Abstract
Alzheimer’s disease is increasing in prevalence disproportionately to longevity, placing enormous burden on individuals, families, healthcare systems and the economy. Pathological brain changes may occur decades before the symptoms of Alzheimer’s disease manifest. With new tools designed to identify at-risk individuals (for example, blood-based biomarkers and polygenic risk assessments), a focus on prevention could begin early and continue throughout life. Targetable risk factors associated mainly with cardiovascular and metabolic health have been established, while mental health factors are increasingly recognized. Given the heterogeneity of the risk and pathogenesis of Alzheimer’s disease, interventions for risk reduction are most effectively determined on an individualized basis. Here we review the evidence for a precision medicine approach to Alzheimer’s disease risk reduction that involves multidisciplinary expertise, with a new emphasis on novel genetic and biomarker advances, and psychological factors. We propose integration of this approach into routine clinical practice and acknowledge obstacles to the widespread practice of preventive neurology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$59.00 per year
only $4.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Gaugler, J. et al. 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 18, 700–789 (2022).
World Health Organization. WHO Dementia Fact Sheet. https://www.who.int/news-room/fact-sheets/detail/dementia (15 March 2023).
World Health Organization. Global Action Plan on the Public Health Response to Dementia 2017–2025. https://www.who.int/publications/i/item/global-action-plan-on-the-public-health-response-to-dementia-2017---2025 (7 December 2017).
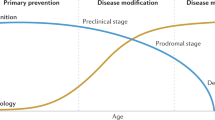
Vermunt, L. et al. Duration of preclinical, prodromal, and dementia stages of Alzheimer’s disease in relation to age, sex and APOE genotype. Alzheimers Dement. 15, 888–898 (2019).
Brookmeyer, R., Abdalla, N., Kawas, C. H. & Corrada, M. M. Forecasting the prevalence of preclinical and clinical Alzheimer’s disease in the United States. Alzheimers Dement. 14, 121–129 (2018).
Fan, L. et al. New insights into the pathogenesis of Alzheimer’s disease. Front. Neurol 10, 1312 (2020).
Pinto, T. C. C. et al. Is the Montreal Cognitive Assessment (MoCA) screening superior to the Mini-Mental State Examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer’s Disease (AD) in the elderly? Int. Psychogeriatr. 31, 491–504 (2019).
Livingston, G. et al. Dementia prevention, intervention and care: 2020 report of the Lancet Commission. Lancet 396, 413–446 (2020).
Rovner, B. W., Casten, R. J., Hegel, M. T. & Leiby, B. Preventing cognitive decline in black individuals with mild cognitive impairment: a randomized clinical trial. JAMA Neurol. 75, 1487–1493 (2018).
Blumenthal, J. A. et al. Lifestyle and neurocognition in older adults with cognitive impairments: a randomized trial. Neurology 92, e212–e223 (2019).
Kivipelto, M. et al. The Finnish Geriatric intervention study to prevent cognitive impairment and disability (FINGER): study design and progress. Alzheimers Dement. 9, 657–665 (2013).
Ngandu, T. et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 385, 2255–2263 (2015).
Liou, Y. J., Tsai, S. J., Bai, Y. M., Chen, T. J. & Chen, M. H. Dementia risk in middle-aged patients with schizophrenia, bipolar disorder and major depressive disorder: a cohort study of 84,824 subjects. Eur. Arch. Psychiatry Clin. Neurosci. 273, 219–227 (2022).
World Health Organization. Optimizing brain health across the life course: WHO position paper. https://www.who.int/publications/i/item/9789240054561 (9 August 2022).
Natalia, S. R. et al. The Brain Health Imperative in the 21st century—a call to action. Neurology 101, 570–579 (2023).
Oboudiyat, C., Glazer, H., Seifan, A., Greer, C. & Isaacson, R. S. Alzheimer’s disease. Semin. Neurol. 33, 313–329 (2013).
Isaacson, R. S. et al. The clinical practice of risk reduction for Alzheimer’s disease: a precision medicine approach. Alzheimers Dement. 14, 1663–1673 (2018).
Seifan, A. & Isaacson, R. The Alzheimer’s Prevention Clinic at Weill Cornell Medical College / New York – Presbyterian Hospital: risk stratification and personalized early intervention. J. Prev. Alzheimers Dis. 2, 254–266 (2015).
Niotis, K., Akiyoshi, K., Carlton, C. & Isaacson, R. Dementia prevention in clinical practice. Semin. Neurol. 42, 525–548 (2022).
Leggett, H. A rare mutation protects against Alzheimer’s disease, Stanford-led research finds. Standford Medicine News https://med.stanford.edu/news/all-news/2022/05/gene-mutation-alzheimers.html (31 May 2022).
Husain, M. A., Laurent, B. & Plourde, M. APOE and Alzheimer’s disease: from lipid transport to physiopathology and therapeutics. Front. Neurosci. 15, 630502 (2021).
Kivipelto, M. et al. Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann. Intern. Med. 137, 149–155 (2002).
Feringa, F. M. & van der Kant, R. Cholesterol and Alzheimer’s disease; from risk genes to pathological effects. Front. Aging Neurosci. 13, 690372 (2021).
Kao, Y.-C., Ho, P.-C., Tu, Y.-K., Jou, I.-M. & Tsai, K.-J. Lipids and Alzheimer’s disease. Int. J. Mol. Sci. 21, 1505 (2020).
Gamba, P. et al. A crosstalk between brain cholesterol oxidation and glucose metabolism in Alzheimer’s disease. Front. Neurosci. 13, 556 (2019).
Power, M. C. et al. Association of midlife lipids with 20-year cognitive change: a cohort study. Alzheimers Dement. 14, 167–177 (2018).
Ishii, M. Apolipoprotein B as a new link between cholesterol and Alzheimer disease. JAMA Neurol. 76, 751–753 (2019).
Gustaw-Rothenberg, K. Dietary patterns associated with Alzheimer’s disease: population based study. Int. J. Environ. Res. Public Health 6, 1335–1340 (2009).
Iwagami, M. et al. Blood cholesterol and risk of dementia in more than 1.8 million people over two decades: a retrospective cohort study. Lancet Healthy Longev. 2, e498–e506 (2021).
Gong, J., Harris, K., Peters, S. A. E. & Woodward, M. Serum lipid traits and the risk of dementia: a cohort study of 254,575 women and 214,891 men in the UK Biobank. eClinicalMedicine 54, 101695 (2022).
Behbodikhah, J. et al. Apolipoprotein B and cardiovascular disease: biomarker and potential therapeutic target. Metabolites 11, 690 (2021).
Tong, J.-h et al. Association of circulating apolipoprotein AI levels in patients with Alzheimer’s disease: a systematic review and meta-analysis. Front. Aging Neurosci. 14, 899175 (2022).
Brautbar, A. & Ballantyne, C. M. Pharmacological strategies for lowering LDL cholesterol: statins and beyond. Nat. Rev. Cardiol. 8, 253–265 (2011).
Olmastroni, E. et al. Statin use and risk of dementia or Alzheimer’s disease: a systematic review and meta-analysis of observational studies. Eur. J. Prev. Cardiol. 29, 804–814 (2021).
Rosoff, D. B. et al. Mendelian randomization study of PCSK9 and HMG-CoA reductase inhibition and cognitive function. J. Am. Coll. Cardiol. 80, 653–662 (2022).
Fassbender, K. et al. Simvastatin strongly reduces levels of Alzheimer’s disease β-amyloid peptides Aβ42 and Aβ40 in vitro and in vivo. Proc. Natl Acad. Sci. USA 98, 5856–5861 (2001).
Martins, I. J. et al. Cholesterol metabolism and transport in the pathogenesis of Alzheimer’s disease. J. Neurochem. 111, 1275–1308 (2009).
Cibičková, L. Statins and their influence on brain cholesterol. J. Clin. Lipidol. 5, 373–379 (2011).
Wisniewski, T., Newman, K. & Javitt, N. B. Alzheimer’s disease: brain desmosterol levels. J. Alzheimers Dis. 33, 881–888 (2013).
Carey, A. & Fossati, S. Hypertension and hyperhomocysteinemia as modifiable risk factors for Alzheimer’s disease and dementia: new evidence, potential therapeutic strategies and biomarkers. Alzheimers Dement. 19, 671–695 (2023).
Lennon, M. J., Makkar, S. R., Crawford, J. D. & Sachdev, P. S. Midlife hypertension and Alzheimer’s disease: a systematic review and meta-analysis. J. Alzheimers Dis. 71, 307–316 (2019).
Abell, J. G. et al. Association between systolic blood pressure and dementia in the Whitehall II cohort study: role of age, duration and threshold used to define hypertension. Eur. Heart J. 39, 3119–3125 (2018).
Williamson, J. D. et al. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. J. Am. Med. Assoc. 321, 553–561 (2019).
Hajjar, I. et al. Effects of candesartan vs lisinopril on neurocognitive function in older adults with executive mild cognitive impairment: a randomized clinical trial. JAMA Netw. Open 3, e2012252 (2020).
Liu, C.-H. et al. Telmisartan use and risk of dementia in type 2 diabetes patients with hypertension: a population-based cohort study. PLoS Med. 18, e1003707 (2021).
Marcum, Z. A., Gabriel, N., Bress, A. P. & Hernandez, I. Association of new use of antihypertensives that stimulate vs inhibit type 2 and 4 angiotensin II receptors with dementia among Medicare beneficiaries. JAMA Netw. Open 6, e2249370 (2023).
Holm, H. et al. Beta-blocker therapy and risk of vascular dementia: a population-based prospective study. Vasc. Pharmacol. 125–126, 106649 (2020).
Beaman, E. E. et al. Blood–brain barrier permeable β-blockers linked to lower risk of Alzheimer’s disease in hypertension. Brain 146, 1141–1151 (2023).
Mergenthaler, P., Lindauer, U., Dienel, G. A. & Meisel, A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 36, 587–597 (2013).
Talbot, K. Brain insulin resistance in Alzheimer’s disease and its potential treatment with GLP-1 analogs. Neurodegener. Dis. Manag. 4, 31–40 (2014).
Arnold, S. E. et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat. Rev. Neurol. 14, 168–181 (2018).
Biessels, G. J., Staekenborg, S., Brunner, E., Brayne, C. & Scheltens, P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 5, 64–74 (2006).
Butterfield, D. A. & Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 20, 148–160 (2019).
Crane, P. K. et al. Glucose levels and risk of dementia. N. Engl. J. Med. 369, 540–548 (2013).
Ethan, D. M. et al. Association between fluctuations in blood lipid levels over time with incident Alzheimer disease and Alzheimer disease related dementias. Neurology https://doi.org/10.1212/WNL.0000000000207595 (2023).
Bartoli, E., Fra, G. P. & Schianca, G. P. C. The oral glucose tolerance test (OGTT) revisited. Eur. J. Intern. Med. 22, 8–12 (2011).
Abdul-Ghani, M. A., Lyssenko, V., Tuomi, T., DeFronzo, R. A. & Groop, L. Fasting versus postload plasma glucose concentration and the risk for future type 2 diabetes: tesults from the Botnia Study. Diabetes Care 32, 281–286 (2009).
American Diabetes Association Professional Practice Committee. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2022. Diabetes Care 45, S17–S38 (2021).
Capozzi, M. E., DiMarchi, R. D., Tschöp, M. H., Finan, B. & Campbell, J. E. Targeting the incretin/glucagon system with triagonists to treat diabetes. Endocr. Rev. 39, 719–738 (2018).
Gilman, C. P. et al. Glucagon-like peptide 1 modulates calcium responses to glutamate and membrane depolarization in hippocampal neurons. J. Neurochem. 87, 1137–1144 (2003).
Perry, T. et al. A novel neurotrophic property of glucagon-like peptide 1: a promoter of nerve growth factor-mediated differentiation in PC12 cells. J. Pharmacol. Exp. Ther. 300, 958–966 (2002).
Nørgaard, C. H. et al. Treatment with glucagon-like peptide-1 receptor agonists and incidence of dementia: data from pooled double-blind randomized controlled trials and nationwide disease and prescription registers. Alzheimers Dement. 8, e12268 (2022).
Boccardi, V., Murasecco, I. & Mecocci, P. Diabetes drugs in the fight against Alzheimer’s disease. Ageing Res. Rev. 54, 100936 (2019).
Nianogo, R. A. et al. Risk factors associated with Alzheimer disease and related dementias by sex and race and ethnicity in the US. JAMA Neurol. 79, 584–591 (2022).
Baumgart, M. et al. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement. 11, 718–726 (2015).
Barnes, D. E. & Yaffe, K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 10, 819–828 (2011).
Racil, G. et al. Effects of high vs. moderate exercise intensity during interval training on lipids and adiponectin levels in obese young females. Eur. J. Appl. Physiol. 113, 2531–2540 (2013).
Søgaard, D. et al. High-intensity interval training improves insulin sensitivity in older individuals. Acta Physiol. 222, e13009 (2018).
Meng, Q., Lin, M. S. & Tzeng, I. S. Relationship between exercise and Alzheimer’s disease: a narrative literature review. Front. Neurosci. 14, 131 (2020).
Liu-Ambrose, T., Barha, C. K. & Best, J. R. Physical activity for brain health in older adults. Appl. Physiol. Nutr. Metab. 43, 1105–1112 (2018).
Suo, C. et al. Therapeutically relevant structural and functional mechanisms triggered by physical and cognitive exercise. Mol. Psychiatry 21, 1633–1642 (2016).
Buchman, A. S., Schneider, J. A., Leurgans, S. & Bennett, D. A. Physical frailty in older persons is associated with Alzheimer disease pathology. Neurology 71, 499–504 (2008).
Buchman, A. S., Wilson, R. S., Boyle, P. A., Bienias, J. L. & Bennett, D. A. Grip strength and the risk of incident Alzheimer’s disease. Neuroepidemiology 29, 66–73 (2007).
Boyle, P. A., Buchman, A. S., Wilson, R. S., Leurgans, S. E. & Bennett, D. A. Association of muscle strength with the risk of Alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Arch. Neurol. 66, 1339–1344 (2009).
Hsu, F.-C. et al. Adiposity is inversely associated with hippocampal volume in African Americans and European Americans with diabetes. J. Diabetes Complications 30, 1506–1512 (2016).
Morris, M. C. et al. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 11, 1007–1014 (2015).
Cherian, L. et al. Mediterranean–Dash Intervention for Neurodegenerative Delay (MIND) diet slows cognitive decline after stroke. J. Prev. Alzheimers Dis. 6, 267–273 (2019).
Scarmeas, N. et al. Mediterranean diet and mild cognitive impairment. Arch. Neurol. 66, 216–225 (2009).
Bartochowski, Z. et al. Dietary interventions to prevent or delay Alzheimer’s disease: what the evidence shows. Curr. Nutr. Rep. 9, 210–225 (2020).
Ventriglio, A. et al. Mediterranean diet and its benefits on health and mental health: a literature review. Clin. Pract. Epidemiol. Ment. Health 16, 156–164 (2020).
Stefaniak, O., Dobrzyńska, M., Drzymała-Czyż, S. & Przysławski, J. Diet in the prevention of Alzheimer’s disease: current knowledge and future research requirements. Nutrients 14, 4564 (2022).
Rapoport, S. I. & Igarashi, M. Can the rat liver maintain normal brain DHA metabolism in the absence of dietary DHA? Prostaglandins Leukot. Essent. Fatty Acids 81, 119–123 (2009).
Grimm, M. O. et al. Docosahexaenoic acid reduces amyloid β production via multiple pleiotropic mechanisms. J. Biol. Chem. 286, 14028–14039 (2011).
Hooijmans, C. et al. Changes in cerebral blood volume and amyloid pathology in aged Alzheimer APP/PS1 mice on a docosahexaenoic acid (DHA) diet or cholesterol enriched Typical Western Diet (TWD). Neurobiol. Dis. 28, 16–29 (2007).
Delrieu, J. et al. Multidomain intervention and/or omega-3 in nondemented elderly subjects according to amyloid status. Alzheimers Dement. 15, 1392–1401 (2019).
Chu, C.-S., Hung, C.-F., Ponnusamy, V. K., Chen, K.-C. & Chen, N.-C. Higher serum DHA and slower cognitive decline in patients with Alzheimer’s disease: two-year follow-up. Nutrients 14, 1159 (2022).
Grosso, G. et al. Omega-3 fatty acids and depression: scientific evidence and biological mechanisms. Oxid. Med. Cell. Longev. 2014, 313570 (2014).
van de Rest, O. et al. Effect of fish oil on cognitive performance in older subjects: a randomized, controlled trial. Neurology 71, 430–438 (2008).
Dangour, A. D. et al. Effect of 2–y n–3 long-chain polyunsaturated fatty acid supplementation on cognitive function in older people: a randomized, double-blind, controlled trial. Am. J. Clin. Nutrition 91, 1725–1732 (2010).
Mazereeuw, G. et al. Omega-3 fatty acids, depressive symptoms, and cognitive performance in patients with coronary artery disease: analyses from a randomized, double-blind, placebo-controlled trial. J. Clin. Psychopharmacol. 36, 436–444 (2016).
Geleijnse, J. M., Giltay, E. J. & Kromhout, D. Effects of n-3 fatty acids on cognitive decline: a randomized, double-blind, placebo-controlled trial in stable myocardial infarction patients. Alzheimers Dement. 8, 278–287 (2012).
Ichinose, T. et al. Intake of docosahexaenoic acid-enriched milk beverage prevents age-related cognitive decline and decreases serum bone resorption marker levels. J. Oleo Sci. 70, 1829–1838 (2021).
Kang, J. H. et al. Marine n–3 fatty acids and cognitive change among older adults in the VITAL randomized trial. Alzheimers Dement. 8, e12288 (2022).
Arellanes, I. C. et al. Brain delivery of supplemental docosahexaenoic acid (DHA): a randomized placebo-controlled clinical trial. EBioMedicine 59, 102883 (2020).
Wang, Q., Zhao, J., Chang, H., Liu, X. & Zhu, R. Homocysteine and folic acid: risk factors for Alzheimer’s disease—an updated meta-analysis. Front. Aging Neurosci. 13, 665114 (2021).
Jernerén, F. et al. Homocysteine status modifies the treatment effect of omega-3 fatty acids on cognition in a randomized clinical trial in mild to moderate Alzheimer’s disease: the OmegAD Study. J. Alzheimers Dis. 69, 189–197 (2019).
Freund-Levi, Y. et al. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD Study: a randomized double-blind trial. Arch. Neurol. 63, 1402–1408 (2006).
Malik, A. et al. ω-3 ethyl ester results in better cognitive function at 12 and 30 months than control in cognitively healthy subjects with coronary artery disease: a secondary analysis of a randomized clinical trial. Am. J. Clin. Nutr. 113, 1168–1176 (2021).
Brickman, A. M. et al. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat. Neurosci. 17, 1798–1803 (2014).
Mastroiacovo, D. et al. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: the Cocoa, Cognition and Aging (CoCoA) Study—a randomized controlled trial. Am. J. Clin. Nutr. 101, 538–548 (2015).
Holland, T. M. et al. Dietary flavonols and risk of Alzheimer dementia. Neurology 94, e1749–e1756 (2020).
Desideri, G. et al. Benefits in cognitive function, blood pressure and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment. Hypertension 60, 794–801 (2012).
Peeke, P. M., Greenway, F. L., Billes, S. K., Zhang, D. & Fujioka, K. Effect of time restricted eating on body weight and fasting glucose in participants with obesity: results of a randomized, controlled, virtual clinical trial. Nutr. Diabetes 11, 6 (2021).
Gudden, J., Arias Vasquez, A. & Bloemendaal, M. The effects of intermittent fasting on brain and cognitive function. Nutrients 13, 3166 (2021).
Lowe, D. A. et al. Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: the TREAT randomized clinical trial. JAMA Intern. Med. 180, 1491–1499 (2020).
Brocchi, A., Rebelos, E., Dardano, A., Mantuano, M. & Daniele, G. Effects of intermittent fasting on brain metabolism. Nutrients 14, 1275 (2022).
Ooi, T. C. et al. Intermittent fasting enhanced the cognitive function in older adults with mild cognitive impairment by inducing biochemical and metabolic changes: a 3-year progressive study. Nutrients 12, 2644 (2020).
Ismail, Z. et al. Psychosis in Alzheimer disease—mechanisms, genetics and therapeutic opportunities. Nat. Rev. Neurol. 18, 131–144 (2022).
Qiu, J., Goldstein, F. C. & Hanfelt, J. J. An exploration of subgroups of neuropsychiatric symptoms in mild cognitive impairment and their risks of conversion to dementia or death. Am. J. Geriatr. Psychiatry 30, 925–934 (2022).
Martin, E. & Velayudhan, L. Neuropsychiatric symptoms in mild cognitive impairment: a literature review. Dement. Geriatr. Cogn. Disord. 49, 146–155 (2020).
Forrester, S. N., Gallo, J. J., Smith, G. S. & Leoutsakos, J. M. Patterns of neuropsychiatric symptoms in mild cognitive impairment and risk of dementia. Am. J. Geriatr. Psychiatry 24, 117–125 (2016).
Singh-Manoux, A. et al. Trajectories of depressive symptoms before diagnosis of dementia: a 28-year follow-up study. JAMA Psychiatry 74, 712–718 (2017).
Jang, Y. J. et al. Additive interaction of mid- to late-life depression and cerebrovascular disease on the risk of dementia: a nationwide population-based cohort study. Alzheimers Res. Ther. 13, 61 (2021).
Yang, W. et al. Association of life-course depression with the risk of dementia in late life: a nationwide twin study. Alzheimers Dement. 17, 1383–1390 (2021).
Modrego, P. J. & Ferrández, J. Depression in patients with mild cognitive impairment increases the risk of developing dementia of Alzheimer type: a prospective cohort study. Arch. Neurol. 61, 1290–1293 (2004).
Dafsari, F. S. & Jessen, F. Depression—an underrecognized target for prevention of dementia in Alzheimer’s disease. Transl. Psychiatry 10, 160 (2020).
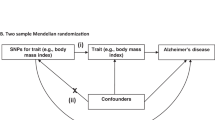
Harerimana, N. V. et al. Genetic evidence supporting a causal role of depression in Alzheimer’s disease. Biol. Psychiatry 92, 25–33 (2022).
Brenowitz, W. D. et al. Depressive symptoms imputed across the life course are associated with cognitive impairment and cognitive decline. J. Alzheimers Dis. 83, 1379–1389 (2021).
Wang, Z.-T. et al. Associations of the rate of change in geriatric depression scale with amyloid and cerebral glucose metabolism in cognitively normal older adults: a longitudinal study. J. Affect. Disord. 280, 77–84 (2021).
Bouter, Y. & Bouter, C. Selective serotonin reuptake inhibitor-treatment does not show beneficial effects on cognition or amyloid burden in cognitively impaired and cognitively normal subjects. Front. Aging Neurosci. 14, 883256 (2022).
Skoog, I. et al. A 9-year prospective population-based study on the association between the APOE*E4 allele and late-life depression in Sweden. Biol. Psychiatry 78, 730–736 (2015).
Sheline, Y. I. et al. An antidepressant decreases CSF Aβ production in healthy individuals and in transgenic AD mice. Sci. Transl. Med. 6, 236re234 (2014).
Cirrito, J. R. et al. Serotonin signaling is associated with lower amyloid-β levels and plaques in transgenic mice and humans. Proc. Natl Acad. Sci. USA 108, 14968–14973 (2011).
Brendel, M. et al. Serotonin selective reuptake inhibitor treatment improves cognition and grey matter atrophy but not amyloid burden during two-year follow-up in mild cognitive impairment and Alzheimer’s disease patients with depressive symptoms. J. Alzheimers Dis. 65, 793–806 (2018).
Burke, S. L., Maramaldi, P., Cadet, T. & Kukull, W. Decreasing hazards of Alzheimer’s disease with the use of antidepressants: mitigating the risk of depression and apolipoprotein E. Int. J. Geriatr. Psychiatry 33, 200–211 (2018).
Tsiouris, J. A., Patti, P. J. & Flory, M. J. Effects of antidepressants on longevity and dementia onset among adults with Down syndrome: a retrospective study. J. Clin. Psychiatry 75, 731–737 (2014).
Bartels, C., Wagner, M., Wolfsgruber, S., Ehrenreich, H. & Schneider, A. Impact of SSRI therapy on risk of conversion from mild cognitive impairment to Alzheimer’s dementia in individuals with previous depression. Am. J. Psychiatry 175, 232–241 (2018).
Alzheimer’s Association. Trajectory Report. https://www.alz.org/help-support/resources/publications/trajectory_report (2022).
Mdawar, B., Ghossoub, E. & Khoury, R. Selective serotonin reuptake inhibitors and Alzheimer’s disease. Neural Regen. Res. 15, 41–46 (2020).
Nykamp, M. J. et al. Opportunities for drug repurposing of serotonin reuptake inhibitors: potential uses in inflammation, infection, cancer, neuroprotection and Alzheimer’s disease prevention. Pharmacopsychiatry 55, 24–29 (2021).
Martínez-Cengotitabengoa, M. & González-Pinto, A. Nutritional supplements in depressive disorders. Actas Esp. Psiquiatr. 45, 8–15 (2017).
Fernández-Rodríguez, R. et al. Does intermittent fasting impact mental disorders? A systematic review with meta-analysis. Crit. Rev. Food Sci. Nutr. https://doi.org/10.1080/10408398.2022.2088687 (2022).
Sussams, R. et al. Psychological stress, cognitive decline and the development of dementia in amnestic mild cognitive impairment. Sci. Rep. 10, 3618 (2020).
Canet, G. et al. Is AD a stress-related disorder? Focus on the HPA axis and its promising therapeutic targets. Front. Aging Neurosci. 11, 269 (2019).
Khalsa, D. S. Stress, meditation and Alzheimer’s disease prevention: where the evidence stands. J. Alzheimers Dis. 48, 1–12 (2015).
Salzman, C. Do benzodiazepines cause Alzheimer’s disease? Am. J. Psychiatry 177, 476–478 (2020).
Irwin, M. R. & Vitiello, M. V. Implications of sleep disturbance and inflammation for Alzheimer’s disease dementia. Lancet Neurol. 18, 296–306 (2019).
Minakawa, E. N., Wada, K. & Nagai, Y. Sleep disturbance as a potential modifiable risk factor for Alzheimer’s disease. Int. J. Mol. Sci. 20, 803 (2019).
Wang, C. & Holtzman, D. M. Bidirectional relationship between sleep and Alzheimer’s disease: role of amyloid, tau, and other factors. Neuropsychopharmacology 45, 104–120 (2020).
Lucey, B. P. It’s complicated: the relationship between sleep and Alzheimer’s disease in humans. Neurobiol. Dis. 144, 105031 (2020).
Saif, N., Sadek, G., Bellara, S., Hristov, H. & Isaacson, R. S. Brain health and dementia risk reduction. Pract. Neurol. https://practicalneurology.com/articles/2019-june/brain-health-dementia-risk-reduction (2019).
Bubu, O. M. et al. Interactive associations of neuropsychiatry inventory-questionnaire assessed sleep disturbance and vascular risk on Alzheimer’s disease stage progression in clinically normal older adults. Front. Aging Neurosci. 13, 763264 (2021).
Shokri-Kojori, E. et al. β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc. Natl Acad. Sci. USA 115, 4483–4488 (2018).
Pase, M. P. et al. Sleep architecture and the risk of incident dementia in the community. Neurology 89, 1244–1250 (2017).
Ooms, S. et al. Effect of 1 night of total sleep deprivation on cerebrospinal fluid β-amyloid 42 in healthy middle-aged men: a randomized clinical trial. JAMA Neurol. 71, 971–977 (2014).
Cassidy-Eagle, E., Siebern, A., Unti, L., Glassman, J. & O’Hara, R. Neuropsychological functioning in older adults with mild cognitive impairment and insomnia randomized to CBT-I or control group. Clin. Gerontol. 41, 136–144 (2018).
Montgomery, P. & Dennis, J. A systematic review of non-pharmacological therapies for sleep problems in later life. Sleep Med. Rev. 8, 47–62 (2004).
Trauer, J. M., Qian, M. Y., Doyle, J. S., Rajaratnam, S. M. & Cunnington, D. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann. Intern. Med. 163, 191–204 (2015).
Alessi, C. et al. Cognitive behavioral therapy for insomnia in older veterans using nonclinician sleep coaches: randomized controlled trial. J. Am. Geriatr. Soc. 64, 1830–1838 (2016).
Liguori, C. et al. Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. JAMA Neurol. 71, 1498–1505 (2014).
Yulug, B., Hanoglu, L. & Kilic, E. Does sleep disturbance affect the amyloid clearance mechanisms in Alzheimer’s disease? Psychiatry Clin. Neurosci. 71, 673–677 (2017).
Lucey, B. P. et al. Suvorexant acutely decreases tau phosphorylation and Aβ in the human CNS. Ann. Neurol. 94, 27–40 (2023).
Ruthirakuhan, M. et al. Use of physical and intellectual activities and socialization in the management of cognitive decline of aging and in dementia: a review. J. Aging Res. 2012, 384875 (2012).
Huang, H. et al. Isolation housing exacerbates Alzheimer’s disease-like pathophysiology in aged APP/PS1 mice. Int. J. Neuropsychopharmacol. 18, pyu116 (2015).
Friedler, B., Crapser, J. & McCullough, L. One is the deadliest number: the detrimental effects of social isolation on cerebrovascular diseases and cognition. Acta Neuropathol. 129, 493–509 (2015).
Stern, Y. What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 8, 448–460 (2002).
Landau, S. M. et al. Association of lifetime cognitive engagement and low β-amyloid deposition. Arch. Neurol. 69, 623–629 (2012).
Olatunji, B. O. et al. Cognitive-behavioral therapy for hypochondriasis/health anxiety: a meta-analysis of treatment outcome and moderators. Behav. Res. Ther. 58, 65–74 (2014).
Ihle, A. et al. The association of leisure activities in middle adulthood with cognitive performance in old age: the moderating role of educational level. Gerontology 61, 543–550 (2015).
Leggieri, M. et al. Music intervention approaches for Alzheimer’s disease: a review of the literature. Front. Neurosci. 13, 132 (2019).
Bae, S. et al. Engagement in lifestyle activities is associated with increased Alzheimer’s disease-associated cortical thickness and cognitive performance in older adults. J. Clin. Med. 9, 1424 (2020).
Valenzuela, M. J. & Sachdev, P. Brain reserve and dementia: a systematic review. Psychol. Med. 36, 441–454 (2006).
Basak, C., Boot, W. R., Voss, M. W. & Kramer, A. F. Can training in a real-time strategy video game attenuate cognitive decline in older adults? Psychol. Aging 23, 765–777 (2008).
Hall, C. B. et al. Cognitive activities delay onset of memory decline in persons who develop dementia. Neurology 73, 356–361 (2009).
Ball, K. et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. J. Am. Med. Assoc. 288, 2271–2281 (2002).
Edwards, J. D. et al. Speed of processing training results in lower risk of dementia. Alzheimers Dement. 3, 603–611 (2017).
Boyle, R. et al. Verbal intelligence is a more robust cross-sectional measure of cognitive reserve than level of education in healthy older adults. Alzheimers Res. Ther. 13, 128 (2021).
Hackett, K. et al. Utility of the NIH Toolbox for assessment of prodromal Alzheimer’s disease and dementia. Alzheimers Dement. 10, 764–772 (2018).
Ribe, A. R. et al. Long-term risk of dementia in persons with schizophrenia: a Danish population-based cohort study. JAMA Psychiatry 72, 1095–1101 (2015).
Cai, L. & Huang, J. Schizophrenia and risk of dementia: a meta-analysis study. Neuropsychiatr. Dis. Treat. 14, 2047–2055 (2018).
Koutsouleris, N. et al. Exploring links between psychosis and frontotemporal dementia using multimodal machine learning: dementia praecox revisited. JAMA Psychiatry 79, 907–919 (2022).
Lin, C. E., Chung, C. H., Chen, L. F. & Chi, M. J. Increased risk of dementia in patients with schizophrenia: a population-based cohort study in Taiwan. Eur. Psychiatry 53, 7–16 (2018).
Jonas, K., Abi-Dargham, A. & Kotov, R. Two hypotheses on the high incidence of dementia in psychotic disorders. JAMA Psychiatry 78, 1305–1306 (2021).
Koppel, J. et al. Haloperidol inactivates AMPK and reduces tau phosphorylation in a tau mouse model of Alzheimer’s disease. Alzheimers Dement. 2, 121–130 (2016).
McCormick, A. V., Wheeler, J. M., Guthrie, C. R., Liachko, N. F. & Kraemer, B. C. Dopamine D2 receptor antagonism suppresses tau aggregation and neurotoxicity. Biol. Psychiatry 73, 464–471 (2013).
DeMichele-Sweet, M. A. A. et al. Genetic risk for schizophrenia and psychosis in Alzheimer disease. Mol. Psychiatry 23, 963–972 (2018).
Dietlin, S. et al. Neuropsychiatric symptoms and risk of progression to Alzheimer’s disease among mild cognitive impairment subjects. J. Alzheimers Dis. 70, 25–34 (2019).
Fischer, C. E. & Agüera-Ortiz, L. Psychosis and dementia: risk factor, prodrome or cause? Int. Psychogeriatr. 30, 209–219 (2018).
Cohen, C. I. Very late-onset schizophrenia-like psychosis: positive findings but questions remain unanswered. Lancet Psychiatry 5, 528–529 (2018).
Berkowitz, C. L. et al. Clinical application of APOE in Alzheimer’s prevention: a precision medicine approach. J. Prev. Alzheimers Dis. 5, 245–252 (2018).
Emrani, S., Arain, H. A., DeMarshall, C. & Nuriel, T. APOE4 is associated with cognitive and pathological heterogeneity in patients with Alzheimer’s disease: a systematic review. Alzheimers Res. Ther. 12, 141 (2020).
Richardson, J. R. et al. Elevated serum pesticide levels and risk for Alzheimer disease. JAMA Neurology 71, 284–290 (2014).
Maddock, J., Cavadino, A., Power, C. & Hyppönen, E. 25-hydroxyvitamin D, APOE ɛ4 genotype and cognitive function: findings from the 1958 British birth cohort. Eur. J. Clin. Nutr. 69, 505–508 (2015).
Tokgöz, S. & Claassen, J. Exercise as potential therapeutic target to modulate Alzheimer’s disease pathology in APOE ε4 carriers: a systematic review. Cardiol. Ther. 10, 67–88 (2021).
Torrandell-Haro, G. et al. Statin therapy and risk of Alzheimer’s and age-related neurodegenerative diseases. Alzheimers Dement. 6, e12108 (2020).
Geifman, N., Brinton, R. D., Kennedy, R. E., Schneider, L. S. & Butte, A. J. Evidence for benefit of statins to modify cognitive decline and risk in Alzheimer’s disease. Alzheimers Res. Ther. 9, 10 (2017).
Lambert, J.-C., Ramirez, A., Grenier-Boley, B. & Bellenguez, C. Step by step: towards a better understanding of the genetic architecture of Alzheimer’s disease. Mol. Psychiatry 28, 2716–2727 (2023).
Jonsson, T. et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med. 368, 107–116 (2012).
Li, J. T. & Zhang, Y. TREM2 regulates innate immunity in Alzheimer’s disease. J. Neuroinflammation 15, 107 (2018).
Krasemann, S. et al. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47, 566–581 (2017).
Yeh, F. L., Wang, Y., Tom, I., Gonzalez, L. C. & Sheng, M. TREM2 binds to apolipoproteins, including APOE and CLU/APOJ, and thereby facilitates uptake of amyloid-beta by microglia. Neuron 91, 328–340 (2016).
Román, G. C., Mancera-Páez, O. & Bernal, C. Epigenetic factors in late-onset Alzheimer’s disease: MTHFR and CTH gene polymorphisms, metabolic transsulfuration and methylation pathways, and B vitamins. Int. J. Mol. Sci. 20, 319 (2019).
Jett, S. et al. Endogenous and exogenous estrogen exposures: how women’s reproductive health can drive brain aging and inform Alzheimer’s prevention. Front. Aging Neurosci. 14, 831807 (2022).
Brinton, R. D., Yao, J., Yin, F., Mack, W. J. & Cadenas, E. Perimenopause as a neurological transition state. Nat. Rev. Endocrinol. 11, 393–405 (2015).
Rocca, W. et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology 69, 1074–1083 (2007).
Phung, T. K. et al. Hysterectomy, oophorectomy and risk of dementia: a nationwide historical cohort study. Dement. Geriatr. Cogn. Disord. 30, 43–50 (2010).
Rettberg, J. R., Yao, J. & Brinton, R. D. Estrogen: a master regulator of bioenergetic systems in the brain and body. Front. Neuroendocrinol. 35, 8–30 (2014).
LeBlanc, E. S., Janowsky, J., Chan, B. K. & Nelson, H. D. Hormone replacement therapy and cognition: systematic review and meta-analysis. J. Am. Med. Assoc. 285, 1489–1499 (2001).
Maki, P. M. Critical window hypothesis of hormone therapy and cognition: a scientific update on clinical studies. Menopause 20, 695–709 (2013).
Saleh, R. N. M., Hornberger, M., Ritchie, C. W. & Minihane, A. M. Hormone replacement therapy is associated with improved cognition and larger brain volumes in at-risk APOE4 women: results from the European Prevention of Alzheimer’s Disease (EPAD) cohort. Alzheimers Res. Ther. 15, 10 (2023).
Shumaker, S. A. et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. J. Am. Med. Assoc. 289, 2651–2662 (2003).
Podcasy, J. L. & Epperson, C. N. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin. Neurosci. 18, 437–446 (2016).
Leeners, B., Geary, N., Tobler, P. N. & Asarian, L. Ovarian hormones and obesity. Hum. Reprod. Update 23, 300–321 (2017).
Lovejoy, J. C., Champagne, C. M., de Jonge, L., Xie, H. & Smith, S. R. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int. J. Obes. 32, 949–958 (2008).
Santos, R. D. et al. Low- and high-density lipoprotein cholesterol goal attainment in dyslipidemic women: the Lipid Treatment Assessment Project (L-TAP) 2. Am. Heart J. 158, 860–866 (2009).
Rabi, D. M. et al. Reporting on sex-based analysis in clinical trials of angiotensin-converting enzyme inhibitor and angiotensin receptor blocker efficacy. Can. J. Cardiol. 24, 491–496 (2008).
Andrew, M. K. & Tierney, M. C. The puzzle of sex, gender and Alzheimer’s disease: why are women more often affected than men? Womens Health 14, 1745506518817995 (2018).
Barha, C. K., Davis, J. C., Falck, R. S., Nagamatsu, L. S. & Liu-Ambrose, T. Sex differences in exercise efficacy to improve cognition: a systematic review and meta-analysis of randomized controlled trials in older humans. Front. Neuroendocrinol. 46, 71–85 (2017).
Mungas, D. in Ethnicity and the Dementias Vol. 2 (eds Yeo, G. & Gallager-Thompson, D.) 93–108 (Routledge, 2013).
Zissimopoulos, J. M., Barthold, D., Brinton, R. D. & Joyce, G. Sex and race differences in the association between statin use and the incidence of Alzheimer disease. JAMA Neurol. 74, 225–232 (2017).
Powell, D. S. et al. The relationship of APOE ε4, race, and sex on the age of onset and risk of dementia. Front. Neurol. 12, 735036 (2021).
Morris, J. C. et al. Assessment of racial disparities in biomarkers for Alzheimer disease. JAMA Neurol. 76, 264–273 (2019).
Manly, J. J. et al. Estimating the prevalence of dementia and mild cognitive impairment in the US: the 2016 health and retirement study harmonized cognitive assessment protocol project. JAMA Neurol. 79, 1242–1249 (2022).
Babulal, G. M. et al. Perspectives on ethnic and racial disparities in Alzheimer’s disease and related dementias: update and areas of immediate need. Alzheimers Dement. 15, 292–312 (2019).
Fu, P. & Yung, K. K. L. Air pollution and Alzheimer’s disease: a systematic review and meta-analysis. J. Alzheimers Dis. 77, 701–714 (2020).
Peters, R. et al. Air pollution and dementia: a systematic review. J. Alzheimers Dis. 70, S145–S163 (2019).
Schikowski, T. & Altuğ, H. The role of air pollution in cognitive impairment and decline. Neurochem. Int. 136, 104708 (2020).
Kravitz-Wirtz, N., Crowder, K., Hajat, A. & Sass, V. The long-term dynamics of racial/ethnic inequality in neighbourhood air pollution exposure, 1990–2009. Du Bois Rev. 13, 237–259 (2016).
Quiroz, Y. T. et al. Addressing the disparities in dementia risk, early detection and care in Latino populations: highlights from the second Latinos & Alzheimer’s Symposium. Alzheimers Dement. 18, 1677–1686 (2022).
Collins, J. C. & Rocco, T. S. Disparities in healthcare for racial, ethnic and sexual minorities. New Dir. Adult Cont. Educ. 2014, 5–14 (2014).
Lin, P.-J. et al. Racial and ethnic differences in knowledge about one’s dementia status. J. Am. Geriatr. Soc. 68, 1763–1770 (2020).
Huggins, L. K. L. et al. Interventions to promote dementia knowledge among racial/ethnic minority groups: a systematic review. J. Am. Geriatr. Soc. 70, 609–621 (2022).
Crawford, K. et al. Golgi apparatus, endoplasmic reticulum and mitochondrial function implicated in Alzheimer’s disease through polygenic risk and RNA sequencing. Mol. Psychiatry 28, 1327–1336 (2023).
Gouveia, C. et al. Genome-wide association of polygenic risk extremes for Alzheimer’s disease in the UK Biobank. Sci. Rep. 12, 8404 (2022).
Deckers, K. et al. Long-term dementia risk prediction by the LIBRA score: a 30-year follow-up of the CAIDE study. Int. J. Geriatr. Psychiatry 35, 195–203 (2020).
Kootar, S. et al. Validation of the CogDrisk instrument as predictive of dementia in four general community-dwelling populations. J. Prev. Alzheimers Dis. 10, 478–487 (2023).
Melis, A. et al. Development and validation of a dementia risk score in the UK Biobank and Whitehall II cohorts. BMJ Mental Health 26, e300719 (2023).
Wilker, E. H., Osman, M. & Weisskopf, M. G. Ambient air pollution and clinical dementia: systematic review and meta-analysis. Brit. Med. J. 381, e071620 (2023).
Harris, K. et al. The impact of routine vaccinations on Alzheimer’s disease risk in persons 65 years and older: a claims-based cohort study using propensity score matching. J. Alzheimers Dis. 95, 703–718 (2023).
Sabia, S. et al. Association of sleep duration in middle and old age with incidence of dementia. Nat. Commun. 12, 2289 (2021).
Baker, E. & Escott-Price, V. Polygenic risk scores in Alzheimer’s disease: current applications and future directions. Front. Digit. Health 2, 14 (2020).
Leonenko, G. et al. Identifying individuals with high risk of Alzheimer’s disease using polygenic risk scores. Nat. Commun. 12, 4506 (2021).
Niotis, K. et al. Feasibility of incorporating direct-to-consumer genomics for individualized dementia risk reduction in clinical practice. J. Gen. Intern. Med. 37, S145–S146 (2022).
Reitz, C., Pericak-Vance, M. A., Foroud, T. & Mayeux, R. A global view of the genetic basis of Alzheimer disease. Nat. Rev. Neurol. 19, 261–277 (2023).
Lake, J. et al. Multi-ancestry meta-analysis and fine-mapping in Alzheimer’s disease. Mol. Psychiatry 28, 3121–3132 (2023).
Smith, R. et al. Clinical utility of tau positron emission tomography in the diagnostic workup of patients with cognitive symptoms. JAMA Neurol. 80, 749–756 (2023).
Joyce, R. C. et al. Blood-based high sensitivity measurements of beta-amyloid and phosphorylated tau as biomarkers of Alzheimer’s disease: a focused review on recent advances. J. Neurol. Neurosurg. Psychiatry 92, 1231–1241 (2021).
Thijssen, E. H. et al. Highly specific and ultrasensitive plasma test detects Abeta(1–42) and Abeta(1–40) in Alzheimer’s disease. Sci. Rep. 11, 9736 (2021).
Schindler, S. E. et al. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 93, e1647–e1659 (2019).
Giudici, K. V. et al. Assessment of plasma amyloid-β42/40 and cognitive decline among community-dwelling older adults. JAMA Netw. Open 3, e2028634 (2020).
Ashton, N. J. et al. Plasma p-tau231: a new biomarker for incipient Alzheimer’s disease pathology. Acta Neuropathol. 141, 709–724 (2021).
Mattsson-Carlgren, N. et al. Prediction of longitudinal cognitive decline in preclinical Alzheimer disease using plasma biomarkers. JAMA Neurol. 80, 360–369 (2023).
Janelidze, S. et al. Associations of plasma phospho-tau217 levels with tau positron emission tomography in early Alzheimer disease. JAMA Neurol. 78, 149–156 (2021).
Mattsson-Carlgren, N. et al. Longitudinal plasma p-tau217 is increased in early stages of Alzheimer’s disease. Brain 143, 3234–3241 (2020).
Cicognola, C. et al. Plasma glial fibrillary acidic protein detects Alzheimer pathology and predicts future conversion to Alzheimer dementia in patients with mild cognitive impairment. Alzheimers Res. Ther. 13, 68 (2021).
Benedet, A. L. et al. Differences between plasma and cerebrospinal fluid glial fibrillary acidic protein levels across the Alzheimer disease continuum. JAMA Neurol. 78, 1471–1483 (2021).
Beyer, L. et al. Amyloid-beta misfolding and GFAP predict risk of clinical Alzheimer’s disease diagnosis within 17 years. Alzheimers Dement. https://doi.org/10.1002/alz.12745 (2022).
Brickman, A. M. et al. Plasma p-tau181, p-tau217, and other blood-based Alzheimer’s disease biomarkers in a multi-ethnic, community study. Alzheimers Dement. 17, 1353–1364 (2021).
Teunissen, C. E. et al. Blood-based biomarkers for Alzheimer’s disease: towards clinical implementation. Lancet Neurol. 21, 66–77 (2022).
Caprioglio, C. et al. Analysis of psychological symptoms following disclosure of amyloid-positron emission tomography imaging results to adults with subjective cognitive decline. JAMA Netw. Open 6, e2250921 (2023).
Simple Finger Prick Test Exemplifies Advances in Alzheimer’s Disease Blood Tests (Alzheimer’s Association, 2023).
Sannemann, L. et al. Neuropsychiatric symptoms in at-risk groups for AD dementia and their association with worry and AD biomarkers—results from the DELCODE study. Alzheimers Res. Ther. 12, 131 (2020).
Lanctôt, K. L. et al. Neuropsychiatric signs and symptoms of Alzheimer’s disease: new treatment paradigms. Alzheimers Dement. 3, 440–449 (2017).
Ismail, Z. & Mortby, M. E. in Mental Health and Illness of the Elderly (eds Chiu, H. & Shulman, K.) 343–368 (Springer, 2017).
Howes, F., Warnecke, E. & Nelson, M. Barriers to lifestyle risk factor assessment and management in hypertension: a qualitative study of Australian general practitioners. J. Hum. Hypertens. 27, 474–478 (2013).
Baucom, K. J. W. et al. Barriers to participation and lifestyle change among lower versus higher income participants in the National Diabetes Prevention Program: lifestyle coach perspectives. Transl. Behav. Med. 12, 860–869 (2022).
Isaacson, R. S. & Saif, N. A missed opportunity for dementia prevention? Current challenges for early detection and modern-day solutions. J. Prev. Alzheimers Dis. 7, 291–293 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT06027320 (2023).
Fuhrer, R., Dufouil, C. & Dartigues, J. F. PAQUID Study. Exploring sex differences in the relationship between depressive symptoms and dementia incidence: prospective results from the PAQUID Study. J. Am. Geriatr. Soc. 51, 1055–1063 (2003).
Szoeke, C. E. et al. The Women’s Healthy Ageing Project: fertile ground for investigation of healthy participants ‘at risk’ for dementia. Int. Rev. Psychiatry 25, 726–737 (2013).
Bromberger, J. T. et al. Major depression during and after the menopausal transition: Study of Women’s Health Across the Nation (SWAN). Psychol. Med. 41, 1879–1888 (2011).
Peavy, G. M. et al. The effects of prolonged stress and APOE genotype on memory and cortisol in older adults. Biol. Psychiatry 62, 472–478 (2007).
Wilson, R. S., Begeny, C. T., Boyle, P. A., Schneider, J. A. & Bennett, D. A. Vulnerability to stress, anxiety, and development of dementia in old age. Am. J. Geriatr. Psychiatry 19, 327–334 (2011).
Epel, E. S. et al. Meditation and vacation effects have an impact on disease-associated molecular phenotypes. Transl. Psychiatry 6, e880 (2016).
Bowling, A. Social networks and social support among older people and implications for emotional well-being and psychiatric morbidity. Int. Rev. Psychiatry 6, 41–58 (1994).
Acknowledgements
R.S.I. has received research funding from the US National Institutes of Health (NIH 4R44AG071416-02).
Author information
Authors and Affiliations
Contributions
K.N., C.S. and R.S.I. contributed equally to the design and oversight of the manuscript. All authors contributed equally to the writing and editing, and reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
R.I. and K.N. are scientific advisors to Retain Health. All other authors declare no competing interests.
Peer review
Peer review information
Nature Mental Health thanks Minerva Carrasquillo, Kirk Daffner and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
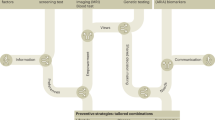
Application of our individualized approach to AD risk reduction management in clinical practice.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Niotis, K., Saperia, C., Saif, N. et al. Alzheimer’s disease risk reduction in clinical practice: a priority in the emerging field of preventive neurology. Nat. Mental Health 2, 25–40 (2024). https://doi.org/10.1038/s44220-023-00191-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44220-023-00191-0