Abstract
Protein misfolding is a major factor of neurodegenerative diseases. Post-mitotic neurons are highly susceptible to protein aggregates that are not diluted by mitosis. Therefore, post-mitotic cells may have a specific protein quality control system. Here, we show that LONRF2 is a bona fide protein quality control ubiquitin ligase induced in post-mitotic senescent cells. Under unperturbed conditions, LONRF2 is predominantly expressed in neurons. LONRF2 binds and ubiquitylates abnormally structured TDP-43 and hnRNP M1 and artificially misfolded proteins. Lonrf2−/− mice exhibit age-dependent TDP-43-mediated motor neuron (MN) degeneration and cerebellar ataxia. Mouse induced pluripotent stem cell-derived MNs lacking LONRF2 showed reduced survival, shortening of neurites and accumulation of pTDP-43 and G3BP1 after long-term culture. The shortening of neurites in MNs from patients with amyotrophic lateral sclerosis is rescued by ectopic expression of LONRF2. Our findings reveal that LONRF2 is a protein quality control ligase whose loss may contribute to MN degeneration and motor deficits.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
RNA-seq data have been deposited in GEO under accession no. GSE179465. Access to DNA sequencing data of patients or healthy individuals is not available to entities without mutual research agreement, as consent for data provision has not been obtained from the individuals. All other data needed to evaluate the conclusions presented here are included in the paper and/or the supplementary materials. All data are available from the corresponding authors.
References
Hipp, M. S., Kasturi, P. & Hartl, F. U. The proteostasis network and its decline in ageing. Nat. Rev. Mol. Cell Biol. 20, 421–435 (2019).
Klaips, C. L., Jayaraj, G. G. & Hartl, F. U. Pathways of cellular proteostasis in aging and disease. J. Cell Biol. 217, 51–63 (2018).
Soto, C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat. Rev. Neurosci. 4, 49–60 (2003).
Soto, C. & Pritzkow, S. Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat. Neurosci. 21, 1332–1340 (2018).
Nakajima, Y. & Suzuki, S. Environmental stresses induce misfolded protein aggregation in plant cells in a microtubule-dependent manner. Int. J. Mol. Sci. 14, 7771–7783 (2013).
Chen, B., Retzlaff, M., Roos, T. & Frydman, J. Cellular strategies of protein quality control. Cold Spring Harb. Perspect. Biol. 3, 1–14 (2011).
Pilla, E., Schneider, K. & Bertolotti, A. Coping with protein quality control failure. Annu. Rev. Cell Dev. Biol. 33, 439–465 (2017).
Amm, I., Sommer, T. & Wolf, D. H. Protein quality control and elimination of protein waste: the role of the ubiquitin-proteasome system. Biochim. Biophys. Acta Mol. Cell. Res. 1843, 182–196 (2014).
Wolff, S., Weissman, J. S. & Dillin, A. Differential scales of protein quality control. Cell 157, 52–64 (2014).
Filbeck, S., Cerullo, F., Pfeffer, S. & Joazeiro, C. A. P. Ribosome-associated quality-control mechanisms from bacteria to humans. Mol Cell 82, 1451–1466 (2022).
Joazeiro, C. A. P. Mechanisms and functions of ribosome-associated protein quality control. Nat. Rev. Mol. Cell Biol. 20, 368–383 (2019).
Ciechanover, A. & Kwon, Y. T. Protein quality control by molecular chaperones in neurodegeneration. Front. Neurosci. 11, 185 (2017).
Ciechanover, A. et al. The ubiquitin-mediated proteolytic system: involvement of molecular chaperones, degradation of oncoproteins, and activation of transcriptional regulators. Cold Spring Harb. Symp. Quant. Biol. 60, 491–501 (1995).
Bercovich, B. et al. Ubiquitin-dependent degradation of certain protein substrates in vitro requires the molecular chaperone Hsc70. J. Biol. Chem. 272, 9002–9010 (1997).
Cuervo, A. M., Dice, J. F. & Knecht, E. A population of rat liver lysosomes responsible for the selective uptake and degradation of cytosolic proteins. J. Biol. Chem. 272, 5606–5615 (1997).
Chiang, H. L., Terlecky, S. R., Plant, C. P. & Dice, J. F. A role for a 70-kiloDalton heat shock protein in lysosomal degradation of intracellular proteins. Science 246, 382–385 (1989).
Jackson, M. P. & Hewitt, E. W. Cellular proteostasis: degradation of misfolded proteins by lysosomes. Essays Biochem. 60, 173–180 (2016).
Kurtishi, A., Rosen, B., Patil, K. S., Alves, G. W. & Møller, S. G. Cellular proteostasis in neurodegeneration. Mol. Neurobiol. 56, 3676–3689 (2019).
Selkoe, D. J. & Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 8, 595–608 (2016).
Kopito, R. R. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 10, 524–530 (2000).
Williams, A. et al. Novel targets for Huntington’s disease in an mTOR-independent autophagy pathway. Nat. Chem. Biol. 4, 295–305 (2008).
Martin, I., Dawson, V. L. & Dawson, T. M. Recent advances in the genetics of Parkinson’s disease. Annu. Rev. Genomics Hum. Genet. 12, 301 (2011).
Prusiner, S. B. Prions. Proc. Natl Acad. Sci. USA 95, 13363–13383 (1998).
Ling, S. C., Polymenidou, M. & Cleveland, D. W. Converging mechanisms in als and FTD: Disrupted RNA and protein homeostasis. Neuron 79, 416–438 (2013).
Prasad, A., Bharathi, V., Sivalingam, V., Girdhar, A. & Patel, B. K. Molecular mechanisms of TDP-43 misfolding and pathology in amyotrophic lateral sclerosis. Front. Mol. Neurosci. 12, 1–36 (2019).
Wood, A., Gurfinkel, Y., Polain, N., Lamont, W. & Lyn Rea, S. Molecular mechanisms underlying TDP-43 pathology in cellular and animal models of ALS and FTLD. Int. J. Mol. Sci. 22, 4705 (2021).
Hasegawa, M. et al. Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann. Neurol. 64, 60–70 (2008).
Neumann, M. et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314, 130–133 (2006).
Connell, P. et al. The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat. Cell Biol. 3, 93–96 (2000).
Nillegoda, N. B. et al. Ubr1 and Ubr2 function in a quality control pathway for degradation of unfolded cytosolic proteins. Mol. Biol. Cell 21, 2102 (2010).
Mishra, A., Godavarthi, S. K., Maheshwari, M., Goswami, A. & Jana, N. R. The ubiquitin ligase E6-AP is induced and recruited to aggresomes in response to proteasome inhibition and may be involved in the ubiquitination of Hsp70-bound misfolded proteins. J. Biol. Chem. 284, 10537 (2009).
Winklhofer, K. F. Parkin and mitochondrial quality control: toward assembling the puzzle. Trends Cell Biol. 24, 332–341 (2014).
Johmura, Y. et al. Senolysis by glutaminolysis inhibition ameliorates various age-associated disorders. Science 371, 265–270 (2021).
Gur, E. & Sauer, R. T. Recognition of misfolded proteins by Lon, a AAA+ protease. Genes Dev. 22, 2267–2277 (2008).
Lee, I. & Suzuki, C. K. Functional mechanics of the ATP-dependent Lon protease- lessons from endogenous protein and synthetic peptide substrates. Biochim. Biophys. Acta Proteins Proteom. 1784, 727–735 (2008).
Wang, N., Gottesman, S., Willingham, M. C., Gottesman, M. M. & Maurizi, M. R. A human mitochondrial ATP-dependent protease that is highly homologous to bacterial Lon protease. Proc. Natl Acad. Sci. USA 90, 11247–11251 (1993).
Voos, W. & Pollecker, K. The mitochondrial lon protease: novel functions off the beaten track? Biomolecules 10, 253 (2020).
Bota, D. A. & Davies, K. J. A. Mitochondrial Lon protease in human disease and aging: Including an etiologic classification of Lon-related diseases and disorders. Free Radic. Biol. Med. 100, 188–198 (2016).
Nishiyama, A. et al. Uhrf1-dependent H3K23 ubiquitylation couples maintenance DNA methylation and replication. Nature 502, 249–253 (2013).
Gupta, R. et al. Firefly luciferase mutants as sensors of proteome stress. Nat. Methods 8, 879–884 (2011).
Miyazaki, Y. et al. A method to rapidly create protein aggregates in living cells. Nat. Commun. 7, 1–7 (2016).
Jongjitwimol, J., Baldock, R. A., Morley, S. J. & Watts, F. Z. Sumoylation of eIF4A2 affects stress granule formation. J. Cell Sci. 129, 2407–2415 (2016).
Protter, D. S. W. & Parker, R. Principles and properties of stress granules. Trends Cell Biol. 26, 668–679 (2016).
Ximerakis, M. et al. Single-cell transcriptomic profiling of the aging mouse brain. Nat. Neurosci. 22, 1696–1708 (2019).
Bampton, A., Gittings, L. M., Fratta, P., Lashley, T. & Gatt, A. The role of hnRNPs in frontotemporal dementia and amyotrophic lateral sclerosis. Acta Neuropathol. 140, 599–623 (2020).
Okano, H. & Morimoto, S. iPSC-based disease modeling and drug discovery in cardinal neurodegenerative disorders. Cell Stem Cell 29, 189–208 (2022).
Masrori, P. & Van Damme, P. Amyotrophic lateral sclerosis: a clinical review. Eur. J. Neurol. 27, 1918–1929 (2020).
Guyenet, S. J. et al. A simple composite phenotype scoring system for evaluating mouse models of cerebellar ataxia. J. Visual. Exp. https://doi.org/10.3791/1787 (2010).
Blum, J. A. et al. Single-cell transcriptomic analysis of the adult mouse spinal cord reveals molecular diversity of autonomic and skeletal motor neurons. Nat. Neurosci. 24, 572–583 (2021).
Kozareva, V. et al. A transcriptomic atlas of mouse cerebellar cortex comprehensively defines cell types. Nature 598, 214–219 (2021).
Park, J. H., Park, H. S., Hong, S. & Kang, S. Motor neurons derived from ALS-related mouse iPS cells recapitulate pathological features of ALS. Exp. Mol. Med. 48, e276 (2016).
Krshnan, L., van de Weijer, M. L. & Carvalho, P. Endoplasmic reticulum-associated protein degradation. Cold Spring Harb. Perspect. Biol. https://doi.org/10.1101/CSHPERSPECT.A041247 (2022).
Chu, J. et al. A mouse forward genetics screen identifies LISTERIN as an E3 ubiquitin ligase involved in neurodegeneration. Proc. Natl Acad. Sci. USA 106, 2097–2103 (2009).
Egawa, N. et al. Drug screening for ALS using patient-specific induced pluripotent stem cells. Sci. Transl. Med. 4, 1–9 (2012).
Ebert, A. D. et al. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature 457, 277–280 (2009).
Lutz, C. Mouse models of ALS: past, present and future. Brain Res. 1693, 1–10 (2018).
Johmura, Y. et al. Necessary and sufficient role for a mitosis skip in senescence induction. Mol. Cell 55, 73–84 (2014).
Nakanishi, M., Robetorye, R. S., Adami, G. R., Pereira-Smith, O. M. & Smith, J. R. Identification of the active region of the DNA synthesis inhibitory gene p21(Sdi1/CIP1/WAF1). EMBO J. 14, 555–563 (1995).
Nakanishi, K. et al. Isozyme-specific role of SAD-A in neuronal migration during development of cerebral cortex. Cerebral Cortex 29, 3738–3751 (2019).
Tomono, T. et al. Highly efficient ultracentrifugation-free chromatographic purification of recombinant AAV serotype 9. Mol. Ther. Methods Clin. Dev. 11, 180–190 (2018).
Johmura, Y. et al. Fbxo22-mediated KDM4B degradation determines selective estrogen receptor modulator activity in breast cancer. J. Clin. Invest. 128, 5603–5619 (2018).
Ruan, L. et al. Cytosolic proteostasis through importing of misfolded proteins into mitochondria. Nature 543, 443–446 (2017).
Shinohara, Y. et al. Effects of neutralizing antibody production on AAV-PHP.B-mediated transduction of the mouse central nervous system. Mol. Neurobiol. 56, 4203–4214 (2019).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Anders, S., Pyl, P. T. & Huber, W. HTSeq-A Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2009).
Johmura, Y. et al. SCF Fbxo22-KDM4A targets methylated p53 for degradation and regulates senescence. Nat. Commun. 7, 10574 (2016).
Kaneko, T., Sakuma, T., Yamamoto, T. & Mashimo, T. Simple knockout by electroporation of engineered endonucleases into intact rat embryos. Sci Rep. 4, 1–5 (2014).
Takeo, T. & Nakagata, N. Reduced glutathione enhances fertility of frozen/thawed C57BL/6 mouse sperm after exposure to methyl-β-cyclodextrin. Biol. Reprod. 85, 1066–1072 (2011).
Brewer, G. J. & Torricelli, J. R. Isolation and culture of adult neurons and neurospheres. Nat. Protoc. 2, 1490–1498 (2007).
Miyoshi, S. et al. DOK7 gene therapy enhances motor activity and life span in ALS model mice. EMBO Mol. Med. 9, 880–889 (2017).
Ueta, R. et al. DOK7 gene therapy enhances neuromuscular junction innervation and motor function in aged mice. iScience 23, 101385 (2020).
Guo, L. et al. A cellular system that degrades misfolded proteins and protects against neurodegeneration. Mol. Cell. 55, 15–30 (2014).
Brooks, B. R., Miller, R. G., Swash, M. & Munsat, T. L. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 1, 293–299 (2000).
Naruse, H. et al. Burden of rare variants in causative genes for amyotrophic lateral sclerosis (ALS) accelerates age at onset of ALS. J. Neurol. Neurosurg. Psychiatry 90, 537–542 (2019).
Ishiura, H. et al. C9ORF72 repeat expansion in amyotrophic lateral sclerosis in the Kii peninsula of Japan. Arch. Neurol. 69, 1154–1158 (2012).
Ando, M. et al. Long-term eradication of extranodal natural killer/T-cell lymphoma, nasal type, by induced pluripotent stem cell-derived Epstein–Barr virus-specific rejuvenated T cells in vivo. Haematologica 105, 796–807 (2020).
Setsu, S. et al. An efficient induction method for human spinal lower motor neurons and high-throughput 1 image analysis at the single cell level. Preprint at bioRxiv https://doi.org/10.1101/2023.04.18.537412 (2023).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Egawa, N. et al. Drug screening for ALS using patient-specific induced pluripotent stem cells. Sci. Transl. Med. 4, 145ra104 (2012).
Fujimori, K. et al. Escape from pluripotency via inhibition of TGF-β/BMP and activation of Wnt signaling accelerates differentiation and aging in hPSC progeny cells. Stem Cell Rep. 9, 1675–1691 (2017).
Goto, K. et al. Simple derivation of spinal motor neurons from ESCs/iPSCs using Sendai virus vectors. Mol. Ther. Methods Clin. Dev. 4, 115–125 (2017).
Acknowledgements
We are grateful to M. Nakanishi (Tokiwa-Bio) for generation of iPS cells and Y. Chiba, M. Ishiura, T. Ando, M. Iwai, M. Kikuchi, S. Omori (University of Tokyo) and S. Nakamura, F. Ozawa, C. Kato (Keio University) and N. Shimakura (Tohoku University) for technical assistance. We also thank H. Inoue (Kyoto University) for kindly donating the hiPS clone (A3411). Histological sections were performed with the help of the Pathology Core Laboratory (University of Tokyo). Confocal microscopy services were provided by the Imaging Core Laboratory (University of Tokyo). Computational resources were provided by the supercomputer system SHIROKANE at the Human Genome Center (University of Tokyo). This study was supported by AMED under grant nos. 21zf0127003 (M.N.), 21cm0106175 (M.N.) and 21gm5010001 (M.N.), 21bm0804027 (H.O.), 22bm0804003 (H.O.), 21ek0109492 (H.O.), 21wm0425009 (H.O.), 214600040 (Y.J.) and by MEXT/JSPS KAKENHI under grant nos. 20H00514 (M.N.), 19H05740 (M.N.), JP18H05026m (Y.J.), JP16H06148 (Y.J.), JP16K15238 (Y.J.), JP22K15736 (S.M.), JP21H05273 (S.M.), JP20H00485 (H.O.) and by the Princess Takamatsu Cancer Research Fund (M.N.), the Uehara Memorial Foundation (S.M.), the Yukihiko Miyata Memorial Trust For Research (S.M.) and Yoshio Koide Grant, Japan ALS Association (S.M.).
Author information
Authors and Affiliations
Contributions
M.N. and Y.J. conceived the idea of the project, D.L., Y.J., T.T. and M.N. planned the experiments, D.L., Y.J,. S.M., M.D., K.N., M.O., Y.T., A.I.Y., H.N,. T.M., Y.T., N.S., M.A., A.N., X.Z., C.K., N.S., A.N., A.S.H., M.M., K.Y. and S.Y. performed the experiments, D.L., Y.J., S.M., M.D., K.N., M.O., A.I.-Y., H.N,. T.M., X.Z., N.S., A.N., M.M., K.Y., Y.F., K.N., S.T., S.Y., Y.Y., S.S., T.O., H.O., T.T. and M.N. analyzed the results, Y.T, N.S., M.A. and A.Y. established iPS cells from patients with ALS and M.N. wrote the manuscript with editing by all the other authors.
Corresponding authors
Ethics declarations
Competing interests
M.N. is a scientific advisor to Airweave and reverSASP Therapeutics and a shareholder of reverSASP Therapeutics. All other authors declare no competing interests.
Peer review
Peer review information
Nature Aging thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
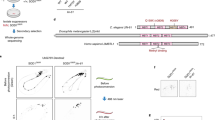
Extended Data Fig. 1 Lonrf2, but not Lonrf1 or Lonrf3, is predominantly expressed in senescent cells.
(a) Structure of LONRF proteins of Homo sapiens (human). Domain structures are indicated. TPR is a protein-protein interaction domain, the RING finger is a domain that binds ubiquitination enzymes and their substrates and functions as a ligase, and the LON substrate binding domain is related to turnover of many misfolded or denatured proteins. (b) qPCR analysis of the indicated genes in uninduced (low passage) or senescent HCA2 cells induced by 9 mM of RO3306 for 24 hrs, 5 mM of nutlin3a for 48 hrs, and then cultured with medium containing 100 nM of BI2536 for 18 days (n-Sen). n = 3. (c) qPCR analysis of the indicated genes in uninduced (low passage) or senescent HCA2 cells induced by 100 nM of doxorubicin for 24 hrs, and then cultured with medium containing 100 nM of BI2536 for 20 days (d-Sen).n = 3. (d) Lysates of d-Sen cells expressing Flag-Lonrf2 in the presence or absence of doxycycline (1 mg/ml) were subjected to immunoblotting using the indicated antibodies. SYPRO orange staining (e) and thioflavin-T (ThT) (10μM) staining (f), of d-Sen cells treated in (d), followed by quantification of fluorescence intensity in the right panel (f) (n = 3). qPCR analysis of LONRF2 (g), SYPRO orange staining (top panel) (h), thioflavin-T (ThT) (10μM) staining (i) in uninduced or d-Sen cells expressing control or LONRF2 shRNA (shLONRF2-1, 2) in the presence of doxycycline (1 μg/ml) (n = 3). Lysates of d-Sen cells in (g) were subjected to immunoblotting with the indicated antibody (bottom panel). Quantification of fluorescence intensity in the right panel (n = 3) (i). (j) HeLa cells were transfected with the indicated plasmids, lysed under denaturing conditions, and subjected to immunoprecipitation with an anti-FLAG M2 affinity gel, followed by immunoblotting with the indicated antibodies. Scale bar: 50 μm (f, i). Data are presented as means ±s.d. of three independent experiments, error bars show s.d. One-way ANOVA with Dunnett’s multiple comparisons post hoc test (g, i) and unpaired two-tailed Student’s t-test (b, c, f). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Extended Data Fig. 2 LONRF2 is required for the effective disassembly of SGs and reduction in the level of misfolded proteins.
(a) Immunoblotting of lysates from MCF7 and AsPC-1 cells expressing the indicated Dox (1 mg/ml)-inducible shRNA for 48 hrs using the indicated antibodies. (b) MCF7 and (c) AsPC-1 cells as in (a) were treated with NaAsO2 (1 mM) for 30 min, washed with PBS, and then subjected to immunofluorescent analysis using anti-G3BP1 antibody and Hoechst33342. Relative populations of stress granule-positive cells (cells with more than five G3BP1-positive foci) were determined (n = 200). (d) Immunoblotting of lysates from A549 cells expressing Dox-inducible Lonrf2 shRNA together with shRNA-resistant Lonrf2-WT or its mutants. (e) A549 cells as in (d) were treated with NaAsO2 (1 mM) for 30 min, washed with PBS, and then subjected to immunohistochemical analysis using anti-G3BP1 antibody and Hoechst33342 at 0 and 120 min after PBS washout. Relative population of stress granule-positive cells (cells with more than five G3BP1-positive foci) were determined (n = 200). (f and g) A549 cells expressing Dox-inducible Lonrf2 shRNA were treated with H2O2 (1 mM) for 60 min (f) or heat shock (43 °C, for 60 min) (g), were recovered, and were then subjected to immunofluorescent analysis using anti-G3BP1 antibody and Hoechst33342 at the indicated times after recovery. Relative population of stress granule-positive cells (cells with more than five G3BP1-positive foci) were determined (n = 200). (h and i) A549 cells treated as in (f and g) were subjected to a pull-down assay with FLAG-LONRF2 affinity beads, followed by immunoblotting using the indicated antibodies. Quantification of the intensity is shown in the right panels. Data are presented as means ±s.d. of three independent experiments, error bars show s.d. Two-tailed one-way analysis of variance (ANOVA) with Dunnett’s multiple comparisons post hoc test (b-c, e-i). **P < 0.01, ***P < 0.001.
Extended Data Fig. 3 Lonrf2−/− mice showed a shorter lifespan than Lonrf2+/+ mice.
(a) Sequence alignment of Lonrf2-WT (+/+) and Lonrf2-KO (−/−) alleles. The 5 depleted base pairs are shown in red. (b) Body weights of Lonrf2+/+ and Lonrf2−/− mice at 21 months of age, (n = 8: 6 males and 2 femals). (c) Representative pictures of Lonrf2+/+ and Lonrf2−/− mice at 21 months of age (d) Survival rates of Lonrf2+/+ (n = 14, 8 males and 6 females), Lonrf2+/− (n = 11, 6 males and 5 females), and Lonrf2−/− mice (n = 11, 4 males and 7 females), data are shown as a percentage of the original mice. Statistical significance was determined with a log-rank (Mantel-Cox) test (p<0.01). Data are presented as means ±s.d. of the indicated independent experiments, error bars show s.d. Unpaired two-tailed Student’s t-test (b).
Extended Data Fig. 4 Lonrf2 is predominantly expressed in neurons.
(a) UMAP clustering of the adult mouse spinal cord (upper) from 43,890 single-cell transcriptomes, visualization of ChAT (middle) and Lonrf2 (lower) expressed populations. (b) Violin plot of Lonrf2 expressed populations in young (16,028 single-cell transcriptomes from 8 young mouse brains) and old (21,041 single-cell transcriptomes from 8 old mouse brains) mice. (c) Violin plot of Lonrf2 expressed populations of cerebellar cortex in 780,553 single nuclei transcriptomes (530,063 profiles from male donors, 4 males at P60, 1 male at E18, 1 male at P0, 2 males at P12; 250,490 profiles from female donors, 2 females of P60, 1 female at P4, 1 female at P8, 2 females at P16). Expression level represents Log-Normalized UMI counts. (d) Left and right figures showed primary motor cortex of 7-week-old mice and 97-week-old mice, respectively. In situ hybridization with antisense probe detected Lonrf2 positive cells in the primary motor cortex of both 7-week-old mice and 97-week-old mice. Sense probe did not yield any specific staining. Small square images showed the higher magnification of the boxed areas. Arrowheads indicated representative mRNA signals. Cortical layers were shown as L1, L2/3, L5, and L6. Scale bars: 100 and 10 mm. Three independent experiments were performed.
Extended Data Fig. 5 Lonrf2−/− mice showed neurodegenerative phenotypes in the cortex.
Immunofluorescent analyses of NeuN (magenta) (a) and Fluoro Jade C (green) (b) -positive cells counterstained with Hoechst or DAPI (blue) using cortical brain from Lonrf2+/+ or Lonrf2−/− mice at 21 months of age. Representative images are shown (left panels). Scale bars: 50 mm. Quantification of NeuN- and Fluoro Jade C-positive cells per square millimeter (right figure) (n = 6, males). Immunofluorescence staining of cortical brain from male (black) and female (red) Lonrf2+/+ or Lonrf2−/− mice at 21 months of age using anti-Ataxin2 (green) (c), anti-G3BP1 (green) (d), or anti-pTDP-43 (green) (e) antibodies, co-staining with anti-NeuN antibodies (magenta) and Hoechst (blue). Representative images are shown (left panels). Scale bars: 10 mm. Quantification of the percentages of Ataxin2, G3BP1 or pTDP-43-positive cells in NeuN-positive cells are shown on the right (n = 8, 6 males and 2 females). (f) Quantification of NeuN-positive, Fluoro Jade C-positive, and percentages of Ataxin-2-positive cells in the striatum of Lonrf2+/+ or Lonrf2−/− mice at 21 months of age are shown (n = 6, males). Data are presented as means ±s.d. of the indicated independent experiments, error bars show s.d. Unpaired two-tailed Student’s t-test (a-f). ***P < 0.001, ****P < 0.0001.
Extended Data Fig. 6 Lonrf2−/− mice showed neurodegenerative phenotypes in the cerebellum.
Immunofluorescent analysis of NeuN (red) (a), Fluoro Jade C (green) (b)-positive cells counterstained with Hoechst or DAPI (blue) using cerebellum from Lonrf2+/+ or Lonrf2−/− mice at 21 months of age. Representative images are shown (left panels). Scale bars: 50 mm. Quantification of NeuN- and Fluoro Jade C-positive cells per square millimeter (right panels) (n = 6, male). Immunofluorescent analysis of Ataxin-2 (red) (c), G3BP1 (green) (d), and pTDP-43 (green)(e) -positive cells counterstained with Hoechst or DAPI (blue) using cerebellum from male (black) and female (red) Lonrf2+/+ or Lonrf2−/− mice at 21 months of age. Representative images are shown (left panels). Scale bars: 10 mm. Percentages of Ataxin-2, G3BP1, and pTDP-43-positive cells in NeuN-positive cells are shown in the right figures, (n = 8, 6 male and 2 female). (f) Representative images of Purkinje cells stained by Calbindin in the cerebellum from Lonrf2+/+ (left panels) or Lonrf2−/− (right panels) mice at 3 months of age (upper panels) and 21 months of age (lower panels). Scale bars: 50 mm. (g) Quantification of Purkinje cells per millimeter of the Purkinje layer in (f), (n = 6, males). (h) Measurement of granular and molecular layer thicknesses in the cerebellum from Lonrf2+/+ or Lonrf2−/− mice at 21 months of age. Data are presented as means ±s.d. of three independent experiments, error bars show s.d. Unpaired two-tailed Student’s t-test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
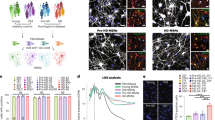
Extended Data Fig. 7 Neurite length shortening in Lonrf2−/− iPS cell-derived neurons is restored by ectopic expression of Lonrf2.
(a) Lonrf2+/+ (left) and Lonrf2−/− (right) mouse iPS cells were positive for alkaline phosphatase staining. Scale bar: 20 mm. (b) Growth curves of established Lonrf2+/+, Lonrf2+/−, and Lonrf2−/− mouse iPS cells. (c) Differentiation rates of Lonrf2+/+, Lonrf2+/−, and Lonrf2−/− neuronal precursors to motor neurons. (d) Representative images of the results shown in Fig. 6f. Anti-Tuj1 antibody (green), anti-ChAT (red) and Hoechst33342 (blue). Scale bar: 50 mm. (e) Representative images of the results shown in Fig. 6f. Cells were infected with AAV-FLAG-Lonrf2 vector (5 × 105 GC/mL) for 12 hrs and cultured for an additional 0 or 14 days. Anti-Tuj1 antibody (green), anti-Flag (gray), anti-ChAT (red), and Hoechst33342 (blue). Scale bar: 50 mm. Data are presented as means ±s.d. of three independent experiments, error bars show s.d.
Extended Data Fig. 8 Lonrf2−/− iPS cell-derived neurons show reduced survival, accumulation of pTDP-43 and G3BP1 aggregates.
Representative images of the results shown in Fig. 6g(a), 6h (b), and 6i (c). TUNEL (green), anti-pTDP-43 (green), anti-G3BP1 (green) and anti-ChAT (red) antibodies. Scale bar: 50 mm (a), 10 mm (b, c).
Extended Data Fig. 9 Ectopic expression of LONRF2 restores mortality and suppresses accumulation in pTDP-43 of Lonrf2−/− iPS cell-derived neurons.
(a) Representative images of the results shown in Fig. 6f. Motor neurons derived from Lonrf2+/+ and Lonrf2−/− iPS cells were stained with anti-LONRF2 (green), anti-FLAG (grey), and anti-ChAT (red) antibodies, counterstained with Hoechst (blue). Representative images of the results shown in Fig. 6g(b) and 6h (c). Cells were infected with AAV-FLAG-Lonrf2 vector (5 × 105 GC/mL) for 12 hrs and cultured for an additional 0 or 14 days. TUNEL (green), anti-pTDP-43 (green), anti-Flag (gray), and anti-ChAT (red). Scale bar: 50 mm (a, b), 10 mm (c).
Extended Data Fig. 10 Lonrf2 is expressed primarily in mouse motor neurons, while both Lonrf1 and Lonrf2 are expressed in human motor neurons derived from iPS cells.
For the human motor neuron differentiation, hiPSCs were transferred to a chemically transitional embryoid-body-like state (CTraS) in AK02N medium (Ajinomoto, Japan) using SB431542, dorsomorphine, and CHIR99021, trypsinized into single cells, and plated on poly-L-lysin and matrigel-coated plates in MN medium (KBM Neural Stem Cell Medium, Kohjin Bio, Japan) with B-27 supplement and Y-27632. Simultaneously, the iPSCs were infected with Sendai virus including Lhx3, Ngn2, and Islet1 (multiplicity of infection = 5, ID Pharma, Tokyo, Japan) on day 0. The medium was changed to motor neuron medium without Y-27632 on day 1 and mRNA of human motor neurons was collected at day 14. Normalized maximum neurite length (a) of motor neurons derived from normal human (201B7 cell line) and two ALS patients with an M337V mutation of TARDBP gene (A3411 cell line) and G4C2 repeat expansion in the C9orf72 gene (C9 148-1) (C9rof72 148-1 cell line) were measured during the experimental course. n = 1, data are presented as the mean relative neurite length in 5 fields/well, error bars show SEM. Mouse motor neurons were induced as shown in Fig. 6 a, mRNA was collected on day of 14 of the assay. qPCR analysis (b) with the indicated primers using RNAs from mouse or human iPS-induced motor neurons. Expression level of LONRF1, 2, 3 was normalized using GAPDH. Set expression level of LONRF1 as 1, relative expression level of LONRF2 and LONRF3 were calculated. The relative expressions are shown. Data are presented as means ±s.d. of three independent experiments, error bars show s.d. Two-tailed one-way analysis with Dunnett’s (a) or Tukey (b) multiple comparisons post hoc test, *P < 0.05, **P < 0.01, ***P < 0.001.
Supplementary information
Supplementary Video 1
Ledge test for WT mouse.
Supplementary Video 2
Ledge test for Lonrf2-KO mouse.
Supplementary Video 3
Hind limb test for WT mouse.
Supplementary Video 4
Hind limb test for Lonrf2-KO mouse.
Supplementary Video 5
Gait test for WT mouse.
Supplementary Video 6
Gait test for Lonrf2-KO mouse.
Supplementary Table 1
Information of primers used in this study.
Supplementary Table 2
Information of antibodies used in this study.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 7
Unprocessed western blots.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed western blots.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, D., Johmura, Y., Morimoto, S. et al. LONRF2 is a protein quality control ubiquitin ligase whose deficiency causes late-onset neurological deficits. Nat Aging 3, 1001–1019 (2023). https://doi.org/10.1038/s43587-023-00464-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-023-00464-4
This article is cited by
-
LONRF2 exerts protein quality control in ageing neurons
Nature Reviews Neurology (2023)
-
LONRF2 is a gatekeeper against protein aggregation in aging neurons
Nature Aging (2023)