Abstract
A defining feature of biology is the use of a multiscale architecture, ranging from molecular networks to cells, tissues, organs, whole bodies, and swarms. Crucially however, biology is not only nested structurally, but also functionally: each level is able to solve problems in distinct problem spaces, such as physiological, morphological, and behavioral state space. Percolating adaptive functionality from one level of competent subunits to a higher functional level of organization requires collective dynamics: multiple components must work together to achieve specific outcomes. Here we overview a number of biological examples at different scales which highlight the ability of cellular material to make decisions that implement cooperation toward specific homeodynamic endpoints, and implement collective intelligence by solving problems at the cell, tissue, and whole-organism levels. We explore the hypothesis that collective intelligence is not only the province of groups of animals, and that an important symmetry exists between the behavioral science of swarms and the competencies of cells and other biological systems at different scales. We then briefly outline the implications of this approach, and the possible impact of tools from the field of diverse intelligence for regenerative medicine and synthetic bioengineering.
Similar content being viewed by others
Introduction
One defining feature of complex life, making it distinct from our current engineered artifacts, is its multiscale nature: there is order in biology across levels of organization, from molecules to cells, tissues, organs, whole organisms, and societies/swarms1,2. Crucially, however, this goes well beyond structural nesting: it is in fact a multiscale competency architecture3,4 because each level solves problems in its own relevant domains (Fig. 1). As evolution facilitated the increase of complexity, living things became composed of layers that cooperate and compete to solve problems in metabolic, physiological, anatomical, and behavioral state spaces (reviewed in refs. 5,6). Biology’s robustness, open-endedness, evolvability, and unique complexity likely depend on the fact that evolution works with an agential material – a substrate with competencies, computational abilities, and homeodynamic setpoints5,7 that strongly influence the structure and function of multicellular forms. Adaptive behavior in new problem spaces3,4 can arise because higher levels of organization can deform the energy landscape for the subunits8, while benefitting from their ability to navigate those landscapes autonomously and without micromanagement.
a Living bodies implement a multiscale competency architecture in which each level of organization, from molecular networks to swarms of animals, navigates specific problem spaces, such as metabolic, physiological, transcriptional, anatomical, and behavioral landscapes. As these diverse subsystems cooperate and compete with each other, their problem-solving dynamics constate adaptive collective intelligence. b Indeed, one way to view evolution of complex forms is as a re-use of many of the same mechanisms and strategies across scales of organization7,72 and problem spaces, which may even extend to high-level navigation of linguistic space. c Concepts from connectionist machine learning, such as artificial neural networks, now provide a rigorous, quantitative understanding of ways in which higher-level information is derived from lower-level subsystems’ inputs in a collective system: for example, input layers receive pixel-level information, but each subsequent layer extracts progressively higher level features in the image8. d The percolation of information across scales is a fundamental aspect of neuroscience: a rat which has learned to press a lever to get a reward is an emergent collective agent, consisting of large numbers of cells, none of which had both experiences on the relevant timescale (interacting with the lever or receiving the nutrients). The cognitive glue that enables emergent agents to support associative memories over their subunits is neural bioelectricity in the case of conventional 3D world behavior, as well as in the traversal of morphospace during development or regeneration27. e Even subcellular components are likely to participate in the scaling of emergent entities from competent parts, as networks as simple as small gene-regulatory circuits or pathways can support several different kinds of learning, including Pavlovian conditioning, when the individual nodes participate in time-dependent stimulus and response patterns225,226,227. f At the level of tissues and organs, collective problem-solving is observed in phenomena such as regulative metamorphosis, in which tadpoles with incorrect arrangements of craniofacial organs still become normal frogs, by novel movements of entire complex structures that operate to reduce distance (error) from current configuration to the normal frog target morphology. This system represents an ideal example of William James’ definition of intelligence as a capacity to achieve specific ends by diverse means as necessary. g Other powerful but poorly-understood examples of collective decision-making include the progressive transformation of a tail transplanted to the flank of an amphibian into a limb: the distal cells (in red)228 slowly become toes, even though in their local environment nothing is wrong (tail tip cells located at the tip of the tail): it is a collective decision that transforms them, flowing down from a perception of anatomical error that is only defined at the whole body-level. Panels a-e created by Jeremy Guay of Peregrine Creative, and used by permission from Refs. 3,5,25,27,225 respectively. Panels f and g are taken with permission from Ref. 151. and228 respectively.
Understanding how the behavior of subunits percolates up toward adaptive processes at higher levels (Fig. 1a–e), and how higher levels of organization constrain and facilitate the behavior of their parts9,10,11,12,13,14,15, is critical not only to basic evolutionary biology but also to the control of system-level outcomes in biomedicine16,17 and to the design of novel engineered systems18,19,20,21,22,23,24. We have previously proposed that this research program can be advanced by exploiting collective intelligence as a crucial symmetry across levels, which enables the tools of behavioral science to be brought to bear on novel unconventional substrates16,17,25,26, especially the capabilities of cell groups in transcriptional, physiological, and anatomical spaces (Fig. 1c, e). Specifically, we have argued that regulative morphogenesis is a kind of behavior of cellular collectives traversing anatomical morphospace (Fig. 1f, g)27,28,29,30,31,32, and others have argued that immune systems33,34, bacterial biofilms35,36,37,38, and many other unconventional substrates39,40,41,42 can be effectively understood and rationally controlled by using techniques from behavioral and cognitive science43.
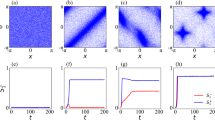
Here, we explore a number of phenomena in biology which illustrate this approach, specifically focusing on two aspects that feature prominently in behavioral science. One is intelligence, in William James’44 sense of a degree of ability to reach the same goal by different means (i.e., problem-solving in changing or novel circumstances). The other is collective decision-making, as studied in the rapidly advancing study of group behavior among swarms45,46,47,48,49,50. This parallel has been explored previously51,52,53,54, and we extend those ideas here with specific references to more recent data revealed by advances in non-invasive imaging and functional cell modulation technology. We emphasize organizational principles that enable not just emergent complexity, but adaptive proto-cognitive systems (problem-solving with respect to adaptive goals and novel circumstances) to appear3,55. A central claim of the emerging field of diverse intelligence is that cognitive capacities (Box. 1) exist on a spectrum: that tools, concepts, and approaches from behavioral sciences can be productively applied to understand and control systems far beyond familiar animals with central nervous systems (without the necessity to attribute advanced, human-level metacognitive traits). We extend James’ definition of intelligence to collectives by considering the perceptual field of an agent: the area in space and time that the agent can survey to find alternative paths to a goal (Fig. 2a, b). As the size of a collective increases its perceptual field increases, improving its ability to find variant paths. (Fig. 2c). It should be noted that while we here focus on animal development, there are also fascinating data of this kind in plants53,56,57,58,59,60,61.
The competency of subunits that flows upward to the collective decision-making is a kind of intelligence – the ability to navigate an environment in an adaptive way that enables specific homeostatic or homeodynamic goals to be met despite novel perturbations or barriers. Non-living objects are capable of simple goal directed behavior, as in the case of a bar magnet moving towards its counterpart (a). This movement can be blocked by an impediment in the direct path of the magnet. Intelligent systems also exhibit goal directedness with the addition of continually surveying their environment in both time and space to find alternate paths to achieve their goal (b). Biological intelligent systems demonstrate increased ability to achieve their (collective) goals despite obstacles by integrating the individual competencies of their components (which can perform tasks in their own space without any inkling of the large-scale goals to which they contribute) (c). This integration enables collectives to survey larger spatial areas and transmit information about possible solutions from one individual to another and likewise expands the capacity of the individual to incorporate information from the past and anticipated future due to the greater computational capacity and broader spatio-temporal perceptual/actuation horizons.
The most familiar examples of collective/swarm intelligence are beehives, ant and termite colonies62,63, and flocks of birds and fish64,65,66. However, it is important to keep in mind that there is no sharp distinction between these collective minds46 and putative centralized ones like those possessed by complex animals and ourselves67 – instead, the biosphere offers a spectrum of architectures including familiar solid brains where the neurons do not move much relative to each other (except in exceptional cases such as metamorphosis68) and so-called “liquid brains” – constructs in which the subunits can implement fluid interactions63,69. Fundamentally, typical brains are a collective of neurons, and provide an experience and functional unity of memories, goals, and preferences because of their interaction dynamics. Thus, one way to view cognitive science is as the study of the collective Intelligence of neurons and other cell types. Understanding how collectives ensure cooperation toward adaptive ends in diverse problem spaces is as much a part of understanding ourselves as of understanding ant colonies. Indeed it has been hypothesized that the remarkable ability of neurons to unify toward a centralized self – the emergent agent that is the subject of memories, preferences, and goals which are not assignable to any of the individual components – is an evolutionary pivot of far earlier cell communication strategies that first solved problems in navigating another domain that requires information processing above the single cell level: anatomical morphospace70. By exploring possible scale-free dynamics in diverse systems, such as viewing the processes of morphogenesis as a kind of behavior of cellular swarms in anatomical space, we may enrich both behavioral neuroscience and developmental/regenerative biology by an influx of new ways of looking at the data28,71,72.
Crucially, despite the clear parallels to the neuroscience of cognition, we here do not make any claims about first-person experience of unconventional collectives73,74,75,76, nor are we saying that the phenomena we describe are of the same degree as familiar human-level cognitive capacities. Instead, we aim to take developmental biology and evolution seriously, and investigate the plesiomorphic, necessarily much more minimal, versions of decision-making and other proto-cognitive functions in multicellular contexts such as regeneration, development, and cancer. We view this as an important step to unify the recent progress in studies of single cell perception/action loops77,78,79,80 with the work on active inference and perceptual control theory currently being developed in neuroscience, robotics, and artificial life81,82,83,84,85,86,87. Specifically, it is essential to move beyond low-level models of information processing, memory, and anticipation in chemical pathways88,89,90 or in single cells91,92,93,94, to understanding the higher-level perceptual landscape of multicellular collectives95,96. This knowledge is essential to improve our ability to explain, control, and re-engineer complex morphological and functional outcomes that today are still outside of our reach25,26. Here, we review interesting examples of the early, simple precursors to the processes that could underlie complex cognitive architectures, and we explore the hypothesis that the porting of tools across disciplines (dissolving artificial barriers between fields) may facilitate further research.
Metazoan somatic cells make collective decisions
Development: transition to multicellularity
A most fundamental example of collectivity is observed during embryogenesis (Fig. 3a). When we observe a blastoderm, we call it an embryo. What precisely are we counting when we say it is 1 embryo? One answer is that the system consists of subunits all cooperating toward a specific path in morphospace: the cells are committed to making a specific functional anatomy corresponding to 1 individual. In effect, we are noticing alignment – both physically, in the sense of planar polarity of cell orientation in a collective97,98,99, and functionally, as is seen in regulative development: if perturbed, the processes of anatomical homeostasis28,100 will attempt to correct and compensate, toward a specific outcome considered normal, across a range of circumstances that includes, but is not limited to, standard development101,102.
a Embryonic blastoderms are considered “1 embryo” even though they are made of thousands of cells because the cells are aligned – both physically, in the sense of planar polarity, and behaviorally, in the sense that they will all cooperate toward one attractor state in anatomical morphospace. However, if the blastoderm is temporarily scratched (a’), each island will, because it doesn’t feel the presence of the others until the scratches heal, form its own embryo103. This results in conjoined twins, triplets, etc. such as these two duck twins shown in b. Indeed (c), this fascinating process demonstrates that 1) the number of embryos emerging from the excitable medium of an embryonic blastoderm is not genetically fixed but determined in real-time by physiological processes, and 2) involves a fundamental process of collective autopoiesis in which each high-level individual (e.g., an embryo) needs to determine the borders between it and its outside world. d Such collective decision-making, which regulates the behavior of the subunits, and its failure modes, is starkly revealed as conjoined twins are known to often exhibit laterality defects (such as heterotaxy) as cells located between two self-organizing embryo collectives may not always decide correctly whether they are the left side of one twin or the right side of the other229. e The same task is solved by individual organ primordia, for example when ectopic eyes are induced by ion channel misexpression150, forming several distinct eyes of normal size instead of one giant eye. Panels a and c were made by Jeremy Guay of Peregrine Creative. Panels b and e produced by co-author M.L. (Tabin lab) and Sherry Aw (Levin lab). Panel d is used with permission from229.
Indeed, the question of how many individuals are present in an embryonic blastoderm is not fixed at 1 by the genetics, because temporary introduction of breaks in the blastoderm (leading to informational isolation of islands of cell masses) results in twins, triplets, etc103. (Fig. 3b–d), showing that the blastoderm is a dynamical excitable medium in which multiple coherent embryos can self-organize. The same is true of organogenesis, such as when an induced large eye-field fragments into a number of individual eyes instead of one large eye (Fig. 3e). Thus, the physiological process that leads to the emergence of integrated collectives, which scientists and conspecifics recognize as discrete individuals is fundamentally dependent on the geometry of interactions (and signaling barriers) present during the early establishment of individuality and the setting of borders between Self and outside world (since every cell is some other cell’s adjacent neighbor).
Cell migration: the group does not go where each of the parts wants to go
One major question about the origin of higher-level individuals from active components (cells, which are themselves not passive agents9,21), is how the behaviors of the higher levels depend on those of the lower-level components. The most obvious scaling mode is linear: the collective does what its individual cells are doing. But a more interesting aspect is that the collective often displays new behaviors or preferences. One example of this in the same space concerns cell migration. In an electric field, keratocytes migrate to the cathode, but fragments of keratocytes migrate to the anode104. Remarkably, individual fragments have the opposite direction of taxis to that of a collective of those fragments (an intact cell). The behaviors of a collective can, even in relatively minimal systems, be a complex and hard-to-predict function of the tendencies of the components. This is a microcosm of the larger issue of competition between wholes and their parts6,105, and of the more general feature of multiscale organization in which collective agents bend the energy landscape for their components to exploit their mechanisms towards distinct ends.
From normal melanocytes to a melanoma phenotype: a collective decision
One failure mode of collective behavior in vivo is cancer106,107. When cells become isolated from the information structure of the tissue, they revert back to an ancient, unicellular transcriptional108 and behavioral phenotype109,110. Exciting work focusing on the biochemical nature of the microenvironment has shown the ability of non-cell-autonomous cues to normalize cancer111,112,113,114,115,116. However, more recent work has focused on bioelectric cues that normally orchestrate multicellular anatomical outcomes117, and the consequences of their disruption (Fig. 4).
Tadpoles of the frog Xenopus laevis (dorsal view of the head) show small numbers of round melanocytes (a) in normal development. However, when the signals from instructor cells118 are interrupted by changing their resting potential, the melanocytes convert to a melanoma-like form: they hyperproliferate, and migrate to fill many regions of the embryo in a phenotype that recapitulates melanoma metastasis (a’). This process can be stimulated at different rates in a population by different reagents (b). Interestingly, while only some percentage of animals convert, the decision is made in a coordinated fashion by every tissue in the animal: individuals are either entirely converted (every melanocyte undergoes the shape and behavioral changes) or are entirely unaffected. This stochastic decision resembles a biased coin toss, but all the tissues of a given animal are linked to the same coin. A model of the pathway (c) was parametrized by a machine learning algorithm which was able to reconstruct the state space (c’) revealing the mechanism of this stochastic decision119,120. This model was then used to predict a novel intervention that would break the concordance among cells – disrupt specifically the collective decision-making. For the first time, partially-pigmented animals were produced (d), showing how AI-discovered pathways fitting biological data can help explain collective decisions made by large numbers of cells in vivo. Panels a-c’ taken with permission from Ref. 119. Panel d taken with permission from Ref. 120.
In the tadpole model, it was shown that normal melanocytes could be driven into a melanoma-like converted phenotype: they over-proliferated, migrated inappropriately to regions normally devoid of melanocytes, invaded the blood vessels and brain, and changed shape into a highly arborized, invasive form118. This could be achieved in the absence of classical carcinogens, oncogenes, or DNA damage, by brief exposure to chemical (chloride ion channel activator drug) or molecular-genetic (GlyCl mutant) targeting of the bioelectric state of a specific cell population: instructor cells which normally keep the melanocytes in their healthy state via serotonergic signaling118. Indeed, targeting only a handful of instructor cells in a region away from the source of melanocyte populations (as confirmed by lineage label) was sufficient to turn the whole tadpole into a hyperpigmented phenotype strongly resembling metastatic melanoma: all of the melanocytes converted, even the ones not close to the GlyCl-activated cells.
The most remarkable thing was that this phenotype is an all-or-none phenomenon. Using different reagents could induce different incidences of hyperpigmentation (conversion) in a cohort of animals, but this was a population-level phenotype: for example, 70% of the animals could be converted, but any given animal was either fully converted or fully normal (Fig. 4a, b). A computational model of the known signaling steps was designed and parametrized (Fig. 4c) to reproduce this all-or-none behavior and fit the experimentally-observed incidence percentages across different perturbations119. The model illustrated how cells navigate biochemical state space (Fig. 4c’) and face specific decision-points at regions of that landscape. The benefit of such a model of collective decision-making is that it can be used to infer interventions. Specifically, the model was used to predict an intervention that would break the concordance of melanocytes within single animals. It suggested two drugs and a dominant negative construct – an experiment that had never been done before - which were then experimentally confirmed to produce the first partially-converted animals (Fig. 4d) seen in almost a decade of experiments in this system120.
Left/right, head/tail: random, but collective anatomical decisions
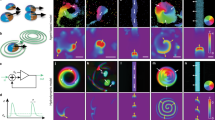
In addition to the 3D and transcriptional/physiological spaces discussed above, one of the most interesting aspects of collective intelligence is the navigation of anatomical morphospace. Cell groups need to make specific decisions about which organ will be built and what shape they must make. This is a fundamentally different problem than identifying gene regulatory networks and differentiation signals. For example (Fig. 5), planarian cells can rebuild a complete worm from any kind of cut or fragment (Fig. 5a). Typical treatments of this problem focus on a fragment within a morphogen gradient, that offers distinct concentrations of an instructive chemical signal that can confer head/tail fate decisions locally to each wound (Fig. 5b). However, the more interesting and fundamental issue is seen when considering just one cut: the cells on either side of the cut will create a head and tail respectively, but they were adjacent neighbors before the cut and located at the same positional information value. In other words, it is actually impossible for an anatomical decision like this to be made locally – the cells of the wound must coordinate with the remaining fragment to get information about where they are located, which way they are facing, and what other structures exist121,122, in order to make adaptive decisions about large-scale growth and form that enable regeneration of normal worms.
a Planaria regenerate a complete worm from even small fragments, which requires large numbers of cells to cooperate to complete a specific path through morphospace, eventually building a correct complex anatomy to very tight tolerances. b Morphogen models are often used to explain the axial polarity of a middle fragment (2 cuts), but if one focuses on a single cut (vertical line marked as P1), it is clear that adjacent cells will have radically different anatomical fates (head vs. tail) despite their identical positional information. The cells must communicate with the rest of the fragment to determine what other structures exist, which way the wound is facing, etc. and then decide which path in morphospace the collective will follow. c If half of the neoblasts of one species of planaria (with a round head) are killed by radiation and replaced with transplanted neoblasts from another species (with a flat head), what shape head would regenerate (and would it ever reach the stop criterion and cease remodeling)? This thought experiment reveals clearly why the science of collective intelligence is a critical complement to molecular genetics: while much single cell-level information is available about the pathways controlling stem cell differentiation, the field has not a single model able to make a prediction for this scenario, because we still lack the conceptual tools and data to understand how collectives of cells make unified decisions. d The tools of dynamical systems theory (top row) and connectionist neuroscience/AI (middle row) are poised to help provide formalisms for understanding how networks of cells can store pattern information and recover it from partial inputs, such as occurs in planarian regeneration (bottom row). e In planaria, one of the modalities that binds individual cells into morphogenetic collectives is bioelectricity117,132: fragments possess a difference in resting potential that determines the number and location of heads. Tracking these patterns using voltage-sensitive fluorescent dyes in functional experiments reveals some aspects of the rules by which the collective makes decisions230. In animals treated with the proton pump inhibitor SCH28080 (SCH column), the bioelectric pattern lacks the depolarization that cells interpret as the make-a-head signal and headless animals result (bottom left). Control fragments have the depolarized (green) signal at one end (middle column) leading to normal 1-headed animals (bottom center). Animals treated with the chloride drug opener Ivermectin (IVM column) exhibit two regions of depolarization resulting in 2-headed animals (bottom right). Interestingly, the green voltage that produces heads in control animals is seen in the middle of the IVM-treated worms, and does not induce heads: only the very depolarized regions (red) become heads. This indicates that the collective is not measuring absolute resting potential values, but (in keeping with the distributed nature of the circuit and the animal-wide signaling) is adopting anatomical organ-level fates driven by the relative difference of regions (i.e., the most-depolarized region is where heads form). f In addition to 0-, 1-, and 2-head worms (which are stable lines that continue to regenerate as 2-headed231), there is another form called Cryptic Worms, also produced by bioelectrical disruption130. These worms show a stochastic phenotype, in which a worm (or indeed, independent pieces of a single worm) will form 2-head or 1-head worms at a 70–30 ratio; the transition diagram with probabilities is shown in panel f; WT = wild-type (1 head); 8OH = octanol which causes the cryptic phenotype; H2O – water (control condition or cutting). The percentages indicate the frequency of each transition. Crucially, while the head/tail decision is stochastic, all of the cells agree on the same outcome (what is never seen is a planarian in which some of the cells in a given region are making a head and others a tail) – the whole region makes the random decision as a single whole. The mechanism is not known but likely involves gap-junctional communication of the voltage signals231 and can be modeled as a kind of perceptual bistability131. Panels a and d made by Jeremy Guay of Peregrine Creative and Alexis Pietak respectively. Panels c, e, f used with permission from Refs. 121,130,230 respectively.
More generally, numerous excellent papers have studied planarian neoblasts and their control networks, as well as the gradients of morphogens that pattern the anterior-posterior, dorso-ventral, and medio-lateral axes123,124,125,126,127. Despite these advances, there is very little understanding of how cells build specific head shapes or how they know when to stop mitosis and morphogenesis when the correct head shape has been achieved. Specifically, for example, no existing model makes a prediction on what will happen if 50% of the neoblasts of a given planarian are replaced with those of a different species and the head is cut off (Fig. 5c). Whether the head will be of the right shape for one of the two species (dominant), or an in-between hybrid form, or in fact continuously cycle between shapes (as each set of neoblasts works to remodel toward the shape they normally make with great fidelity), cannot yet be derived from the properties of single cell regulatory pathways – it is a collective decision about navigating the space of possible head shapes128,129 (Fig. 5d).
Indeed, modification of cell:cell communication during regeneration can cause genetically-normal fragments to produce heads appropriate to other species of planaria128,129 – visiting attractors in morphospace normally reserved for other genetic lineages. More specifically, several perturbations targeting the bioelectric control circuit (Fig. 5e) have shown randomization of outcome: such Cryptic planaria are destabilized, and fragments (even from the same parent worm) will form 1-head and 2-head forms at a set frequency of ~1:2130 (Fig. 5f). This phenomenon highlights collective decision-making because this randomization is at the level of the population: each individual animal has clear heads and tails, not tissue speckled with cells of different identity. In other words, the randomization of bioelectric state131,132 and the downstream morphogen gradients is interpreted with respect to anterior-posterior organ identity by collectives, not by individual cells.
The left-right axis in vertebrates shows a similar phenomenon (Fig. 6). Consistent asymmetries across the midline first show up in the chick embryo around the primitive streak and Hensen’s node133. A number of treatments, including targeting of the bioelectric134,135,136,137,138 or downstream biochemical139,140,141,142 pathways, result in randomization of molecular and anatomical consequences of symmetry breakage and orientation143,144,145,146,147,148. The animals display, in addition to the normal L:R identity, double-right or double-left (isomerism), or reversed (situs inversus) patterns of lateral identity markers, followed by heterotaxy of the heart and viscera. Remarkably, while many of these treatments randomize outcomes, the randomization is once again above the level of the individual: any given embryo has a consistent identity on the L and R side, and all of the cells agree. In all of the many studies on perturbation of the LR pathway, we are aware of only one that actually breaks the concordance: disruption of the planar polarity pathway by down-regulating VANGL signaling149 leads to a speckling on both sides of the midline, consistent with individual cells within a single lateral domain disagreeing on whether they should have L or R identity (Fig. 6a–d).
The expression of Sonic hedgehog in the Hensen’s node of early chick embryos is a marker (purple color) of left-right asymmetry: in normal embryos, it is only expressed on the left side (a, red arrowhead). By perturbing upstream events, such as the voltage gradient that determines lateral identity138, it is possible to induce a percentage of right-sided (b) or bilateral (c) expression. However, in all these cases, all of the cells on one side of the node agree on their identity, even if the identity is stochastically determined within a cohort of embryos. There is one known perturbation – disruption of planar polarity149 – that breaks the concordance and results in a speckled appearance (d) in which individual cells within a lateral compartment disagree about which side they are on. e Ectopic eyes can be produced in frog embryos, even far from the anterior neurectoderm such as on the gut (red arrowhead), by microinjection of potassium channel mRNAs that induce a specific state that is interpreted by cells as an eye induction signal150. The information content of this inducer is very low – as in the endogenous eye spot in the head151, there is a simple voltage pattern that cannot encode all of the nuances of a complex vertebrate eye structure. This simple spatial pattern is read out by the cellular collective to make an organ-level decision. Interestingly, if very few cells are injected (f), the blue cells are lineage-labeled to indicate the presence of the ectopic ion channel), normal-sized lenses can be produced because the voltage-modified cells recruit their wild-type neighbors to participate in the organogenesis. However, this does not always work because the surrounding cells, in a cancer-suppression mechanism, are meanwhile exerting influence to shut down the ectopic eye induction, and sometimes no eye tissue appears at all. This is an example of collective decisions competing to determine the path through morphospace that will be taken and once a choice is made, all of the cells fall into line. Panels a, c, d, e, f taken with permission from Ref. 3,149,150,232 respectively.
Eye or skin: competition in DNKir6 injected animals, and size control
A number of collective decisions are mediated by bioelectric signaling, which coordinates cells in the body as a likely precursor to its role in coordinating neurons in the brain toward the emergence of a coherent, problem-solving Self27,70. One example of this at the organ level concerns the induction of whole ectopic eyes in the frog embryo by misexpression of ion channels150 whose activity sets up a voltage gradient similar to that of the eye spot which normally determines their location in the head151. As with previous examples, this is a signal to the collective, setting organ-level identity, not micromanaging the differentiation of the many cells which need to be produced and placed with exquisite precision to make a normal vertebrate eye. Interestingly, this signaling has another built-in competency: recruitment. If very few cells are injected with the channel (attached), they will often recruit their neighbors (Fig. 6e, f) to help them complete the task. This is a kind of secondary instruction, where we instruct a group of cells to make an eye, and they recruit the others (which were never directly manipulated), including all of the necessary downstream morphogenetic steps. This recruitment of individuals to accomplish a high-level goal is seen in other collective systems like ant colonies152,153, which often call in helpers when a task is large. The ability to recruit participants to complete tasks may be a central competency of collective intelligence that works across scales, from cells to swarms of entire organisms7.
The neural crest acts as an intelligently migrating collective
The neural crest is a cell population that arises between the neural plate and the non-neural ectoderm before migrating throughout the body to produce a constellation of cell types including head mesenchyme, peripheral nervous system and melanocytes154. Neural crest cells (NCCs) must successfully traverse the complex and rapidly changing embryonic body before identifying their target location and integrating with nearby tissue. The energy landscape that they traverse is more complex than it initially appears, however. While each cell navigates a fairly simple dorsal-ventral cartesian space towards a goal destination, the neural crest collective is navigating morphospace to create properly spaced, symmetrical facial structures. When the cell-level navigation of cartesian space is put in opposition to the collective-level navigation of morphospace, the collective supersedes the behavior of the individual to achieve the organism-level morphogenetic target of forming a functional, symmetrical face as we describe in the examples below.
Neural crest migration is intelligent
Grafting and ablation experiments underscore the collective ability of the neural crest to accomplish its morphogenetic goals despite some novel circumstances. Axolotls regulate the number of cells, compensating for too few or too many155. Neural crest cells (and in some cases neural tube cells156,157) regulatively adapt their migratory behavior to compensate for the loss of NCCs in nearby or contralateral branchial arches158,159. This re-routed migration suggests that individual cells can leverage the perceptual field of the neural crest cell collective to determine the movements that they should take to contribute to proper system level morphogenesis (Fig. 7). In an especially striking example of the NCCs’ intelligent capability to achieve their ontogenic goals in challenging environments, mouse NCCs grafted into chicken embryos will successfully navigate the forming embryonic face and form teeth160.
Regulative compensatory migration in migrating neural crest cells demonstrates collective intelligence by placing the goal of the individual and the goal of the collective in opposition (a). During normal development neural crest cells integrate signaling cues to migrate dorsal to ventral along the forming embryonic body. When an arch is ablated, cells will move anteriorly or posteriorly instead of dorsoventrally to fill this missing arch thus achieving the correct target morphology of the collective behavior through movement patterns contrary to the individual cell’s normal optimal path158,159. Applying our perceptual field framework, the expanded collective perceptual field contains a more attractive path to achieving the cell’s goal than its individual perceptual field does, and thus is undergoes compensatory rather than normal migration. Inter-domain grafting experiments demonstrate the expanded time-domain perceptual field of a collective intelligence (b). When grafted from one Hox domain to another, individual rhombomere or neural crest cells will lose the memory of their original Hox gene inductive event and adopt the expression pattern of their neighbors161,162. Similarly grafted collectives, in contrast, maintain their previously induced state suggesting increased memory due to their collective intelligence.
Neural crest intelligence is collective
Individual cells transposed from one anterior-posterior axial domain to another will change their gene expression to match their neighbors159,161,162. In contrast, groups of cells transposed along the anterior-posterior axis maintain their original gene expression, thus resisting the inductive effects of the surrounding tissue159,161,162. Within the context of our perceptual field model, the increased positional memory and resistance to neighbor effects suggests that cell collectives have an expanded perceptual cone in the posterior time (history) dimension (Fig. 7). While individual cells rapidly lose their memory of past inductive cues, collectives are better able to maintain a consistent identity in a noisy developmental environment159,161,162. As with the re-routing post arch ablation example above, this example suggests that the cell-level behavior is subordinate to the collective-level behavior. When collective-level behaviors are put in opposition by grafting of collectives these is no clear hierarchy, and original fate is maintained.
Cells intelligently segment the vertebrate body axis via the segmentation clock
Another well-studied example of collective intelligence is the vertebrate segmentation clock comprising coordinated oscillations of Notch pathway target genes to establish segmental boundaries of the early vertebrate embryo163. The segmentation clock exhibits functional robustness to interventions, consistent with James’ definition of intelligence, because of the ability to take different paths through morphospace to correctly partition tissue into uniform, correctly sized segments. Clever experiments radically altering the geometry of the tissue test its intelligence by forcing it to explore a variant morphospatial landscape.
Segmentation is intelligent
In whole embryos, coordinated oscillations will re-emerge following chemically induced disruption and resume producing properly spaced segments164, though the complexity of the developing organism make it challenging to determine if this re-emergence is intrinsic to the segmenting tissue or imposed upon it by other tissues. Recent work with paraxial mesoderm explants165 further emphasizes the remarkable intelligence of this system. In the context of James’ framework for intelligence, the goal states are 1) coordinated oscillatory gene expression and 2) morphological segmentation, and the obstacle is the severe geometric transformation from 3D tube to 2D sheet. In the 2D geometry, oscillatory gene expression waves manifest as outwardly propagating rings that successfully effect segmentation of the outer edge of the explant. While these segments manifest as serially repeated spheres in the 3D in vivo environment, 2D cultures form segments circumscribing the explant’s circumference (Fig. 8a). Segmentation can be re-capitulated from embryonic stem cells in culture by production of trunk-like organoids termed gastruloids166,167, which arrive at a segmented target morphology despite a very different ontogenic history than normal trunk cells.
When the tissue is dissociated, cells quickly stop oscillating, though coordinated oscillations re-emerge in cells that are dissociated and re-aggregated, indicating that collectivity is essential to this intelligent behavior169 (a). Cells grafted between tissues in different phases of the segmental oscillator will synchronize with their neighbors in the host tissue. Within our perceptual field framework, this synchronization suggests that the system level memory (i.e., where they were in the oscillation) is conferred upon the grafted individual cells for whom the spatial and temporal cues are now mismatched (b).
Segmentation intelligence is collective
The ability of the segmentation clock to intelligently navigate its morphospatial landscape has also been tested by forcing it into a state that it would never normally adopt by grafting out of phase cells into oscillating tissue. The segmentation clock functions to coordinate a collection of cells to organize into a large super-cellular structure. Consistent with this function, collectivity is necessary for the segmentation clock to function. When wild type cells are grafted into mutant non-cycling fish, they express the normally oscillatory gene her1, but it does not cycle168. Similarly, pre-somitic mesoderm cells do not oscillate when cultured independently, but will resume oscillating when cultured collectively169. This loss of oscillation can be partially rescued by addition of external FGF, potentially mimicking the effects of high cell density169 and implicating collectivity in stem cell maintenance. Most directly, cells hetero-grafted from tissue in one phase of the clock into a group of cells in a different phase will synchronize to the phase of their lateral neighbors170, (Fig. 8b). In the context of our perceptual field model, the grafted cells benefit from the expanded memory and predictive power of their neighbors to determine their correct position in the clock (Fig. 8b). These cells then adjust their intrinsic oscillatory dynamics to entrain to their neighbors, thus completing their task despite an internal configuration with novel hardware components which do not have an evolutionary history of living together in a single organism.
Intelligence in bacterial communities
Though bacteria are unicellular, they often form into large biofilms that exhibit fascinating physiological and morphological collective properties171,172,173,174. Interestingly, much as bioelectric networks are used in metazoan systems to bind individual cells together to large-scale morphogenetic projects, bacteria likewise exploit electrical signaling across space and time to coordinate175,176. Cells within the biofilm (and even between biofilms) exhibit bioelectrically-coordinated oscillatory growth patterns that favor the health of the collective at the expense of their own individual fitness177,178, and bioelectric signals coordinate metabolism among distant cells within the biofilm38. These bioelectric signals help recruit new bacteria to the biofilm, even across species179, and can be optogenetically controlled to evoke long-lasting changes on bacterial behavior – a collective memory35.
Exciting recent work has identified a mechanism similar to the vertebrate segmentation clock in bacterial biofilms responding to nitrogen stress mediated by a negative feedback loop180. The similarities between this system and the vertebrate segmentation clock point to further roles for this phenomenon in collective intelligence, and the manifestation of similar molecular logic circuits in distant clades suggests that such collective intelligence is a much more widespread phenomenon than is currently appreciated. Furthermore, the parameter space is neither cartesian space nor morphospace as in our previous examples, but physiological space. The bacterial cells intelligently adjust their individual physiologies to achieve an optimal collective physiology. The capacity of such simple organisms to collectively navigate physiology space using paradigms recapitulated in multicellular organisms highlights the deep cruciality of such navigation to the emergence of complex tissue and points to the necessity of understanding how such navigation occurs during animal development and pathology.
Conclusion
Cell and developmental biology offer very rich fodder for the emerging field of diverse intelligence: discovering a vast spectrum of problem-solving capacities in novel substrates and at unconventional spatiotemporal scales. Because of life’s multi-scale competency architecture, a fundamental aspect of intelligence is collective behavior: all intelligences appear to be made of parts, connected by mechanisms implementing policies that bind the competent components into a cooperative (and competitive6) computational medium that solves problems in new spaces and at higher scales. The harnessing of individual cell behaviors toward regulative morphogenesis (navigating anatomical morphospace), and system-level physiological robustness (traversing physiological space) are especially interesting examples. Indeed, it could be argued that a unique signature of Life is a causal architecture in which the problem-solving competency of the whole is greater than that of its parts). Evolution seems to be particularly good at finding ways to scale the cognitive light cone of cells3,4,5,181 to achieve spectacular capabilities for gracefully and adaptively handling complexity, novelty, and noise at large scale.
A key aspect of collective intelligence of cell groups is binding subunits’ activities to the same target morphology – a kind of discrete (e.g., head vs. tail) outcome whereas the components have states that range over many continuous quantities. In axial patterning (left-right, anterior-posterior), collective decision-making enables large numbers of cells in a compartment to agree on an organ-scale anatomical fate despite stochastic influences upstream. And it is seen that a decision with respect to morphogenetic outcome, and harnessing cells to the same decision, are orthogonal functions with distinct mechanisms that can be experimentally dissociated.
Importantly, the definition of intelligence as the ability to reach the same endpoint despite internal or external changes emphasizes not only robustness (successful use of novel navigational policies to overcome perturbations) but also its failure modes. Numerous ways of targeting of its sensory, memory, decision-making, or other components can de-rail the performance of a collective intelligence, resulting in birth defects and malformations. This is quite consistent with the proposed symmetry between the behavioral and developmental domains, because computational neuroscience and cognitive science are replete with interesting ways to think about how cognitive systems make mistakes. The use of tools and concepts across fields has begun, including attempts to understand cancer as a dissociative identity disorder of the morphological collective intelligence109, the use of serotonin reuptake inhibitors and hallucinogens to perturb non-neural development182,183, the modeling of the unstable phenotypes in planarian regeneration as perceptual bistability131, and the finding that some visual illusions that plague vertebrate nervous systems are recapitulated in collective intelligences such as ants184,185. We expect that many concepts from the behavioral sciences that explain failures of learning, recall, Bayesian updating of dynamic signaling models, attention, arousal, and perception will find application in explaining and controlling defects in navigation of anatomical space towards healthy, optimal outcomes.
Another hallmark of collective intelligence is the ability of the higher-level agent to make decisions based on extended patterns of information. For example, in the frog embryo brain, it is the spatial difference in voltage between regions that drives downstream gene expression, not the absolute value of any cells186,187. In other words, cells have to read whole cell fields and recognize specific patterns to determine what to do – the collectivity is seen in the input, as well as the output, of cell groups’ behaviors.
Future work is essential to understand how higher-order entities (organisms, organs, tissues, etc.) distort the energy landscape for their subunits, benefitting from their competencies to navigate spaces of which the subunits are unaware. This underlies the harnessing of cellular signaling and computational abilities to regulative development and regeneration, which implement organ-level homeostatic loops that keep large-scale order against cellular defections (aging and cancer106,188) and injury189. Living matter is a kind of agential material with the ability to propagate information across scales – a phenomenon which has many implications for evolution9, and for bioengineering21.
Many tools are becoming available which increase insight and cross-fertilization of approaches across disciplines. Examples include optogenetic interrogation of single cell78,190,191,192,193 and embryonic194,195,196,197,198,199 dynamics, as well as the very elegant electrotactic ‘SCHEEPDOG’ system which is able to precisely steer collectives of keratinocytes using patterned dynamic electric fields200 that distinguish between collective and individual cell behaviors. In addition to technologies, important additions are conceptual tools, such as the active inference framework201,202,203 and tools of causal information theory204,205,206,207,208,209,210,211,212, which will have many applications in the biological sciences.
Future work in this area will also continue to be enriched by advances in the collective intelligence of animal behavior46,213 as well as in the field of swarm robotics214,215,216,217. Additional directions for investigation include: how conflict (competition) is used for coordination in collectives6,218, and how propagation of shared stress181,219,220 and the sharing of cellular memories via gap junctions4,109 establish higher-order individuals.
One of the most exciting aspects of this emerging field is the way in which collective intelligence serves as a focal point for exploring the symmetries between developmental biology and neuroscience26. This ranges from the use of cognitive science formalisms to understand morphogenesis and its disorders55,131,221 to the questions of how many human Selves can be sustained by the excitable medium of a human brain67 and the parallels to the multiple bodies that can emerge from a single embryonic blastoderm103.
Many of the same mechanisms (e.g., electrophysiological networks) and control policies are re-used by evolution to bind neurons to collective behavior and animal navigation of 3D space and to bind pre-neural cells to move the body configuration in morphospace5,70. Turing was prescient in studying both intelligence and the chemical basis of self-organization222,223, as the problem of self-organization in familiar neural-based intelligences may have much in common with the problem of self-organizing a non-neural collective intelligence of morphogenesis224. If true, a number of fields can look forward to exciting advances. Cancer, a kind of dissociative identity disorder of the somatic collective intelligence109, limitations in regenerative ability, and many physiological disorders could all be advanced by techniques that exploit not just the low-level mechanisms, but also the higher-level decision-making of life16,17. Neuroscience can benefit from a glimpse into the evolutionary past of the brain’s remarkable capabilities, while developmental biology and bioengineering can borrow the practical and conceptual tools of neuroscience which is likely to be about much more basic principles than the function of classical neurons. Understanding how evolution works in an agential, multiscale material (where it can take advantage of cross-level computation) will nicely complement the efforts of engineers to build and control swarms of robots and AI systems, but who as yet largely work with passive matter where competency exists only at one scale.
Taken together, collective intelligence is an extremely exciting and interdisciplinary emerging field that spans from the most fundamental philosophical problems of the parts-whole relationship to advancing fundamental and applied discovery in a number of important subfields.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
References
Shellard, A. & Mayor, R. Rules of collective migration: from the wildebeest to the neural crest. Philos. Trans. R. Soc. Lond. B Biol. Sci. 375, 20190387 (2020).
Lawton, A. K. et al. Regulated tissue fluidity steers zebrafish body elongation. Development 140, 573–582 (2013).
Levin, M. Technological approach to mind everywhere: an experimentally-grounded framework for understanding diverse bodies and minds. Front Syst. Neurosci. 16, 768201 (2022).
Levin, M. The computational boundary of a “Self”: Developmental bioelectricity drives multicellularity and scale-free cognition. Front Psychol. 10, 2688 (2019).
Fields, C. & Levin, M. Competency in navigating arbitrary spaces as an invariant for analyzing cognition in diverse embodiments. Entropy 24, 819 (2022).
Gawne, R., McKenna, K. Z. & Levin, M. Competitive and coordinative interactions between body parts produce adaptive developmental outcomes. Bioessays 42, e1900245 (2020).
Fields, C. & Levin, M. Scale-free biology: integrating evolutionary and developmental thinking. Bioessays 42, e1900228 (2020).
Watson, R., Levin, M. & Buckley, C. L. Design for an individual: connectionist approaches to the evolutionary transitions in individuality. Front. Ecol. Evol. 10, 823588 (2022).
Levin, M. Darwin’s agential materials: evolutionary implications of multiscale competency in developmental biology. Cell Mol. Life Sci. 80, 142 (2023).
Noble, D. Genes and causation. Philos. Trans. A Math. Phys. Eng. Sci. 366, 3001–3015 (2008).
Ellis, G. F. R. On the nature of causation in complex systems. Trans. R. Soc. South Afr. 63, 69–84 (2008).
Auletta, G., Ellis, G. F. & Jaeger, L. Top-down causation by information control: from a philosophical problem to a scientific research programme. J. R. Soc. Interface 5, 1159–1172 (2008).
Walker, S., Cisneros, L. & Davies, P. C. W. Evolutionary transitions and top-down causation. Artif. Life 13, 283–290 (2012).
Scerri, E. R. Top-down causation regarding the chemistry-physics interface: a sceptical view. Interface Focus 2, 20–25 (2012).
Okasha, S. Emergence, hierarchy and top-down causation in evolutionary biology. Interface Focus 2, 49–54 (2012).
Mathews, J., Chang, A. J., Devlin, L. & Levin, M. Cellular signaling pathways as plastic, proto-cognitive systems: Implications for biomedicine. Patterns 4, 100737 (2023).
Lagasse, E. & Levin, M. Future medicine: from molecular pathways to the collective intelligence of the body. Trends Mol. Med 29, 687–710 (2023).
Sample, M. et al. Multi-cellular engineered living systems: building a community around responsible research on emergence. Biofabrication 11, 043001 (2019).
Kamm, R. D. et al. Perspective: The promise of multi-cellular engineered living systems. Apl. Bioeng. 2, 040901 (2018).
Davies, J. A. & Glykofrydis, F. Engineering pattern formation and morphogenesis. Biochem Soc. Trans. 48, 1177–1185 (2020).
Davies, J. & Levin, M. Synthetic morphology with agential materials. Nat. Rev. Bioeng. 1, 46–59 (2023).
Ebrahimkhani, M. R. & Levin, M. Synthetic living machines: A new window on life. iScience 24, 102505 (2021).
Ebrahimkhani, M. R. & Ebisuya, M. Synthetic developmental biology: build and control multicellular systems. Curr. Opin. Chem. Biol. 52, 9–15 (2019).
Velazquez, J. J., Su, E., Cahan, P. & Ebrahimkhani, M. R. Programming morphogenesis through systems and synthetic biology. Trends Biotechnol. 36, 415–429 (2018).
Pezzulo, G. & Levin, M. Top-down models in biology: explanation and control of complex living systems above the molecular level. J. R. Soc. Interface 13, 20160555 (2016).
Pezzulo, G. & Levin, M. Re-membering the body: applications of computational neuroscience to the top-down control of regeneration of limbs and other complex organs. Integr. Biol. (Camb.) 7, 1487–1517 (2015).
Levin, M. Bioelectric networks: the cognitive glue enabling evolutionary scaling from physiology to mind. Anim. Cogn. 26, 1865–1891 (2023).
Levin, M. in Evolution “on Purpose” : Teleonomy in Living Systems (ed P. A. Corning, Kauffman, S. A., Noble, D., Shapiro, J. A., Vane-Wright, R. I., Pross, A.) 175-198 (MIT Press, 2023).
Stone, J. R. The spirit of D’arcy Thompson dwells in empirical morphospace. Math. Biosci. 142, 13–30 (1997).
Raup, D. M. & Michelson, A. Theoretical morphology of the coiled shell. Science 147, 1294–1295 (1965).
Abzhanov, A. et al. The calmodulin pathway and evolution of elongated beak morphology in Darwin’s finches. Nature 442, 563–567 (2006).
Ollé-Vila, A., Duran-Nebreda, S., Conde-Pueyo, N., Montañez, R. & Solé, R. A morphospace for synthetic organs and organoids: the possible and the actual. Integr. Biol. (Camb.) 8, 485–503 (2016).
Okabe, Y. & Medzhitov, R. Tissue biology perspective on macrophages. Nat. Immunol. 17, 9–17 (2016).
Ciaunica, A., Shmeleva, E. V. & Levin, M. The brain is not mental! coupling neuronal and immune cellular processing in human organisms. Front Integr. Neurosci. 17, 1057622 (2023).
Yang, C. Y. et al. Encoding membrane-potential-based memory within a microbial community. Cell Syst. 10, 417–423 e413 (2020).
Martinez-Corral, R., Liu, J., Prindle, A., Süel, G. M. & Garcia-Ojalvo, J. Metabolic basis of brain-like electrical signalling in bacterial communities. Philos. Trans. R. Soc. Lond. B Biol. Sci. 374, 20180382 (2019).
Larkin, J. W. et al. Signal percolation within a bacterial community. Cell Syst. 7, 137–145 e133 (2018).
Prindle, A. et al. Ion channels enable electrical communication in bacterial communities. Nature 527, 59–63 (2015).
Baluška, F., Reber, A. S. & Miller, W. B. Jr. Cellular sentience as the primary source of biological order and evolution. Biosystems 218, 104694 (2022).
Baluška, F., Miller, W. B. & Reber, A. S. Cellular and evolutionary perspectives on organismal cognition: from unicellular to multicellular organisms. Biol. J. Linn. Soc. 139, 503–513 (2023).
Reber, A. S. & Baluška, F. Cognition in some surprising places. Biochem Biophys. Res Commun. 564, 150–157 (2021).
Baluška, F. & Levin, M. On having no head: cognition throughout biological systems. Front Psychol. 7, 902 (2016).
Abramson, C. I. & Levin, M. Behaviorist approaches to investigating memory and learning: A primer for synthetic biology and bioengineering. Commun. Integr. Biol. 14, 230–247 (2021).
James, W. The principles of psychology. (H. Holt and company, 1890).
Couzin, I. D. Collective cognition in animal groups. Trends Cogn. Sci. 13, 36–43 (2009).
Couzin, I. Collective minds. Nature 445, 715 (2007).
Heylighen, F. Self-organization in Communicating Groups: The Emergence of Coordination, Shared References and Collective Intelligence. Underst Complex Syst., 117–149, https://doi.org/10.1007/978-3-642-32817-6 (2013).
Wheeler, W. M. The ant‐colony as an organism. J. Morphol. 22, 307–325 (1911).
Engel, D. & Malone, T. W. Integrated information as a metric for group interaction. PLoS One 13, e0205335 (2018).
Sasaki, T. & Biro, D. Cumulative culture can emerge from collective intelligence in animal groups. Nat. Commun. 8, 15049 (2017).
Camley, B. A. & Rappel, W. J. Physical models of collective cell motility: from cell to tissue. J. Phys. D. Appl Phys. 50, 113002 (2017).
Solé, R. et al. Synthetic collective intelligence. Biosystems 148, 47–61 (2016).
Baluška, F., Lev-Yadun, S. & Mancuso, S. Swarm intelligence in plant roots. Trends Ecol. Evol. 25, 682–683 (2010).
Deisboeck, T. S. & Couzin, I. D. Collective behavior in cancer cell populations. Bioessays 31, 190–197 (2009).
Pio-Lopez, L., Kuchling, F., Tung, A., Pezzulo, G. & Levin, M. Active inference, morphogenesis, and computational psychiatry. Front Comput Neurosci. 16, 988977 (2022).
Davis, G. V. et al. Toward uncovering an operating system in plant organs. Trends Plant Sci. S1360-1385, 00365–00365 (2023).
Calvo, P., Baluška, F. & Sims, A. “Feature Detection” vs. “Predictive Coding” Models of plant behavior. Front Psychol. 7, 1505 (2016).
Baluška, F., Volkmann, D. & Menzel, D. Plant synapses: actin-based domains for cell-to-cell communication. Trends Plant Sci. 10, 106–111 (2005).
Johnston, I. G. & Bassel, G. W. Identification of a bet-hedging network motif generating noise in hormone concentrations and germination propensity in Arabidopsis. J. R. Soc. Interface 15, 20180042 (2018).
Bassel, G. W. Information processing and distributed computation in plant organs. Trends Plant Sci. 23, 994–1005 (2018).
Topham, A. T. et al. Temperature variability is integrated by a spatially embedded decision-making center to break dormancy in Arabidopsis seeds. Proc. Natl Acad. Sci. USA 114, 6629–6634 (2017).
Marais, E. N. The soul of the white ant. (Penguin, 1973).
Piñero, J. & Solé, R. Statistical physics of liquid brains. Philos. Trans. R. Soc. Lond. B Biol. Sci. 374, 20180376 (2019).
Ward, A. J. W. & Webster, M. M. Mid-sized groups perform best in a collective decision task in sticklebacks. Biol. Lett. 15, 20190335 (2019).
Bai, Y., Tang, Z. H. & Fu, S. J. Numerical ability in fish species: preference between shoals of different sizes varies among singletons, conspecific dyads and heterospecific dyads. Anim. Cogn. 22, 133–143 (2019).
Ward, A. J., Sumpter, D. J., Couzin, I. D., Hart, P. J. & Krause, J. Quorum decision-making facilitates information transfer in fish shoals. Proc. Natl Acad. Sci. USA 105, 6948–6953 (2008).
Braude, S. E. First person plural: multiple personality and the philosophy of mind. (Rowman & Littlefield Publishers, 1995).
Blackiston, D. J., Shomrat, T. & Levin, M. The stability of memories during brain remodeling: A perspective. Commun. Integr. Biol. 8, e1073424 (2015).
Solé, R., Moses, M. & Forrest, S. Liquid brains, solid brains. Philos. Trans. R. Soc. Lond. B Biol. Sci. 374, 20190040 (2019).
Fields, C., Bischof, J. & Levin, M. Morphological coordination: a common ancestral function unifying neural and non-neural signaling. Physiology 35, 16–30 (2020).
Manicka, S. & Levin, M. The Cognitive Lens: a primer on conceptual tools for analysing information processing in developmental and regenerative morphogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 374, 20180369 (2019).
Fields, C. & Levin, M. Regulative development as a model for origin of life and artificial life studies. Biosystems 229, 104927 (2023).
Friston, K. J., Wiese, W. & Hobson, J. A. Sentience and the origins of consciousness: from Cartesian duality to Markovian Monism. Entropy 22, 516 (2020).
Tononi, G. Consciousness as integrated information: a provisional manifesto. Biol. Bull. 215, 216–242 (2008).
Tononi, G., Boly, M., Massimini, M. & Koch, C. Integrated information theory: from consciousness to its physical substrate. Nat. Rev. Neurosci. 17, 450–461 (2016).
Tononi, G. & Koch, C. Consciousness: here, there and everywhere? Philos. Trans. R. Soc. Lond. B Biol. Sci. 370, 20140167 (2015).
Bugaj, L. J., O’Donoghue, G. P. & Lim, W. A. Interrogating cellular perception and decision making with optogenetic tools. J. Cell Biol. 216, 25–28 (2017).
Wilson, M. Z., Ravindran, P. T., Lim, W. A. & Toettcher, J. E. Tracing information flow from Erk to target gene induction reveals mechanisms of dynamic and combinatorial control. Mol. Cell 67, 757–769 e755 (2017).
Karin, O., Swisa, A., Glaser, B., Dor, Y. & Alon, U. Dynamical compensation in physiological circuits. Mol. Syst. Biol. 12, 886 (2016).
Koseska, A. & Bastiaens, P. I. Cell signaling as a cognitive process. EMBO J. 36, 568–582 (2017).
Ramstead, M. J. D., Constant, A., Badcock, P. B. & Friston, K. J. Variational ecology and the physics of sentient systems. Phys. Life Rev. 31, 188–205 (2019).
Friston, K. A free energy principle for a particular physics. arXiv:1906.10184 (2019). <https://ui.adsabs.harvard.edu/abs/2019arXiv190610184F>
Badcock, P. B., Friston, K. J., Ramstead, M. J. D., Ploeger, A. & Hohwy, J. The hierarchically mechanistic mind: an evolutionary systems theory of the human brain, cognition, and behavior. Cogn. Affect Behav. Neurosci. 19, 1319–1351 (2019).
Badcock, P. B., Friston, K. J. & Ramstead, M. J. D. The hierarchically mechanistic mind: A free-energy formulation of the human psyche. Phys. Life Rev. 31, 104–121 (2019).
Ramstead, M. J. D., Badcock, P. B. & Friston, K. J. Answering Schrodinger’s question: A free-energy formulation. Phys. Life Rev. 24, 1–16 (2018).
Pezzulo, G., Rigoli, F. & Friston, K. J. Hierarchical active inference: a theory of motivated control. Trends Cogn. Sci. 22, 294–306 (2018).
Sengupta, B., Tozzi, A., Cooray, G. K., Douglas, P. K. & Friston, K. J. Towards a neuronal gauge theory. Plos Biol. 14, e1002400 (2016).
Chakravarthy, S. V. & Ghosh, J. On Hebbian-like adaptation in heart muscle: a proposal for ‘cardiac memory’. Biol. Cyber. 76, 207–215 (1997).
Katz, Y. & Fontana, W. Probabilistic inference with polymerizing biochemical circuits. Entropy 24, 629 (2022).
Katz, Y., Springer, M. & Fontana, W. Embodying probabilistic inference in biochemical circuits. arXiv:1806.10161 (2018). https://arxiv.org/abs/1806.10161.
Mitchell, A. et al. Adaptive prediction of environmental changes by microorganisms. Nature 460, 220–224 (2009).
Saigusa, T., Tero, A., Nakagaki, T. & Kuramoto, Y. Amoebae anticipate periodic events. Phys. Rev. Lett. 100, 018101 (2008).
Boussard, A., Delescluse, J., Pérez-Escudero, A. & Dussutour, A. Memory inception and preservation in slime moulds: the quest for a common mechanism. Philos. Trans. R. Soc. Lond. B Biol. Sci. 374, 20180368 (2019).
Vladimirov, N. & Sourjik, V. Chemotaxis: how bacteria use memory. Biol. Chem. 390, 1097–1104 (2009).
Kuchling, F., Friston, K., Georgiev, G. & Levin, M. Integrating variational approaches to pattern formation into a deeper physics: Reply to comments on “Morphogenesis as Bayesian inference: A variational approach to pattern formation and manipulation in complex biological systems”. Phys. Life Rev. 33, 125–128 (2020).
Kuchling, F., Friston, K., Georgiev, G. & Levin, M. Morphogenesis as Bayesian inference: A variational approach to pattern formation and control in complex biological systems. Phys. Life Rev. 33, 88–108 (2020).
Muñoz-Soriano, V., Belacortu, Y. & Paricio, N. Planar cell polarity signaling in collective cell movements during morphogenesis and disease. Curr. Genomics 13, 609–622 (2012).
Devenport, D. The cell biology of planar cell polarity. J. Cell Biol. 207, 171–179 (2014).
Devenport, D. & Fuchs, E. Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. Nat. cell Biol. 10, 1257–1268 (2008).
Pinet, K., Deolankar, M., Leung, B. & McLaughlin, K. A. Adaptive correction of craniofacial defects in pre-metamorphic Xenopus laevis tadpoles involves thyroid hormone-independent tissue remodeling. Development 146, dev175893 (2019).
Pinet, K. & McLaughlin, K. A. Mechanisms of physiological tissue remodeling in animals: Manipulating tissue, organ, and organism morphology. Dev. Biol. 451, 134–145 (2019).
Vandenberg, L. N., Adams, D. S. & Levin, M. Normalized shape and location of perturbed craniofacial structures in the Xenopus tadpole reveal an innate ability to achieve correct morphology. Dev. Dyn. 241, 863–878 (2012).
Lutz, H. Sur la production experimentale de la polyembryonie et de la monstruosite double chez les oiseaux. Archs Anat. Microsc. Morph. Exp. 38, 79–144 (1949).
Sun, Y. et al. Keratocyte fragments and cells utilize competing pathways to move in opposite directions in an electric field. Curr. Biol. 23, 569–574 (2013).
Roux, W. Der Kampf der Theile im Organismus. (W. Engelmann, 1881).
Rubin, H. Cancer as a dynamic developmental disorder. Cancer Res 45, 2935–2942 (1985).
Waddington, C. H. Cancer and the theory of organisers. Nature 135, 606–608 (1935).
Davies, P. C. & Lineweaver, C. H. Cancer tumors as Metazoa 1.0: tapping genes of ancient ancestors. Phys. Biol. 8, 015001 (2011).
Levin, M. Bioelectrical approaches to cancer as a problem of the scaling of the cellular self. Prog. Biophys. Mol. Biol. 165, 102–113 (2021).
Moore, D., Walker, S. I. & Levin, M. Cancer as a disorder of patterning information: computational and biophysical perspectives on the cancer problem. Converg. Sci. Phys. Oncol. 3, 043001 (2017).
Maffini, M. V., Calabro, J. M., Soto, A. M. & Sonnenschein, C. Stromal regulation of neoplastic development: age-dependent normalization of neoplastic mammary cells by mammary stroma. Am. J. Pathol. 167, 1405–1410 (2005).
Kasemeier-Kulesa, J. C. et al. Reprogramming multipotent tumor cells with the embryonic neural crest microenvironment. Dev. Dyn. 237, 2657–2666 (2008).
Illmensee, K. & Mintz, B. Totipotency and normal differentiation of single teratocarcinoma cells cloned by injection into blastocysts. Proc. Natl Acad. Sci. USA 73, 549–553 (1976).
Mintz, B. & Illmensee, K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc. Natl Acad. Sci. USA 72, 3585–3589 (1975).
Bissell, M. J. & Labarge, M. A. Context, tissue plasticity, and cancer: Are tumor stem cells also regulated by the microenvironment? Cancer Cell 7, 17–23 (2005).
Kenny, P. A. & Bissell, M. J. Tumor reversion: correction of malignant behavior by microenvironmental cues. Int J. Cancer 107, 688–695 (2003).
Levin, M. Bioelectric signaling: Reprogrammable circuits underlying embryogenesis, regeneration, and cancer. Cell 184, 1971–1989 (2021).
Blackiston, D., Adams, D. S., Lemire, J. M., Lobikin, M. & Levin, M. Transmembrane potential of GlyCl-expressing instructor cells induces a neoplastic-like conversion of melanocytes via a serotonergic pathway. Dis. Model Mech. 4, 67–85 (2011).
Lobikin, M. et al. Serotonergic regulation of melanocyte conversion: A bioelectrically regulated network for stochastic all-or-none hyperpigmentation. Sci. Signal 8, ra99 (2015).
Lobo, D., Lobikin, M. & Levin, M. Discovering novel phenotypes with automatically inferred dynamic models: a partial melanocyte conversion in Xenopus. Sci. Rep. 7, 41339 (2017).
Lobo, D., Beane, W. S. & Levin, M. Modeling planarian regeneration: a primer for reverse-engineering the worm. PLoS Comput Biol. 8, e1002481 (2012).
Levin, M., Pietak, A. M. & Bischof, J. Planarian regeneration as a model of anatomical homeostasis: Recent progress in biophysical and computational approaches. Semin. Cell Dev. Biol. 87, 125–144 (2019).
Hill, E. M. & Petersen, C. P. Positional information specifies the site of organ regeneration and not tissue maintenance in planarians. Elife 7, e33680 (2018).
Saló, E. et al. Planarian regeneration: achievements and future directions after 20 years of research. Int J. Dev. Biol. 53, 1317–1327 (2009).
Cebrià, F. Regenerating the central nervous system: how easy for planarians! Dev. Genes Evol. 217, 733–748 (2007).
Sheiman, I. M. & Kreshchenko, I. D. Regeneration of planarians: experimental object. Ontogenez 46, 3–12 (2015).
Gentile, L., Cebrià, F. & Bartscherer, K. The planarian flatworm: an in vivo model for stem cell biology and nervous system regeneration. Dis. Model Mech. 4, 12–19 (2011).
Sullivan, K. G., Emmons-Bell, M. & Levin, M. Physiological inputs regulate species-specific anatomy during embryogenesis and regeneration. Commun. Integr. Biol. 9, e1192733 (2016).
Emmons-Bell, M. et al. Gap junctional blockade stochastically induces different species-specific head anatomies in genetically wild-type Girardia Dorotocephala Flatworms. Int. J. Mol. Sci. 16, 27865–27896 (2015).
Durant, F. et al. Long-term, stochastic editing of regenerative anatomy via targeting endogenous bioelectric gradients. Biophys. J. 112, 2231–2243 (2017).
Pezzulo, G., LaPalme, J., Durant, F. & Levin, M. Bistability of somatic pattern memories: stochastic outcomes in bioelectric circuits underlying regeneration. Philos. Trans. R. Soc. Lond. B Biol. Sci. 376, 20190765 (2021).
Durant, F. et al. The role of early bioelectric signals in the regeneration of planarian anterior/posterior polarity. Biophys. J. 116, 948–961 (2019).
Levin, M. Left-right asymmetry in embryonic development: a comprehensive review. Mech. Dev. 122, 3–25 (2005).
Pai, V. P. et al. HCN4 ion channel function is required for early events that regulate anatomical left-right patterning in a nodal and lefty asymmetric gene expression-independent manner. Biol. Open 6, 1445–1457 (2017).
Aw, S. et al. The ATP-sensitive K(+)-channel (K(ATP)) controls early left-right patterning in Xenopus and chick embryos. Dev. Biol. 346, 39–53 (2010).
Aw, S., Adams, D. S., Qiu, D. & Levin, M. H,K-ATPase protein localization and Kir4.1 function reveal concordance of three axes during early determination of left-right asymmetry. Mech. Dev. 125, 353–372 (2008).
Adams, D. S. et al. Early, H+-V-ATPase-dependent proton flux is necessary for consistent left-right patterning of non-mammalian vertebrates. Development 133, 1657–1671 (2006).
Levin, M., Thorlin, T., Robinson, K. R., Nogi, T. & Mercola, M. Asymmetries in H+/K+-ATPase and cell membrane potentials comprise a very early step in left-right patterning. Cell 111, 77–89 (2002).
Wang, S. et al. Chick Pcl2 regulates the left-right asymmetry by repressing Shh expression in Hensen’s node. Development 131, 4381–4391 (2004).
Kelly, K. A., Wei, Y. & Mikawa, T. Cell death along the embryo midline regulates left-right sidedness. Dev. Dyn. 224, 238–244 (2002).
García-Castro, M. I., Vielmetter, E. & Bronner-Fraser, M. N-Cadherin, a cell adhesion molecule involved in establishment of embryonic left-right asymmetry. Science 288, 1047–1051 (2000).
Branford, W. W., Essner, J. J. & Yost, H. J. Regulation of gut and heart left-right asymmetry by context-dependent interactions between Lefty and BMP4 signaling. Dev. Biol. 223, 291–306 (2000).
Vandenberg, L. N., Lemire, J. M. & Levin, M. It’s never too early to get it Right: A conserved role for the cytoskeleton in left-right asymmetry. Commun. Integr. Biol. 6, e27155 (2013).
Vandenberg, L. N. & Levin, M. Far from solved: a perspective on what we know about early mechanisms of left-right asymmetry. Dev. Dyn. 239, 3131–3146 (2010).
Vandenberg, L. N. & Levin, M. Perspectives and open problems in the early phases of left-right patterning. Semin Cell Dev. Biol. 20, 456–463 (2009).
Raya, A. & Izpisua Belmonte, J. C. Unveiling the establishment of left-right asymmetry in the chick embryo. Mech. Dev. 121, 1043–1054 (2004).
Yost, H. J. Establishment of left-right asymmetry. Int Rev. Cytol. 203, 357–381 (2001).
Ramsdell, A. F. & Yost, H. J. Molecular mechanisms of vertebrate left-right development. Trends Genet 14, 459–465 (1998).
Zhang, Y. & Levin, M. Left-right asymmetry in the chick embryo requires core planar cell polarity protein Vangl2. Genesis 47, 719–728 (2009).
Pai, V. P., Aw, S., Shomrat, T., Lemire, J. M. & Levin, M. Transmembrane voltage potential controls embryonic eye patterning in Xenopus laevis. Development 139, 313–323 (2012).
Vandenberg, L. N., Morrie, R. D. & Adams, D. S. V-ATPase-dependent ectodermal voltage and pH regionalization are required for craniofacial morphogenesis. Dev. Dyn. 240, 1889–1904 (2011).
Planqué, R., van den Berg, J. B. & Franks, N. R. Recruitment strategies and colony size in ants. PLoS One 5, e11664 (2010).
Reid, C. R., Sumpter, D. J. & Beekman, M. Optimisation in a natural system: Argentine ants solve the Towers of Hanoi. J. Exp. Biol. 214, 50–58 (2011).
Szabó, A. & Mayor, R. Mechanisms of neural crest migration. Annu Rev. Genet 52, 43–63 (2018).
Zarzosa, A. et al. Axolotls with an under- or oversupply of neural crest can regulate the sizes of their dorsal root ganglia to normal levels. Dev. Biol. 394, 65–82 (2014).
Sechrist, J., Nieto, M. A., Zamanian, R. T. & Bronner-Fraser, M. Regulative response of the cranial neural tube after neural fold ablation: spatiotemporal nature of neural crest regeneration and up-regulation of Slug. Development 121, 4103–4115 (1995).
Scherson, T., Serbedzija, G., Fraser, S. & Bronner-Fraser, M. Regulative capacity of the cranial neural tube to form neural crest. Development 118, 1049–1062 (1993).
Couly, G., Grapin-Botton, A., Coltey, P. & Le Douarin, N. M. The regeneration of the cephalic neural crest, a problem revisited: the regenerating cells originate from the contralateral or from the anterior and posterior neural fold. Development 122, 3393–3407 (1996).
Le Douarin, N. M., Creuzet, S., Couly, G. & Dupin, E. Neural crest cell plasticity and its limits. Development 131, 4637–4650 (2004).
Mitsiadis, T. A., Chéraud, Y., Sharpe, P. & Fontaine-Pérus, J. Development of teeth in chick embryos after mouse neural crest transplantations. Proc. Natl Acad. Sci. USA 100, 6541–6545 (2003).
Trainor, P. & Krumlauf, R. Plasticity in mouse neural crest cells reveals a new patterning role for cranial mesoderm. Nat. cell Biol. 2, 96–102 (2000).
Trainor, P. A. & Krumlauf, R. Patterning the cranial neural crest: hindbrain segmentation and Hox gene plasticity. Nat. Rev. Neurosci. 1, 116–124 (2000).
Pourquié, O. Vertebrate segmentation: from cyclic gene networks to scoliosis. Cell 145, 650–663 (2011).
Uriu, K., Liao, B. K., Oates, A. C. & Morelli, L. G. From local resynchronization to global pattern recovery in the zebrafish segmentation clock. Elife 10, e61358 (2021).
Diaz-Cuadros, M. & Pourquie, O. In vitro systems: A new window to the segmentation clock. Dev. Growth Differ. 63, 140–153 (2021).
Veenvliet, J. V. et al. Mouse embryonic stem cells self-organize into trunk-like structures with neural tube and somites. Science 370, eaba4937 (2020).
van den Brink, S. C. et al. Single-cell and spatial transcriptomics reveal somitogenesis in gastruloids. Nature 582, 405–409 (2020).
Holley, S. A., Geisler, R. & Nusslein-Volhard, C. Control of her1 expression during zebrafish somitogenesis by a delta-dependent oscillator and an independent wave-front activity. Genes Dev. 14, 1678–1690 (2000).
Webb, A. B. et al. Persistence, period and precision of autonomous cellular oscillators from the zebrafish segmentation clock. Elife 5, e08438 (2016).
Horikawa, K., Ishimatsu, K., Yoshimoto, E., Kondo, S. & Takeda, H. Noise-resistant and synchronized oscillation of the segmentation clock. Nature 441, 719–723 (2006).
Jacob, E. B., Aharonov, Y. & Shapira, Y. Bacteria harnessing complexity. Biofilms 1, 239–263 (2005).
Shapiro, J. A. Thinking about bacterial populations as multicellular organisms. Annu Rev. Microbiol 52, 81–104 (1998).
Lyon, P. The cognitive cell: bacterial behavior reconsidered. Front Microbiol 6, 264 (2015).
Gloag, E. S. et al. Stigmergy co-ordinates multicellular collective behaviours during Myxococcus xanthus surface migration. Sci. Rep. 6, 26005 (2016).
Reguera, G. et al. Extracellular electron transfer via microbial nanowires. Nature 435, 1098–1101 (2005).
Nielsen, L. P., Risgaard-Petersen, N., Fossing, H., Christensen, P. B. & Sayama, M. Electric currents couple spatially separated biogeochemical processes in marine sediment. Nature 463, 1071–1074 (2010).
Liu, J. et al. Metabolic co-dependence gives rise to collective oscillations within biofilms. Nature 523, 550–554 (2015).
Liu, J. et al. Coupling between distant biofilms and emergence of nutrient time-sharing. Science 356, 638–642 (2017).
Humphries, J. et al. Species-independent attraction to biofilms through electrical signaling. Cell 168, 200–209 e212 (2017).
Chou, K. T. et al. A segmentation clock patterns cellular differentiation in a bacterial biofilm. Cell 185, 145–157 e113 (2022).
Pio-Lopez, L., Bischof, J., LaPalme, J. V. & Levin, M. The scaling of goals from cellular to anatomical homeostasis: an evolutionary simulation, experiment and analysis. Interface Focus 13, 20220072 (2023).
Geber, W. F. Congenital malformations induced by mescaline, lysergic acid diethylamide, and bromolysergic acid in the hamster. Science 158, 265–267 (1967).
Sullivan, K. G. & Levin, M. Neurotransmitter signaling pathways required for normal development in Xenopus laevis embryos: a pharmacological survey screen. J. Anat. 229, 483–502 (2016).
Sakiyama, T. & Gunji, Y. P. The Muller-Lyer illusion in ant foraging. PLoS One 8, e81714 (2013).
Sakiyama, T. & Gunji, Y. P. The Kanizsa triangle illusion in foraging ants. Biosystems 142-143, 9–14 (2016).
Pai, V. P. et al. Endogenous gradients of resting potential instructively pattern embryonic neural tissue via Notch signaling and regulation of proliferation. J. Neurosci. 35, 4366–4385 (2015).
Pai, V. P., Lemire, J. M., Chen, Y., Lin, G. & Levin, M. Local and long-range endogenous resting potential gradients antagonistically regulate apoptosis and proliferation in the embryonic CNS. Int J. Dev. Biol. 59, 327–340 (2015).
Rubin, H. Ordered heterogeneity and its decline in cancer and aging. Adv. Cancer Res 98, 117–147 (2007).
Harris, A. K. The need for a concept of shape homeostasis. Biosystems 173, 65–72 (2018).
Shin, Y. et al. Spatiotemporal control of intracellular phase transitions using light-activated optoDroplets. Cell 168, 159–171 e114 (2017).
Johnson, H. E. et al. The Spatiotemporal limits of developmental Erk signaling. Dev. Cell 40, 185–192 (2017).
Toettcher, J. E., Weiner, O. D. & Lim, W. A. Using optogenetics to interrogate the dynamic control of signal transmission by the Ras/Erk module. Cell 155, 1422–1434 (2013).
Toettcher, J. E., Gong, D., Lim, W. A. & Weiner, O. D. Light-based feedback for controlling intracellular signaling dynamics. Nat. Methods 8, 837–839 (2011).
Chernet, B. T., Adams, D. S., Lobikin, M. & Levin, M. Use of genetically encoded, light-gated ion translocators to control tumorigenesis. Oncotarget 7, 19575–19588 (2016).
Adams, D. S. et al. Bioelectric signalling via potassium channels: a mechanism for craniofacial dysmorphogenesis in KCNJ2-associated Andersen-Tawil Syndrome. J. Physiol. 594, 3245–3270 (2016).
Adams, D. S., Lemire, J. M., Kramer, R. H. & Levin, M. Optogenetics in Developmental Biology: using light to control ion flux-dependent signals in Xenopus embryos. Int J. Dev. Biol. 58, 851–861 (2014).
Adams, D. S., Tseng, A. S. & Levin, M. Light-activation of the Archaerhodopsin H(+)-pump reverses age-dependent loss of vertebrate regeneration: sparking system-level controls in vivo. Biol. Open 2, 306–313 (2013).
Wolf, A. E., Heinrich, M. A., Breinyn, I. B., Zajdel, T. J. & Cohen, D. J. Short-term bioelectric stimulation of collective cell migration in tissues reprograms long-term supracellular dynamics. PNAS Nexus 1, pgac002 (2022).
Cohen, D. J., Nelson, W. J. & Maharbiz, M. M. Galvanotactic control of collective cell migration in epithelial monolayers. Nat. Mater. 13, 409–417 (2014).
Zajdel, T. J., Shim, G., Wang, L., Rossello-Martinez, A. & Cohen, D. J. SCHEEPDOG: Programming electric cues to dynamically herd large-scale cell migration. Cell Syst. 10, 506–514 e503 (2020).
Kirchhoff, M., Parr, T., Palacios, E., Friston, K. & Kiverstein, J. The Markov blankets of life: autonomy, active inference and the free energy principle. J. R. Soc. Interface 15, 20170792 (2018).
Constant, A., Ramstead, M. J. D., Veissière, S. P. L., Campbell, J. O. & Friston, K. J. A variational approach to niche construction. J. R. Soc. Interface 15, 20170685 (2018).
Allen, M. & Friston, K. J. From cognitivism to autopoiesis: towards a computational framework for the embodied mind. Synthese 195, 2459–2482 (2018).
Varley, T. F. & Hoel, E. Emergence as the conversion of information: a unifying theory. Philos. Trans. A Math. Phys. Eng. Sci. 380, 20210150 (2022).
Hoel, E. & Levin, M. Emergence of informative higher scales in biological systems: a computational toolkit for optimal prediction and control. Commun. Integr. Biol. 13, 108–118 (2020).
Hoel, E. P. in Wandering Towards a Goal: How Can Mindless Mathematical Laws Give Rise to Aims and Intention? (eds A. Aguirre, B. Foster, & Z. Merali) 63-76 (Springer International Publishing, 2018).
Hoel, E. P. When the Map Is Better Than the Territory. Entropy-Switz 19, https://doi.org/10.3390/e19050188 (2017).
Albantakis, L., Marshall, W., Hoel, E. P. & Tononi, G. What caused what? An irreducible account of actual causation using dynamical causal networks. Entropy 21, 459 (2019).
Hoel, E. P., Albantakis, L., Marshall, W. & Tononi, G. Can the macro beat the micro? Integrated information across spatiotemporal scales. Neurosci. Conscious 2016, niw012 (2016).
Hoel, E. P., Albantakis, L. & Tononi, G. Quantifying causal emergence shows that macro can beat micro. Proc. Natl Acad. Sci. USA 110, 19790–19795 (2013).
Wang, X. R., Miller, J. M., Lizier, J. T., Prokopenko, M. & Rossi, L. F. Quantifying and tracing information cascades in swarms. PLoS One 7, e40084 (2012).
Lizier, J. T., Heinzle, J., Horstmann, A., Haynes, J. D. & Prokopenko, M. Multivariate information-theoretic measures reveal directed information structure and task relevant changes in fMRI connectivity. J. Comput Neurosci. 30, 85–107 (2011).
Sridhar, V. H. et al. The geometry of decision-making in individuals and collectives. Proc. Natl Acad. Sci. USA 118, e2102157118 (2021).
Rahwan, I. et al. Machine behaviour. Nature 568, 477–486 (2019).
Slavkov, I. et al. Morphogenesis in robot swarms. Sci. Robot 3, eaau9178 (2018).
Brambilla, M., Ferrante, E., Birattari, M. & Dorigo, M. Swarm robotics: a review from the swarm engineering perspective. Swarm Intell.-Us 7, 1–41 (2013).
Barca, J. C. & Sekercioglu, Y. A. Swarm robotics reviewed. Robotica 31, 345–359 (2013).
Smiley, P. & Levin, M. Competition for finite resources as coordination mechanism for morphogenesis: An evolutionary algorithm study of digital embryogeny. Biosystems 221, 104762 (2022).
Beloussov, L. V., Louchinskaia, N. N. & Stein, A. A. Tension-dependent collective cell movements in the early gastrula ectoderm of Xenopus laevis embryos [English]. Dev. Genes Evol. 210, 92–104 (2000).
Peters, A., McEwen, B. S. & Friston, K. Uncertainty and stress: Why it causes diseases and how it is mastered by the brain. Prog. Neurobiol. 156, 164–188 (2017).
Pezzulo, G. Disorders of morphogenesis as disorders of inference: Comment on “Morphogenesis as Bayesian inference: A variational approach to pattern formation and control in complex biological systems” by Michael Levin et al. Phys. Life Rev. 33, 112–114 (2020).
Turing, A. M. The chemical basis of morphogenesis. Philos. T R. Soc. B 237, 37–72 (1952).
Turing, A. M. Computing machinery and intelligence. Mind 59, 433–460 (1950).
Grossberg, S. Communication, Memory, and Development. in Progress in Theoretical Biology Vol. 5 (eds R. Rosen & F. Snell) 183-232 (1978).
Biswas, S., Clawson, W. & Levin, M. Learning in transcriptional network models: computational discovery of pathway-level memory and effective interventions. Int J. Mol. Sci. 24, 285 (2023).
Biswas, S., Manicka, S., Hoel, E. & Levin, M. Gene regulatory networks exhibit several kinds of memory: quantification of memory in biological and random transcriptional networks. iScience 24, 102131 (2021).
Watson, R. A., Buckley, C. L., Mills, R. & Davies, A. in Artificial Life Conference XII. 194–201 (2010).
Farinella-Ferruzza, N. The transformation of a tail into a limb after xenoplastic transformation. Experientia 15, 304–305 (1956).
Levin, M., Roberts, D. J., Holmes, L. B. & Tabin, C. Laterality defects in conjoined twins. Nature 384, 321 (1996).
Beane, W. S., Morokuma, J., Adams, D. S. & Levin, M. A chemical genetics approach reveals H,K-ATPase-mediated membrane voltage is required for planarian head regeneration. Chem. Biol. 18, 77–89 (2011).
Oviedo, N. J. et al. Long-range neural and gap junction protein-mediated cues control polarity during planarian regeneration. Dev. Biol. 339, 188–199 (2010).
Fukumoto, T., Kema, I. P. & Levin, M. Serotonin signaling is a very early step in patterning of the left-right axis in chick and frog embryos. Curr. Biol. 15, 794–803 (2005).
Moore, D. G., Valentini, G., Walker, S. I. & Levin, M. Inform: Efficient information-theoretic analysis of collective behaviors. Front. Robot AI 5, 60 (2018).
Yokawa, K. et al. Anaesthetics stop diverse plant organ movements, affect endocytic vesicle recycling and ROS homeostasis, and block action potentials in Venus flytraps. Ann. Bot. 122, 747–756 (2018).
Ciszak, M., Masi, E., Baluška, F. & Mancuso, S. Plant shoots exhibit synchronized oscillatory motions. Commun. Integr. Biol. 9, e1238117 (2016).
Gyurkó, D. M. et al. Adaptation and learning of molecular networks as a description of cancer development at the systems-level: potential use in anti-cancer therapies. Semin Cancer Biol. 23, 262–269 (2013).
Csermely, P. et al. Learning of signaling networks: molecular mechanisms. Trends Biochem. Sci. 45, 284–294 (2020).
Acknowledgements
We thank Douglas Blackiston, Randall Ellis, and Patrick Erickson for their helpful comments on the manuscript, as well as Julia Poirier for editorial assistance. M.L. gratefully acknowledges support of the Guy Foundation Family Trust (103733-00001), of grants 62212 and 62230 from the John Templeton Foundation (the opinions expressed in this publication are those of the author(s) and do not necessarily reflect the views of the John Templeton Foundation), of the Templeton World Charity Foundation (TWCF0606) and of the Air Force Office of Scientific Research under award number FA9550-22-1-0465, Cognitive & Computational Neuroscience program.
Author information
Authors and Affiliations
Contributions
M.L. and P.M. wrote this Perspective together, including working on the text and creating the figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare the following competing interests: Tufts University has a sponsored research agreement with a company, Astonishing Labs, to fund projects relevant to the collective intelligence of cells.
Peer review
Peer review information
Communications Biology thanks Timothy Jackson and Ricard Solé for their contribution to the peer review of this work. Primary Handling Editor: Manuel Breuer. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
McMillen, P., Levin, M. Collective intelligence: A unifying concept for integrating biology across scales and substrates. Commun Biol 7, 378 (2024). https://doi.org/10.1038/s42003-024-06037-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-024-06037-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.