Abstract
Alzheimer’s disease (AD) is a major healthcare challenge with no curative treatment at present. To address this challenge, we need a paradigm shift, where we focus on pre-dementia stages of AD. In this Perspective, we outline a strategy to move towards a future with personalized medicine for AD by preparing for and investing in effective and patient-orchestrated diagnosis, prediction and prevention of the dementia stage. While focusing on AD, this Perspective also discusses studies that do not specify the cause of dementia. Future personalized prevention strategies encompass multiple components, including tailored combinations of disease-modifying interventions and lifestyle. By empowering the public and patients to be more actively engaged in the management of their health and disease and by developing improved strategies for diagnosis, prediction and prevention, we can pave the way for a future with personalized medicine, in which AD pathology is stopped to prevent or delay the onset of dementia.
Similar content being viewed by others
Main
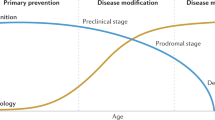
Alzheimer’s disease (AD) is among this century’s major healthcare challenges. It is characterized by progressive decline in the individual’s cognitive abilities. Worldwide, 55 million patients suffer from dementia1. AD is the most common cause of dementia. Therefore we focus on AD, but we also discuss studies that do not specify the cause of dementia and acknowledge that much of the reasoning holds for other pathologies causing dementia as well. While most prevalence studies do not specify dementia subtype, advances of biomarkers for AD pathology enable more precise estimates of dementia due to AD at 32 million worldwide2. In fact, dementia is only the late stage of a disease that takes years to develop in the brain. Biomarkers allow us to estimate the size of populations in pre-dementia stages of AD; first estimates indicate 69 million individuals with mild cognitive impairment (MCI) due to AD and >300 million individuals having preclinical AD2,3. MCI refers to the prodromal stage of AD in which there is some cognitive impairment that does not suffice for a diagnosis of dementia. Preclinical AD refers to the presence of AD pathology in individuals without any signs or symptoms. It is not yet clear, however, whether all individuals with preclinical AD progress to symptomatic AD and dementia. Nonetheless, the biomarker-based prevalence estimates of the AD continuum illustrate that we need to disentangle the concepts of AD from dementia4,5,6. While AD refers to the disease or pathological process that takes years to develop, dementia refers to a late-stage, detrimental outcome of this disease. There is no cure for AD yet, nor for other dementia-causing diseases. In a large proportion of patients with AD, a diagnosis is only made at a late disease stage. The same holds for other dementia-causing diseases. As a result, care is too often untimely and insufficiently adjusted to patients’ needs, resulting in frustrations in patients as well as health professionals, high costs and a compromised quality of life7. Treatment strategies for AD that lead to even small delays in onset of dementia and progression of the disease or enable self-management of patients would not only considerably reduce the prevalence of dementia but also the individual and socioeconomic burden8,9. It is therefore critical to bring therapy and support to individuals in as timely and adequately a manner as possible.
AD pathology should be targeted before the onset of dementia. Research has shown that AD develops in the course of 20–30 years10,11. By the time AD manifests as dementia, the brain can no longer be rescued. This provides a huge window of opportunity for preventive action. To optimally employ these possibilities, we need a paradigm shift with a focus on (1) individual characteristics and preferences and (2) the stages before dementia to ultimately (3) prevent progression to dementia. Effective deployment of preventive strategies requires timely identification of individuals who would benefit the most. Further development of diagnostic tests to detect early AD pathophysiological changes, also capturing differences in pathological pathways between patients, is therefore warranted. Individual preferences and patient-reported outcomes should be the starting point for high-quality individualized care12,13.
Based on observations in the consultation room—where the information need of patients boils down to three questions: ‘Doctor, what diagnosis do I have?’, ‘What can I expect?’ and ‘What can I do?’—in this Perspective, we outline the preparatory steps to ready society for a future with personalized medicine for AD. We discuss the need for preventive strategies, outlining the importance of both disease-modifying drugs and lifestyle interventions. We also reflect on the importance of timely and molecular diagnosis of AD, where blood-based biomarkers, genetic information and digital tools can be incorporated into the AD diagnosis framework. Moving toward earlier stages of the disease, personal risk profiles should provide prognostic information on outcomes that matter to patients. Finally, we recommend promoting patient-orchestrated care by engaging older adults (at risk of) AD throughout their health and disease management, with a keen eye for an inclusionary approach to keep healthcare affordable and accessible. As such, we describe a direction for the future in which patient-orchestrated AD healthcare entails accurate and timely diagnosis with prediction of meaningful outcomes to ultimately achieve prevention of dementia.
Prevention
Dementia risk reduction is one of the strategic action areas of the World Health Organization’s global action plan on dementia, outlining steps to be taken on a global, national and regional level14. To ultimately reduce the global burden of AD and other causes of dementia, we should move the needle forward to pre-dementia stages of disease. The report identified a wide gap between the need for prevention and treatment and the actual provision of services. It stresses the need for a collective effort to understand how we can prevent or at least delay onset of the disease. Prevention encompasses both pharmacological and non-pharmacological strategies. These are not mutually exclusive but rather complementary strategies. For complex diseases such as AD, we need to employ every possible strategy that may help to reduce the disease burden, that is, focus on risk reduction, while also developing disease-modifying treatment, with the ultimate question of what works for whom.
Pharmacological interventions
In the dementia stage, it is too late to rescue the brain, and most pharmacological treatment is therefore aimed at slowing the progression of symptoms, in fact, tertiary prevention. For future pharmaceutical strategies to be most effective, however, they probably should be provided in the pre-dementia stages. After aducanumab in 2021, the approval of lecanemab by the Food and Drug Administration at the beginning of 2023 heralds the second drug with disease-modifying properties15,16. This marks a milestone heralding the dawn of a new era, in which drugs that alter the biological disease pathways in AD are a realistic opportunity. Market access of these drugs will impact the patient journey, not only in terms of treatment, but also in terms of diagnosis, monitoring and prognosis. In particular, this illustrates the necessity to focus on underlying pathology, rather than syndrome outcome. There are a number of challenges to address related to integrating this new class of drugs in clinical care. We need to obtain better understanding of their clinical impact as well as the putative side effects. Risk–benefit assessment for use of the drugs, that is, who will benefit most while taking into account who is at most risk of side effects, needs to take place on an individual basis. One might argue that, initially, treatment should only take place in certified centers with sufficient expertise. At the same time, we need to make sure that healthcare remains accessible and sustainable. Clear stop criteria, that is, stopping when all amyloid has been removed or when the drug is not successful in doing so, will be indispensable to keep costs at bay.
Despite the positive news on anti-amyloid treatment, we know that AD is far too complex a disease to be stopped by targeting amyloid only. The drug pipeline is much wider, however, as there are currently 143 drugs being studied in clinical trials, the large majority of which are disease-modifying therapies17. Non-amyloid targeting targets include tau, inflammation, synaptic plasticity and many others. Compared to clinical stage interventions, the portfolio of targets for treatment in preclinical studies is even broader, with an increasing focus on targets associated with AD risk genes, including apolipoprotein E (ApoE) and lipids, lysosomal–endosomal targets and proteostasis18. This further illustrates how developments in treatment strategies also impact the needs in diagnosis, for example, that genetic workup will have a role in future diagnosis, as a starting point to identify suitable treatment strategies.
Lifestyle interventions
It is estimated that up to 40% of dementia risk is attributable to 12 modifiable risk factors19. Risk factors vary across the lifespan, for example, from less education in early life, hypertension and obesity in midlife and social isolation, depression and physical inactivity in later life. It is not clear whether they relate to AD pathology specifically or rather to all-cause dementia. Neither is it entirely clear whether all 12 factors indeed constitute risk factors or whether some of them (for example, depression and social isolation) are in fact early features of disease. Nonetheless, these modifiable factors offer attractive targets for intervention.
Several large population-based cohort studies indicate that prevalence of all-cause dementia is increasing less than expected, and age-specific incidence is even declining in the Western world20,21. A recent post-mortem study showed a decline in vascular pathology but not in pathological AD diagnosis over a period of 25 years22. This suggests that, even in those with AD pathology, lifestyle-targeting interventions may help to prevent clinical manifestation of disease. The increasing number of studies examining modifiable risk factors has created momentum for developing intervention strategies to maintain cognitive health and ultimately prevent dementia. The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) study took as a starting point not one single modifiable risk factor but rather a multi-domain approach and showed somewhat improved cognitive functioning23. These findings are now being replicated through multiple studies testing similar intervention strategies in diverse populations and settings. This collaborative action of the World-Wide FINGERS Network is an example of how international and national collaboration drives strengthening evidence and moving the field forward24.
It is yet unknown whether lifestyle interventions still delay cognitive decline when AD pathology has started to accumulate, although there is some evidence suggesting that this may be the case25,26. Therefore, there is a need to better understand the value of lifestyle improvements in people that worry about their memory or have MCI. The beneficial effect of lifestyle interventions may be attributable to improving resilience against pathological processes27. Resilience encompasses cognitive reserve, or adaptability of cognitive processes to pathology, brain maintenance, referring to reduced accrual of pathology over time, and brain reserve, directly related to the individual’s structural characteristics of the brain. To what extent these concepts are specific to AD pathology or rather generic to any type of brain pathology remains to be discovered.
There are several challenges in the translation of multicomponent lifestyle interventions into practice. We need to improve the evidence base on potential modifiable risk factors in the clinical context to set realistic expectations. In addition, one of the major challenges is to reach under-represented groups that have, for example, an ethnic or cultural minority status, low socioeconomic status or low levels of education28. Tailoring lifestyle interventions in practice to the knowledge, needs and preferences of these under-represented high-risk groups is essential. Secondly, communicating about dementia prevention comes with important ethical challenges, for example, to avoid ‘blaming the victim’. Strategies based on fear and stigma, such as those used in anti-smoking campaigns, should be avoided to not increase the stigma that dementia already has for people currently living with dementia28. In addition, implementing multicomponent lifestyle interventions in practice is complex and requires collaborative capacity to take collective action on a societal level. Preventive action for a disease that mostly occurs in late life should already start in midlife. Collaborating with established prevention programs in the public health domain and primary care setting, for example, by teaming up with cardiovascular disease prevention, could facilitate this.
Public participant involvement and recruitment
The dawn of the first effective strategies underlines and increases the need for clinical research in both patients and at-risk groups. Finding enough participants is an important bottleneck to finding effective intervention strategies29. Moreover, the lack of diversity in populations participating in clinical trials may be an important explanation of the limited breakthroughs in intervention strategies that are translated into clinical practice, given the disparities in disease risk and burden in some communities. Therefore, it is essential that clinical trials enroll diverse populations30. Remote strategies may facilitate the recruitment of diverse populations31.
Online platforms such as Brain Health Registry, Join Dementia Research or https://Hersenonderzoek.nl can help to involve citizens and patients to accelerate research32,33,34. In addition to providing more effective recruitment and making it easier to reach diverse populations, these platforms actively engage individuals with research, which ultimately leads to better treatment and care. Taking public and patient views into account from the start of the project will also lead to better translation of findings to clinical practice. The online platform to support communication in diagnosis (https://www.adappt.health) is an example, in which we combined rigorous statistical modeling based on biomarkers with a process of co-creation to determine how these models should be translated into clinical practice35. More recently, the response to coronavirus disease 2019 (COVID-19) has shown that research can be a major part of management for a large number of patients and leads to constant updating of best practices.
In the Netherlands, we recently initiated the ABOARD (short for ‘a personalized medicine approach for AD’) cohort, a societal initiative aiming for large-scale engagement of citizens and patients with research (Box 1). The ABOARD cohort takes a direct-to-participant recruitment strategy, putting research participants themselves in an active position. This is further reinforced by the installment of a participant panel that ensures active involvement of the end users. Recruitment is supported by a Facebook campaign from Alzheimer Nederland (the Dutch Alzheimer society). In this way, the ABOARD cohort achieves large-scale engagement of citizens; it provides data to study trajectories of disease in a real-life, national sample. And finally, it lays the foundation for national rollout of future studies.
Translation to clinical practice
There are a number of prerequisites for translation of effective preventive strategies, whether lifestyle or pharmacological interventions, into clinical practice. First, an accurate, etiological diagnosis is essential to start treatment. Second, when diagnoses need to be made before the stage of dementia to allow preventive action, adequate information on prediction and prognosis becomes highly relevant. A citizen wants to know what to expect, to prepare for what is ahead and to make balanced decisions with regard to potential risks and benefits of proposed preventive strategies. Finally, this illustrates that we need to empower the public and patients to be more actively engaged in the management of their own health and disease. When we think of a future with a broader array of potential preventive strategies, the associated risks and benefits of which may differ depending on individuals’ characteristics, also taking into account their preferences and needs, it becomes clear that shared decision making should become more common practice in the diagnosis and management of AD.
Diagnosis
The diagnosis of AD dementia is based on clinical criteria. Patients with cognitive complaints or changes in behavior present to the primary care physician, who performs cognitive tests and initial examinations and can decide to refer the patient to a memory clinic for further diagnostic investigation20. Clinical diagnosis relies on careful history taking from the patient and family by a skilled clinician. A cognitive screening test, such as Mini-Mental State Examination (MMSE) or Montreal Cognitive Assessment (MoCA), is useful to obtain a crude indication of cognitive functioning. Patients can be referred to a memory clinic for a more thorough investigation. Standard diagnostic workup includes neuropsychological investigation and inventory of activities of daily living20. There is a need for an inclusive approach, ensuring that tests and questionnaires have validity across language and cultural barriers36. Imaging and routine laboratory tests serve to rule out other causes of cognitive decline. Diagnosis and management plans are decided by consensus in a multidisciplinary meeting.
In addition to this routine diagnostic practice, there have been tremendous advances in both imaging and fluid-based diagnostic tests providing evidence for underlying AD pathophysiology. This has culminated in the launch of the National Institute on Aging and Alzheimer’s Association (NIA-AA) research framework, which provides a biological definition of AD5 (Box 2). This framework provides syndrome staging of cognitive impairment (subjective cognitive decline, MCI and dementia). It also categorizes AD biomarkers based on the ATN classification (as summarized in Table 1 and Box 2), where A refers to amyloid pathology (cerebrospinal fluid (CSF) amyloid-β or amyloid positron emission tomography (PET)), T refers to tau pathology (CSF phosphorylated tau (p-tau)/total tau or tau PET) and N refers to neurodegeneration (CSF neurofilament light chain protein (NfL) or [18F]Fluorodeoxyglucose PET (FDG-PET) or magnetic resonance imaging (MRI)). There is ongoing debate about the ATN framework, which is currently being revised, as it does not capture the full complexity of AD-related pathophysiology. For example, synaptic loss and inflammation, also part of AD pathophysiology, are not accounted for. Moreover, the clinical syndrome in most patients results from mixed pathologies, for example, with co-occurring cerebrovascular disease, α-synucleiopathy or TAR DNA-binding protein 43 (TDP-43). The NIA-AA research framework does not account for mixed pathology. Nor do any other sets of diagnostic criteria, for that matter. There is an urgent need for criteria and guidelines for diagnosing mixed types of pathologies, particularly when we are moving toward a future with disease-modifying treatment.
Currently, a diagnosis is the starting point for initiating appropriate care and symptomatic treatment. In a future with personalized medicine, the choice of disease-modifying strategy is directly related to the presence of a specific type of pathology. This highlights a need for molecular diagnosis.
Molecular biomarkers
A key to delaying the onset of dementia is an early and timely detection of AD-associated pathophysiological processes. The different AD-associated pathophysiological processes, namely, amyloid and tau pathology as well as neurodegeneration, can be identified using different biomarker modalities (as summarized in Table 1). Each modality of testing presents with advantages as well as limitations in capturing the spatiotemporal progression of AD pathology with a degree of affordability, accessibility and scalability37. For example, while structural MRI provides high spatial resolution and simultaneous presentation of information on multiple pathologies (for example, cerebrovascular pathology), it is not specific for the AD pathophysiological processes. PET tracers allow visualization and quantification of the spatial distribution of amyloid and tau pathology, but they provide information on a single type of pathology (for example, amyloid or tau). Moreover, the costs and infrastructural requirements are high, which limit their utility38. CSF-based biomarkers present the opportunity to evaluate multiple markers from one sample, which is scalable and a cost-effective option compared to neuroimaging; however, there is no localization of pathology and the invasive nature of the lumbar puncture is a limitation39. More recently, blood-based biomarkers have developed rapidly that provide an opportunity to detect multiple markers that are more affordable, accessible and scalable, compared to all other biomarker modalities, albeit unable to provide information on the localization of pathophysiological processes40. Hence, there is potential for blood-based biomarkers in the future as a biomarker modality for screening and monitoring the disease and treatment response41. Despite being highly promising, blood-based biomarkers are not ready for use in a clinical setting. Challenges in translating blood-based biomarkers to clinical practice include identifying the most promising biomarkers (and combinations thereof) and effective measurement platforms, prospective validation in real-life populations, in vitro diagnostic assay development and activities to obtain regulatory approval and refunding40. While validation studies are still ongoing, a first step toward implementation is the recent definition of appropriate use recommendations42.
Future developments in molecular diagnosis also contribute to further refinement of the diagnosis. AD is a highly heterogeneous disease, with multiple pathological pathways involved. Refining diagnosis for patient stratification is a next step toward personalized medicine of AD. Patient stratification using CSF-proteomic-based strategies has resulted in subgroups of AD with (1) hyperplasticity, (2) innate immune activation and (3) blood–brain barrier dysfunction43. It is likely that, when pathways from initial brain changes to late-stage dementia vary between individuals, this variability affects treatment response and/or risk of side effects.
In addition, knowledge on the genetic determinants of AD is quickly increasing. To date, >80 risk and protective genes for AD have been identified, most with only very small effect sizes when evaluated on their own44. The APOE gene is the most important risk gene for AD. Other risk variants, for example, TREM2 and SORL1, although far less common, also confer strongly increased risks. The effects of all risk genes can be combined in a polygenic risk score. To date, genetics are not part of the routine diagnostic workup and are only performed when the family history is highly suggestive of a mutation. It is conceivable that genomics will be incorporated in the diagnostic workup of the future, however44,45,46. Of note, genetic makeup not only predisposes for an increased risk but can also explain reduced risk or resilience47,48,49. In a future with personalized medicine, certain genetic variants may predict treatment response for pharmaceutical strategies, both in terms of benefit and risk. As an example, homozygous APOE ε4 carriers have a strongly increased risk of severe side effects of anti-amyloid treatment, while the observed benefit may be less15. Finally, genetic variants that reveal specific pathways to be involved, such as TREM2 or SORL1, may be the starting point of targeted therapeutic solutions50,51,52.
These developments show that we are making the transition to a biomarker-based diagnosis of AD. In the future, this may further develop to (1) a biomarker-based diagnostic fingerprint of different pathophysiological processes (mixed pathology) and (2) a more fine-grained diagnosis of AD, doing justice to the heterogeneity of the disease beyond the common ground of abnormal amyloid and tau. Detection of AD pathology across the spectrum (preclinical AD to dementia) provides a window of opportunity for therapeutic intervention to delay or even prevent the onset of AD dementia.
Digital tools
In addition to the swift developments in molecular diagnosis, careful characterization of the patient in terms of their clinical, cognitive and behavioral functioning remains key. In this context, digital biomarkers are very promising53,54.
Digital tools include online cognitive tests and questionnaires that resemble their paper-and-pencil equivalents55,56. Digital tests and questionnaires have the advantage of increased reliability and potentially increased sensitivity, as they allow extraction of many more data points than paper-and-pencil administration of a similar test. They could be cost saving, as they require less-skilled staff to administer. Computer-adapted testing versions of these tests shorten the administration time. In this way, they help to make the patient journey more patient friendly. Online cognitive tests also provide the possibility for the same set of tests and questionnaires to be provided at home, at the primary care setting and at the more specialized setting. By harmonizing the patient journey in this way, monitoring the disease and tracking progression becomes easier.
In addition, increased digitalization of society and improved data-analysis methods (for example, with use of artificial intelligence) open up innovative opportunities for digital biomarkers that can be obtained from, for example, wearables or voice recording57,58,59. These tests could be performed remotely at home, and they have ecological validity, as they test actual behavior in the home situation. Digital biomarkers could serve as a self-test to funnel to additional medical care, to monitor disease progression remotely as part of the follow-up visits after diagnosis and to monitor treatment response, potentially even increasing adherence to the program.
A European survey among professionals, patients and family members showed that a considerable majority had a positive attitude toward digital tools60. User friendliness and improved accuracy are main factors stimulating the adoption of a tool. Inadequate integration with electronic patient records and fear of losing important clinical information were most frequently indicated as barriers. Many patients and care partners showed interest in the possibility of using the tools themselves. Nonetheless, digital tools are still not used frequently in clinical practice. In addition to scaling practical hurdles and barriers, this also shows an urgent need for education of professionals and empowerment of patients and care partners. Use and development of digital tools has increased considerably during the COVID-19 pandemic, when testing remotely was a necessity when patients could not be seen in the clinic61. Finally, digital testing may contribute to making healthcare accessible also to low-literacy populations, particularly when cognitive testing is integrated in their daily lives, for example, by analyzing changes in use of their mobile devices or speech31.
The future diagnostic workup
The future diagnostic workup has a stepped or funneled approach, needed to keep healthcare accessible to an increasing number of patients. The specific diagnostic strategy could vary, depending on patients’ preferences and needs regarding diagnosis, prediction and prevention (Fig. 1). For example, one patient may want to know as much as possible about their genetic makeup and biomarker results to optimally prepare for the future, enroll in clinical trials or know their eligibility for disease-modifying treatment. For another patient, it may suffice to know that, at this time, cognitive impairment and daily functioning are still sufficiently intact and that they do not qualify for a syndrome diagnosis of dementia.
We are making the transition from a patient journey focused on diagnosis and post-dementia care to a patient journey in which diagnostic biomarkers increasingly serve the purpose of prediction and monitoring, and (preventive) treatment. Throughout the patient journey, information provision, when possible supported by e-tools, is key. This should entail information about what can be expected from the patient journey itself and information about the disease. In addition, there should be information about available options for diagnosis, prediction and prevention before embarking on testing or treatment, and information about what the results of specific tests mean for the individual lives of patients after testing and treatment. A prominent role for shared decision making promotes diagnosis and treatment to be more strongly aligned with the preferences, needs and wishes of patients and their families. The patient journey encompasses different settings (at home, primary care, secondary care and tertiary care) and may vary depending on disease stage (cognitively normal, mild cognitive impairment, dementia). Memory clinics mainly have a role for symptomatic patients, while Brain Health Services are an emerging concept that may be closer to primary care and may cater to cognitively normal citizens84. In the future patient journey, the themes of prediction, identifying the optimal preventive strategy, monitoring disease progression (including side effects) and evaluating treatment response become increasingly relevant. Diagnosis has a more funneled approach. Individualized risk profiles can be based on different types of determinants, depending on an individual’s disease characteristics and preferences. Treatment strategies have a stronger focus on prevention, encompassing both targeting of lifestyle (primary prevention), disease-modifying treatment (secondary prevention), symptomatic treatment (which could be referred to as tertiary prevention) and care. ARIA, amyloid-related imaging abnormalities; PRO, patient-reported outcome.
Initial testing should involve easily accessible and scalable tools that allow reliable ruling out of AD when negative, preventing the need for further expensive testing. Current testing in primary care mostly entails risk factor assessment, with medical history and a cognitive screening test. In the future, digital biomarkers in combination with blood tests could further improve this process. The initial tests can be used for more effective referral to specialist memory clinics for further, in depth diagnostic testing, for example, with more invasive or expensive tools such as MRI, CSF biomarkers or PET scans. In addition, memory clinics can provide further refinement of diagnosis, for example, based on proteomics or genomics. Finally, we foresee that computer-based tools, for example, making use of artificial intelligence solutions, will enable clinicians to extract maximum information from the available diagnostic test results. This will reduce practice variation and improve accuracy but also deliver answers in an understandable way to both the professional and the patient. Timely and precise diagnosis will lead to a reduction in patient burden, costs and length of the diagnostic process and reduce the healthcare burden and costs in specialist settings (which are, by definition, more expensive).
Prediction
A diagnosis is not the end point, but rather the beginning of the rest of the disease trajectory. Given that AD is a progressive disorder, patients want to know what they can expect62,63. Available prediction models are mostly based on community-based studies (cardiovascular risk factors and lifestyle) or selected research populations (biomarker based). The former have relevance for the general population or general practitioner setting and often refer to lifetime risk of dementia64,65,66, while the latter pertain to a tertiary memory clinic setting and short-term risk67,68. Prediction models should be clear about the time frame for which they make predictions.
Biomarker-based prognosis
With the use of biomarkers, prediction of dementia has become more accurate, particularly in the MCI stage, providing a view on a future with individualized risk predictions67,68. Prediction models in the stage of cognitively normal are less generalizable and, for that reason, more difficult to translate to the individual level. Nonetheless, cognitively normal individuals who are positive for both amyloid and tau based on PET (hence, A+T+) have a 50% probability to progress to a symptomatic stage in the short term, while progression rates are very low when A and T are not both positive. These data emphasize that biomarkers hold important prognostic information69,70.
Prediction of outcomes that matter
When we diagnose AD before the stage of dementia, a diagnosis in fact becomes a prognosis, as patients and their families are worried about the detrimental clinical outcome, rather that the molecular nature of the disease. Most modeling efforts predict the outcome of dementia. In a disease trajectory that takes decades to unfold, onset dementia will not be the key reference to commencing treatment anymore, as the recently approved medications can be prescribed to patients with MCI and mild dementia. In addition, with respect to prognosis, other outcomes may have even more relevance from the perspective of patients and their families. In an effort to identify patient-relevant outcomes, we asked patients and caregivers which outcomes a hypothetical future medicine should prevent71. The core list of prognostic information relevant to both patients and care partners included items mostly related to cognitive decline, dependency and physical health (Fig. 2). This information should guide modeling efforts and trial design.
Alzheimer’s disease (AD) includes the clinical stages of preclinical AD (including subjective cognitive decline), MCI and mild, moderate and severe dementia. Most prediction studies take cognitively normal individuals or patients with MCI as the starting point and predict progression to dementia. Yet, onset of dementia is in fact rather an arbitrary moment in a disease trajectory that takes decades to unfold. In a former study, we identified 13 outcomes that matter to patients and care partners, which may occur somewhere in the course of the disease71. Together, these meaningful outcome define the clinical trajectory of AD. To cater to the need of prognostic information for patients and their families, future studies should focus on prediction of these outcomes that matter.
Prediction in different settings
Much work still needs to be accomplished: (1) risk models should be applicable in primary, secondary and tertiary care and be generalizable beyond research settings to the ‘typical patient’, (2) findings should be interpretable at the individual level, and (3) outcomes should reflect what really matters to patients. Development of generalizable, flexible and patient-relevant prediction models is essential to provide tailored prognostic information. Ultimately, individualized risk predictions will identify which individuals benefit most from which preventive strategies.
Patient-orchestrated care
With the number of options in diagnosis, prediction and prevention of AD rapidly increasing, it becomes ever more important to take the preferences, wishes and needs of patients and their families as a starting point for providing care. Patients and their families being actively involved in the management of their own health and disease can contribute to keeping healthcare affordable and sustainable.
Ethical aspects
Now that it becomes possible to diagnose AD before onset of dementia, a next question is whether it is ethical to make such a diagnosis or inform individuals about their biomarker status or future risk of dementia72. Such knowledge may cause distress, as an exact prognosis cannot be provided and there is currently no curative treatment. Yet, is it ethical to withhold available information about AD risk when a person asks for it? The uncertainty of not knowing the cause of memory problems may be equally burdensome. Moreover, a diagnosis may provide an opportunity for preventive action to delay the start of dementia, help individuals and care partners to prepare for the future and allow participation in dementia-prevention trials. Of note, we only refer here to patients seeking help for their perceived problems at primary care or a memory clinic (that is, MCI or subjective cognitive decline). There is no reason to think that, at short notice, a widespread screening program in the general community would be useful. Nonetheless, individuals vary in their personal considerations regarding diagnosis, prediction and prevention of AD, and the question is how we can best accommodate these differences.
Shared decision making
Empirical evidence on the implications of a pre-dementia diagnosis for the well-being of individuals is largely lacking, whereas such information can inform organization of the patient journey. Nevertheless, the weighing of the pros and cons of an early diagnosis and the decision of whether to initiate testing or not that follows from such deliberation ultimately remains a highly individual process. Patients should be facilitated to articulate their voice in this decision as part of a shared decision-making process. Shared decision making refers to clinicians and patients (and/or their care partners) working together to decide which care plan best fits individual patients and their lives, given that there is more than one reasonable option73. To facilitate a process of shared decision making, we need to provide the public and patients with information to be able to make informed decisions. This information should include but not be limited to terminology (difference between AD and dementia), the advantages and disadvantages of existing diagnostic tests, possibility of misdiagnosis and mixed pathology, difficulty in personalized prognostication and risks and benefits associated with different treatment strategies.
Tailoring information to promote an inclusive approach
Some individuals run the risk of being less informed and less involved in decision making than others as a result of diversity in cultural background, health and e-health literacy, and/or educational attainment. Special attention is warranted for the needs and preferences of these more vulnerable individuals to ensure that their perspective is also taken into account in the organization of care. Heterogeneity in needs and preferences regarding information and participation in decision making also result from individual differences in psychological characteristics such as coping style or tolerance for uncertainty as well as, for example, living situation62. All of this requires that care is tailored to individual patients’ needs and preferences. Such tailoring is easier if patients (and their care partners) are in the lead. In former studies, we found that patients and their families hardly ask for additional information during diagnostic consultations, while afterward many still report a need for information74. To foster information provision, we developed a topic list and animation videos (https://www.adappt.health) that empower patients by informing them what to expect at the memory clinic and inviting them to think about the questions that they would like to ask63.
Communication
Customization also necessitates optimal communication between care providers and patients. Given the current lack of curative treatment and uncertainty of outcomes of early diagnostic testing, clinicians are reluctant to provide risk information, arguing that this would burden patients75. By stark contrast, many patients and care partners explicitly prefer to receive this probabilistic information, as it can help them prepare for the future63,76. To make well-informed choices, patients and care partners need to be able to understand and recall diagnostic and prognostic information in a way that allows them to make decisions and engage in preventive action that is in line with their needs and values. Hence best-practice recommendations are urgently needed to disclose results of new diagnostic tests, including the risk of dementia77,78,79,80. Such communication between healthcare professionals and patients can be supported by digital tools (see Fig. 3 for example). Online tools may help clinicians to provide information in an individualized and understandable way, thereby improving information retention and empowering patients35,81,82,83. Successful implementation of such tools in clinical practice calls for a co-creation process involving professionals as well as patients and care partners, considering diversity in needs, preferences and abilities.
Screenshots of https://ADappt.health. Communication about risk and probability is challenging, because this information is hard to understand for patients and their families and difficult to explain for professionals. Yet, we can learn from other research fields such as oncology or cardiovascular disease, where there is substantial information about best-practice risk communication85. Here we provide an example of the communication sheet at https://ADappt.health, which facilitates communication about the risk of dementia for patients with mild cognitive impairment, including use of natural numbers, graphical representation of risk, neutral framing and plain language35. It is recommended to provide patients with written information about their diagnosis and prognosis79. The communication sheet can therefore be printed for the patient to take home.
Concluding remarks
AD, being the major cause of dementia, is one of the largest healthcare challenges of our century. As such, AD is a major concern for us all, either as individuals living with or at risk of the disease, their family members and caregivers or professionals who encounter individuals with dementia in clinical practice and care. The sheer size of the population facing AD, the trend toward more active involvement of patients, families and citizens in the management of their own health and disease, in combination with the swift scientific progress in diagnosis, prediction and prevention, results in momentum for the field. We see the first AD disease-modifying treatments at the horizon, illustrating that we are swiftly moving toward a new era. Moreover, insight in the putative effect of lifestyle interventions is increasing, providing implications for actionability.
The next step is understanding how we can move toward a future of personalized medicine for AD, a future that will include not only technical and neuroscientific innovations but also has to find answers to ethical dilemmas, socioeconomic consequences and personal considerations, a dialog that we must embrace as a society. In this dialog, countries can learn from each other. Nonetheless, healthcare is largely organized by country; hence, it is essential to also conduct the dialog by country, involving all relevant stakeholders. In the Netherlands, we initiated the ABOARD project to provide a platform for this cross-sectoral dialog and to take the necessary preparatory steps for a future with personalized medicine (Box 1).
The imminent changes that convert AD into a treatable disease profoundly impact the entire patient journey. We need to address questions such as how to keep healthcare accessible and how to ensure scalability of new solutions for diagnosis, prediction and prevention. Figure 1 provides an outline of the patient journey of the future. Dementia risk assessment and easily accessible monitoring of cognitive function may already start at home, when citizens increasingly want to know what they can do themselves. When signs and symptoms warrant a physician visit, there will be a funneled approach toward accurate and molecular diagnosis, which is the starting point of tailored prevention strategies. This comes with additional challenges, such as how to monitor side effects, how to ensure equal access to care, how to evaluate treatment response and, particularly, how to identify those individuals who would benefit most from which intervention. Throughout the patient journey, adequate and easily digestible information is crucial. Educating professionals to optimally navigate their patients through this journey and to support a process of shared decision making is a necessary prerequisite. Finally, providing information to patients and their families about what to expect from the patient journey in terms of diagnostic tests, information about the disease and disease trajectory, and information about different types of prevention strategies is crucial to work toward patient-orchestrated care.
To see this future come to fruition, we need to invest in research in precise and molecular diagnosis and personalized risk profiles providing information on a person’s likely trajectory of disease, which together form the basis for the selection of preventive strategies. To facilitate this, integrating shared decision making throughout the patient journey is crucial, and tools to support both patients and their families and professionals to effectively engage in such a process are dearly needed.
In conclusion, we provide an outlook on a future with personalized medicine for AD, in which patients and care partners are empowered and more actively engaged in the management of their health and disease and in which tailored combinations of lifestyle interventions and disease-modifying treatment are provided in a timely fashion to target AD pathology to prevent or delay the onset of dementia.
References
WHO. Global Status Report on the Public Health Response to Dementia. Report No. ISBN 978-92-4-003324-5 (World Health Organization, 2021).
Gustavsson, A. et al. Global estimates on the number of persons across the Alzheimer’s disease continuum. Alzheimers Dement. 19, 658–670 (2022).
Jack, C. R. Jr. et al. Prevalence of biologically vs clinically defined Alzheimer spectrum entities using the National Institute on Aging-Alzheimer’s Association research framework. JAMA Neurol. 76, 1174–1183 (2019).
Knopman, D. S., Petersen, R. C. & Jack, C. R. Jr. A brief history of “Alzheimer disease”: multiple meanings separated by a common name. Neurology 92, 1053–1059 (2019).
Jack, C. R. Jr. et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14, 535–562 (2018).
van der Flier, W. M. & Scheltens, P. The ATN framework—moving preclinical Alzheimer disease to clinical relevance. JAMA Neurol. 79, 968–970 (2022).
van der Roest, H. G. et al. Subjective needs of people with dementia: a review of the literature. Int. Psychogeriatr. 19, 559–592 (2007).
Wimo, A., Guerchet, M., Ali, G. C., Wu, Y. T. & Prina, M. World Alzheimer Report 2015: the Global Impact of Dementia (Alzheimer’s Disease International, 2015).
Brookmeyer, R., Gray, S. & Kawas, C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am. J. Public Health 88, 1337–1342 (1998).
Bateman, R. J. et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N. Engl. J. Med. 367, 795–804 (2012).
Jansen, W. J. et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA 313, 1924–1938 (2015).
Weldring, T. & Smith, S. M. Patient-reported outcomes (PROs) and patient-reported outcome measures (PROMs). Health Serv. Insights 6, 61–68 (2013).
Hofman, C. S. et al. Comparing the health state preferences of older persons, informal caregivers and healthcare professionals: a vignette study. PLoS ONE 10, e0119197 (2015).
World Health Organization. Global Action Plan on the Public Health Response to Dementia 2017–2025. Report No. ISBN 978-92-4-151348-7 (World Health Organization, 2017).
van Dyck, C. H. et al. Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. 388, 9–21 (2023).
Budd Haeberlein, S. et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J. Prev. Alzheimers Dis. 9, 197–210 (2022).
Cummings, J. et al. Alzheimer’s disease drug development pipeline: 2022. Alzheimers Dement. 8, e12295 (2022).
van Bokhoven, P. et al. The Alzheimer’s disease drug development landscape. Alzheimers Res. Ther. 13, 186 (2021).
Livingston, G. et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446 (2020).
Wu, Y. T. et al. The changing prevalence and incidence of dementia over time—current evidence. Nat. Rev. Neurol. 13, 327–339 (2017).
Stephan, B. C. M. et al. Secular trends in dementia prevalence and incidence worldwide: a systematic review. J. Alzheimers Dis. 66, 653–680 (2018).
Grodstein, F., Leurgans, S. E., Capuano, A. W., Schneider, J. A. & Bennett, D. A. Trends in postmortem neurodegenerative and cerebrovascular neuropathologies over 25 years. JAMA Neurol. 20, e225416 (2023).
Ngandu, T. et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 385, 2255–2263 (2015).
Kivipelto, M. et al. World-Wide FINGERS Network: a global approach to risk reduction and prevention of dementia. Alzheimers Dement. 16, 1078–1094 (2020).
Solomon, A. et al. Effect of the apolipoprotein E genotype on cognitive change during a multidomain lifestyle intervention: a subgroup analysis of a randomized clinical trial. JAMA Neurol. 75, 462–470 (2018).
Muller, S. et al. Relationship between physical activity, cognition, and Alzheimer pathology in autosomal dominant Alzheimer’s disease. Alzheimers Dement. 14, 1427–1437 (2018).
Stern, Y. et al. Mechanisms underlying resilience in ageing. Nat. Rev. Neurosci. 20, 246 (2019).
Steyaert, J. et al. Putting primary prevention of dementia on everybody’s agenda. Aging Ment. Health 25, 1376–1380 (2021).
Fargo, K. N., Carrillo, M. C., Weiner, M. W., Potter, W. Z. & Khachaturian, Z. The crisis in recruitment for clinical trials in Alzheimer’s and dementia: an action plan for solutions. Alzheimers Dement. 12, 1113–1115 (2016).
Grill, J. D., Sperling, R. A. & Raman, R. What should the goals be for diverse recruitment in Alzheimer clinical trials? JAMA Neurol. 79, 1097–1098 (2022).
Weiner, M. W. et al. Increasing participant diversity in AD research: plans for digital screening, blood testing, and a community-engaged approach in the Alzheimer’s Disease Neuroimaging Initiative 4. Alzheimers Dement. 19, 307–317 (2023).
Aisen, P. et al. Registries and cohorts to accelerate early phase Alzheimer’s trials. A report from the E.U./U.S. clinical trials in Alzheimer’s Disease Task Force. J. Prev. Alzheimers Dis. 3, 68–74 (2016).
Weiner, M. W. et al. The Brain Health registry: an internet-based platform for recruitment, assessment, and longitudinal monitoring of participants for neuroscience studies. Alzheimers Dement. 14, 1063–1076 (2018).
Zwan, M. D. et al. Dutch Brain Research registry for study participant recruitment: design and first results. Alzheimers Dement. 7, e12132 (2021).
van Maurik, I. S. et al. Development and usability of ADappt: web-based tool to support clinicians, patients, and caregivers in the diagnosis of mild cognitive impairment and Alzheimer disease. JMIR Form. Res. 3, e13417 (2019).
Franzen, S. et al. Neuropsychological assessment in the multicultural memory clinic: development and feasibility of the TULIPA battery. Clin. Neuropsychol. 37, 60–80 (2023).
Festari, C. et al. European consensus for the diagnosis of MCI and mild dementia: preparatory phase. Alzheimers Dement. https://doi.org/10.1002/alz.12798 (2022).
Ashton, N. J. et al. The validation status of blood biomarkers of amyloid and phospho-tau assessed with the 5-phase development framework for AD biomarkers. Eur. J. Nucl. Med. Mol. Imaging 48, 2140–2156 (2021).
Leuzy, A. et al. 2020 update on the clinical validity of cerebrospinal fluid amyloid, tau, and phospho-tau as biomarkers for Alzheimer’s disease in the context of a structured 5-phase development framework. Eur. J. Nucl. Med. Mol. Imaging 48, 2121–2139 (2021).
Teunissen, C. E. et al. Blood-based biomarkers for Alzheimer’s disease: towards clinical implementation. Lancet Neurol. 21, 66–77 (2022).
Pontecorvo, M. J. et al. Association of donanemab treatment with exploratory plasma biomarkers in early symptomatic Alzheimer disease: a secondary analysis of the TRAILBLAZER-ALZ randomized clinical trial. JAMA Neurol. 79, 1250–1259 (2022).
Hansson, O. et al. The Alzheimer’s Association appropriate use recommendations for blood biomarkers in Alzheimer’s disease. Alzheimers Dement. 18, 2669–2686 (2022).
Tijms, B. M. et al. Pathophysiological subtypes of Alzheimer’s disease based on cerebrospinal fluid proteomics. Brain 143, 3776–3792 (2020).
Bellenguez, C. et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet. 54, 412–436 (2022).
Jansen, I. E. et al. Genome-wide meta-analysis for Alzheimer’s disease cerebrospinal fluid biomarkers. Acta Neuropathol. 144, 821–842 (2022).
van der Lee, S. J. et al. The effect of APOE and other common genetic variants on the onset of Alzheimer’s disease and dementia: a community-based cohort study. Lancet Neurol. 17, 434–444 (2018).
van der Lee, S. J. et al. A nonsynonymous mutation in PLCG2 reduces the risk of Alzheimer’s disease, dementia with Lewy bodies and frontotemporal dementia, and increases the likelihood of longevity. Acta Neuropathol. 138, 237–250 (2019).
Jonsson, T. et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature 488, 96–99 (2012).
Ebenau, J. L. et al. Risk of dementia in APOE ε4 carriers is mitigated by a polygenic risk score. Alzheimers Dement. 13, e12229 (2021).
Guerreiro, R. et al. TREM2 variants in Alzheimer’s disease. N. Engl. J. Med. 368, 117–127 (2013).
Jonsson, T. et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med. 368, 107–116 (2013).
Holstege, H. et al. Characterization of pathogenic SORL1 genetic variants for association with Alzheimer’s disease: a clinical interpretation strategy. Eur. J. Hum. Genet. 25, 973–981 (2017).
Ohman, F., Hassenstab, J., Berron, D., Scholl, M. & Papp, K. V. Current advances in digital cognitive assessment for preclinical Alzheimer’s disease. Alzheimers Dement. 13, e12217 (2021).
Chan, J. Y. C., Yau, S. T. Y., Kwok, T. C. Y. & Tsoi, K. K. F. Diagnostic performance of digital cognitive tests for the identification of MCI and dementia: a systematic review. Ageing Res. Rev. 72, 101506 (2021).
Rhodius-Meester, H. F. M. et al. cCOG: a web-based cognitive test tool for detecting neurodegenerative disorders. Alzheimers Dement. 12, e12083 (2020).
Jutten, R. J. et al. Detecting functional decline from normal aging to dementia: development and validation of a short version of the Amsterdam IADL Questionnaire. Alzheimers Dement. 8, 26–35 (2017).
Piau, A., Wild, K., Mattek, N. & Kaye, J. Current state of digital biomarker technologies for real-life, home-based monitoring of cognitive function for mild cognitive impairment to mild Alzheimer disease and implications for clinical care: systematic review. J. Med. Internet Res. 21, e12785 (2019).
Tavabi, N. et al. Cognitive digital biomarkers from automated transcription of spoken language. J. Prev. Alzheimers Dis. 9, 791–800 (2022).
Lam, K. H. et al. Smartphone-derived keystroke dynamics are sensitive to relevant changes in multiple sclerosis. Eur. J. Neurol. 29, 522–534 (2022).
van Gils, A. M. et al. Assessing the views of professionals, patients, and care partners concerning the use of computer tools in memory clinics: international survey study. JMIR Form. Res. 5, e31053 (2021).
Kaye, J. et al. Using digital tools to advance Alzheimer’s drug trials during a pandemic: the EU/US CTAD Task Force. J. Prev. Alzheimers Dis. 8, 513–519 (2021).
Kunneman, M. et al. Patients’ and caregivers’ views on conversations and shared decision making in diagnostic testing for Alzheimer’s disease: the ABIDE project. Alzheimers Dement. 3, 314–322 (2017).
Fruijtier, A. D. et al. ABIDE Delphi study: topics to discuss in diagnostic consultations in memory clinics. Alzheimers Res. Ther. 11, 77 (2019).
Licher, S. et al. Development and validation of a dementia risk prediction model in the general population: an analysis of three longitudinal studies. Am. J. Psychiatry 176, 543–551 (2019).
Exalto, L. G. et al. Midlife risk score for the prediction of dementia four decades later. Alzheimers Dement. 10, 562–570 (2014).
Kivipelto, M. et al. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. 5, 735–741 (2006).
van Maurik, I. S. et al. Biomarker-based prognosis for people with mild cognitive impairment (ABIDE): a modelling study. Lancet Neurol. 18, 1034–1044 (2019).
Karikari, T. K. et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 19, 422–433 (2020).
Strikwerda-Brown, C. et al. Association of elevated amyloid and tau positron emission tomography signal with near-term development of Alzheimer disease symptoms in older adults without cognitive impairment. JAMA Neurol. 79, 975–985 (2022).
Ossenkoppele, R. et al. Amyloid and tau PET-positive cognitively unimpaired individuals are at high risk for future cognitive decline. Nat. Med. 28, 2381–2387 (2022).
Mank, A. et al. Identifying relevant outcomes in the progression of Alzheimer’s disease; what do patients and care partners want to know about prognosis? Alzheimers Dement. 7, e12189 (2021).
van der Schaar, J. et al. Considerations regarding a diagnosis of Alzheimer’s disease before dementia: a systematic review. Alzheimers Res. Ther. 14, 31 (2022).
Kunneman, M., Montori, V. M., Castaneda-Guarderas, A. & Hess, E. P. What is shared decision making? (and what it is not). Acad. Emerg. Med. 23, 1320–1324 (2016).
Visser, L. N. C. et al. Clinician–patient communication during the diagnostic workup: the ABIDE project. Alzheimers Dement. 11, 520–528 (2019).
Visser, L. N. C. et al. Clinicians’ communication with patients receiving a MCI diagnosis: the ABIDE project. PLoS ONE 15, e0227282 (2020).
Vanderschaeghe, G., Schaeverbeke, J., Vandenberghe, R. & Dierickx, K. Amnestic MCI patients’ perspectives toward disclosure of amyloid PET results in a research context. Neuroethics 10, 281–297 (2017).
Fruijtier, A. D. et al. Identifying best practices for disclosure of amyloid imaging results: a randomized controlled trial. Alzheimers Dement. 19, 285–295 (2022).
Ketchum, F. B. et al. Moving beyond disclosure: stages of care in preclinical Alzheimer’s disease biomarker testing. Alzheimers Dement. 18, 1969–1979 (2022).
Grill, J. D. et al. Communicating mild cognitive impairment diagnoses with and without amyloid imaging. Alzheimers Res. Ther. 9, 35 (2017).
Lingler, J. H. et al. Development of a standardized approach to disclosing amyloid imaging research results in mild cognitive impairment. J. Alzheimers Dis. 52, 17–24 (2016).
Babapour Mofrad, R. et al. Cerebrospinal fluid collection: an informative animation video for patients and caregivers. Alzheimers Dement. 11, 435–438 (2019).
Gruters, A. A. A. et al. An exploratory study of the development and pilot testing of an interactive visual tool of neuropsychological test results in memory clinics. J. Alzheimers Dis. 79, 1157–1170 (2021).
van Gils, A. M. et al. Development and design of a diagnostic report to support communication in dementia: co-creation with patients and care partners. Alzheimers Dement. 14, e12333 (2022).
Altomare, D. et al. Brain Health Services: organization, structure, and challenges for implementation. A user manual for Brain Health Services—part 1 of 6. Alzheimers Res. Ther. 13, 168 (2021).
Visser, L. N. C. et al. Dementia risk communication. A user manual for Brain Health Services—part 3 of 6. Alzheimers Res. Ther. 13, 170 (2021).
Jessen, F. et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 10, 844–852 (2014).
Acknowledgements
The authors are work package leads on the ABOARD project. ABOARD is a public–private partnership receiving funding from ZonMw (73305095007) and Health~Holland, Topsector Life Sciences & Health (PPP allowance, LSHM20106). Partners in ABOARD are Amsterdam UMC, locations VUmc and AMC, MUMC+, Erasmus MC, UMC Radboud, UMCG, TU Delft, Inholland, Vilans, Pharos, HealthRI, Jeroen Bosch Ziekenhuis, Medisch Centrum Leeuwarden, Zorg Innovatie Forum, Pharmo–STIZON, Alzheimer Nederland, Hersenstichting, KBO-PCOB, PGGM, Zorgverzekeraars Nederland, CZ, Zilveren Kruis, Neurocast, Philips, ADx NeuroSciences, Castor, Vereniging Innovatieve Geneesmiddelen, Roche NL, Biogen NL, Novartis NL and the Brain Research Center. ABOARD also receives funding from Edwin Bouw Fonds and Gieskes-Strijbis Fonds. W.M.v.d.F., E.M.A.S. and C.E.T. are project leads on TAP-Dementia, a ZonMw-funded project (10510032120003) to optimize diagnosis of dementia, part of the Dutch National Dementia Strategy. W.M.v.d.F. is a recipient of JPND-funded EU-FINGERS (ZonMw-Memorabel 733051102) and ADDITION (ZonMw 733051083). Research of Alzheimer Center Amsterdam is part of the neurodegeneration research program of Amsterdam Neuroscience. Alzheimer Center Amsterdam is supported by funding from Stichting Alzheimer Nederland and Stichting VUmc. The chair of W.M.v.d.F. is supported by the Pasman Stichting.
Author information
Authors and Affiliations
Contributions
M.E.d.V. and M.B. drafted the section on prevention; C.E.T. drafted the section on diagnosis; E.M.A.S. drafted the section on patient-orchestrated care; W.M.v.d.F. drafted the remaining sections and critically revised the overall manuscript.
Corresponding author
Ethics declarations
Competing interests
Research programs of W.M.v.d.F. have been funded by ZonMw, NWO, EU-FP7, EU-JPND, Alzheimer Nederland, Hersenstichting CardioVascular Onderzoek Nederland, Health~Holland, Topsector Life Sciences & Health, Stichting Dioraphte, Gieskes-Strijbis Fonds, Stichting Equilibrio, Edwin Bouw Fonds, Pasman Stichting, Stichting Alzheimer and the Neuropsychiatrie Foundation, Philips, Biogen MA, Novartis NL, Life-MI, AVID, Roche, Eisai, Fujifilm and Combinostics. W.M.v.d.F. holds the Pasman chair. W.M.v.d.F. is a recipient of ABOARD, which is a public–private partnership receiving funding from ZonMw (73305095007) and Health~Holland, Topsector Life Sciences & Health (PPP allowance, LSHM20106). W.M.v.d.F. has performed contract research for Biogen MA and Boehringer Ingelheim. All funding is paid to her institution. W.M.v.d.F. has been an invited speaker at Biogen MA, Danone, Eisai, WebMD Neurology (Medscape), Springer Healthcare, the European Brain Council and Novo Nordisk. W.M.v.d.F. is a consultant to the Oxford Health Policy Forum, Roche and Biogen MA. W.M.v.d.F. participated on the advisory boards of Biogen MA, Roche and Eli Lilly. All funding is paid to her institution. W.M.v.d.F. is member of the steering committee of PAVE and Think Brain Health. W.M.v.d.F. was an associate editor of Alzheimer’s Research and Therapy in 2020–2021. W.M.v.d.F. is an associate editor at Brain. M.E.d.V. reports no disclosures. E.M.A.S. reports no disclosures. M.B. is the scientific director of Alzheimer Nederland. Research of C.E.T. is supported by the European Commission (Marie Curie International Training Network, grant agreement 860197 (MIRIADE), Innovative Medicines Initiatives 3TR (Horizon 2020, grant 831434), EPND (IMI 2 Joint Undertaking (JU), grant 101034344) and JPND (bPRIDE)), the National MS Society (Progressive MS Alliance), the Alzheimer Association, Health Holland, the Dutch Research Council (ZonMw), the Alzheimer Drug Discovery Foundation, the Selfridges Group Foundation and Alzheimer Netherlands. C.E.T. is a recipient of ABOARD, which is a public–private partnership receiving funding from ZonMw (73305095007) and Health~Holland, Topsector Life Sciences & Health (PPP allowance, LSHM20106). C.E.T. has a collaboration contract with ADx NeuroSciences, Quanterix and Eli Lilly and performed contract research or received grants from AC Immune, AXON Neuroscience, BioConnect, Bioorchestra, Brainstorm Therapeutics, Celgene, EIP Pharma, Eisai, Fujirebio, Grifols, Instant NanoBiosensors, Merck, Novo Nordisk, PeopleBio, Roche, Siemens, Toyama and Vivoryon. C.E.T. serves on editorial boards of Medidact Neurologie–Springer, Alzheimer Research and Therapy, Neurology: Neuroimmunology and Neuroinflammation. C.E.T. had speaker contracts for Roche, Grifols and Novo Nordisk. All funding is paid to her institution.
Peer review
Peer review information
Nature Aging thanks Paresh Malhotra and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
van der Flier, W.M., de Vugt, M.E., Smets, E.M.A. et al. Towards a future where Alzheimer’s disease pathology is stopped before the onset of dementia. Nat Aging 3, 494–505 (2023). https://doi.org/10.1038/s43587-023-00404-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-023-00404-2
This article is cited by
-
Modulation of hippocampal protein expression by a brain penetrant biologic TNF-α inhibitor in the 3xTg Alzheimer’s disease mice
Journal of Translational Medicine (2024)
-
Predicting cognitive scores from wearable-based digital physiological features using machine learning: data from a clinical trial in mild cognitive impairment
BMC Medicine (2024)
-
Use of a digital tool to support the diagnostic process in memory clinics–a usability study
Alzheimer's Research & Therapy (2024)
-
Recognition of cognitive dysfunction in hospitalised older patients: a flash mob study
BMC Geriatrics (2024)
-
Treatments for AD: towards the right target at the right time
Nature Reviews Neurology (2023)