H. R. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1β-converting enzyme. Cell 75, 641–652 (1993).
Cory, S., Roberts, A. W., Colman, P. M. & Adams, J. M. Targeting BCL-2-like proteins to kill cancer cells. Trends Cancer 2, 443–460 (2016).
Nakamura, T. et al. Phase separation of FSP1 promotes ferroptosis. Nature 619, 371–377 (2023).
Ryan, S. K. et al. Therapeutic inhibition of ferroptosis in neurodegenerative disease. Trends Pharmacol. Sci. 44, 674–688 (2023).
Tonnus, W. et al. Dysfunction of the key ferroptosis-surveilling systems hypersensitizes mice to tubular necrosis during acute kidney injury. Nat. Commun. 12, 4402 (2021).
Singh, J., Habean, M. L. & Panicker, N. Inflammasome assembly in neurodegenerative diseases. Trends Neurosci. 65, 885–904 (2023).
Fetter, T., de Graaf, D. M., Claus, I. & Wenzel, J. Aberrant inflammasome activation as a driving force of human autoimmune skin disease. Front. Immunol. 14, 1190388 (2023).
Vontell, R. T. et al. Identification of inflammasome signaling proteins in neurons and microglia in early and intermediate stages of Alzheimer’s disease. Brain Pathol. 33, e13142 (2023).
Parmar, D. V. et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of the oral NLRP3 inflammasome inhibitor ZYIL1: first-in-human phase 1 studies (single ascending dose and multiple ascending dose). Clin. Pharmacol. Drug Dev. 12, 202–211 (2023).
Ambrus-Aikelin, G. et al. JT002, a small molecule inhibitor of the NLRP3 inflammasome for the treatment of autoinflammatory disorders. Sci. Rep. 13, 13524 (2023).
Xie, T. et al. Structural basis of RIP1 inhibition by necrostatins. Structure 21, 493–499 (2013).
Chen, L. et al. Advances in RIPK1 kinase inhibitors. Front. Pharmacol. 13, 976435 (2022).
Feng, X. et al. Receptor-interacting protein kinase 3 is a predictor of survival and plays a tumor suppressive role in colorectal cancer. Neoplasma 62, 592–601 (2015).
Yan, J., Wan, P., Choksi, S. & Liu, Z. G. Necroptosis and tumor progression. Trends Cancer 8, 21–27 (2022).
Seifert, L. et al. The necrosome promotes pancreatic oncogenesis via CXCL1 and Mincle-induced immune suppression. Nature 532, 245–249 (2016).
Acknowledgements
The work of J.Y. is supported, in part, by the China National Natural Science Foundation (82188101, 21837004, 91849204 and 92049303), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB39030200), the Shanghai Municipal Science and Technology Major Project (grant no. 2019SHZDZX02), and the Shanghai Key Laboratory of Aging Studies (19DZ2260400).
Author information
Authors and Affiliations
Contributions
The manuscript was written and revised by J.Y. with input from D.O.
Corresponding authors
Ethics declarations
Competing interests
D.O. is an employee of Sanofi.
Peer review
Peer review information
Nature Reviews Molecular Cell Biology thanks Andreas Linkermann and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- AMPK
-
AMPK is a highly conserved sensor of intracellular adenosine nucleotide levels and is activated when the AMP-to-ATP ratio is elevated under energy-stress conditions. AMPK activation promotes catabolic pathways to generate more ATP and inhibits anabolic pathways.
- Amyloid-β plaques
-
An important pathological hallmark in the brain of patients with Alzheimer disease. Amyloid-β plaques contain fibrillar polymers of the amyloid-β cleavage products of APP protein as well as other components. Amyloid-β plaques can propagate and spread via prion-like self-assembly to drive neurodegeneration.
- Apoptosome
-
A heptameric APAF1 protein complex shaped as a wheel-shaped structure with sevenfold symmetry that can drive the activation of caspase-9, which in turn cleaves and activates pro-caspase-3, in intrinsic apoptosis.
- Autoimmune lymphoproliferative syndrome
-
A lymphoproliferative disease characterized by defects in the control of lymphocyte numbers that lead to enlargement of the lymph nodes (lymphadenopathy), the liver (hepatomegaly) and the spleen (splenomegaly).
- Blood–brain barrier
-
A property of the blood vessels in the central nervous system that enables them to regulate the movement of molecules and cells between the blood and the brain, preventing many macromolecules from entering the brain through diffusion.
- Centromere
-
A centromere is the point of attachment of the kinetochore, which is a structure that anchors the mitotic spindle during mitosis.
- Damage-associated molecular patterns
-
Intracellular molecules with pro-inflammatory and immunogenic activity that are discharged to extracellular space as the result of damage to the cell membrane from necrotic cell death.
- Efferocytosis
-
Process that mediates the removal of apoptotic cells by phagocytic cells and non-professional phagocytic cells.
- Flippases
-
ABC transporter or P4-type ATPase families of transmembrane lipid transporter proteins to facilitate the movement of phospholipid molecules between the two leaflets of the cell plasma membrane.
- Freidrich ataxia
-
A rare genetic disorder that leads to progressive movement disability.
- Glutathione
-
Glutathione is a linear tripeptide of l-glutamine, l-cysteine and glycine with strong antioxidant activity.
- Granzyme A
-
A serine protease present in cytotoxic T cells.
- Integrin
-
Heterodimeric transmembrane receptors that mediate cell–cell and cell–extracellular matrix adhesion.
- NF-κB
-
Nuclear factor-κ light chain enhancer of activated B cells is a family of highly conserved transcription factors that regulate many important cellular responses, including inflammation, proliferation, cellular growth and apoptosis.
- NOD family
-
Nucleotide oligomerization domain (NOD) proteins NOD1 and NOD2, which can enable detection of intracellular bacteria and promote their clearance through initiation of the pro-inflammatory transcriptional programme and other host defence responses such as autophagy.
- Normoxic condition
-
Having a normal oxygen concentration; typically 20–21% in the atmosphere or in tissue culture flasks.
- Protomers
-
Structural units of an oligomeric protein.
- Signal peptide
-
A peptide segment of 20–30 amino acids that acts as the N-terminal sorting signal that targets the linked protein to the secretory pathway in eukaryotes and prokaryotes.
- Ub acceptor site
-
Ubiquitin is a 76-amino acid polypeptide that can be attached to proteins through the formation of an isopeptide bond between its carboxyl terminus and the Ub acceptor site, which can be the ɛ-amino group of lysin side chains on target proteins for K63, K48 and K11 ubiquitination or the N-terminal methionine as in M1-linked ubiquitination.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yuan, J., Ofengeim, D. A guide to cell death pathways. Nat Rev Mol Cell Biol 25, 379–395 (2024). https://doi.org/10.1038/s41580-023-00689-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41580-023-00689-6