Abstract
Vagus nerve signaling is a key component of the gut-brain axis and regulates diverse physiological processes that decline with age. Gut to brain vagus firing patterns are regulated by myenteric intrinsic primary afferent neuron (IPAN) to vagus neurotransmission. It remains unclear how IPANs or the afferent vagus age functionally. Here we identified a distinct ageing code in gut to brain neurotransmission defined by consistent differences in firing rates, burst durations, interburst and intraburst firing intervals of IPANs and the vagus, when comparing young and aged neurons. The aminosterol squalamine changed aged neurons firing patterns to a young phenotype. In contrast to young neurons, sertraline failed to increase firing rates in the aged vagus whereas squalamine was effective. These results may have implications for improved treatments involving pharmacological and electrical stimulation of the vagus for age-related mood and other disorders. For example, oral squalamine might be substituted for or added to sertraline for the aged.
Similar content being viewed by others
Introduction
Intestinal vagal afferents are the rapid information superhighway through which chemosensory information is relayed from the gut to the brain. Moreover, perhaps because of its huge sensory surface, the gut supplies the majority of vagal afferent fibres to the brain1. Despite this, little is known about how vagal afferent function alters in the aged although dystrophic anatomical changes have been observed in vagal afferents innervating the intestine2.
The primary target of vagal innervation in the murine gut is the myenteric plexus, while direct vagal innervation of the mucosa and submucosa are sparse or absent1. Indeed the densest innervation of the intestinal epithelial layer cells3,4 is supplied by the enteric nervous system, which provides more than 90% of sensory neuropeptide containing fibres to the mucosal layer. While there are several functional classes of neurons within the myenteric plexus, only one class, the intrinsic primary afferents (IPANs) is both chemo- and mechanosensitive serving as an intramural sensory gatekeeper relaying the signals originating from luminal contents to the afferent vagus nerve5.
It has been proposed, based on age-related changes in calcium-binding proteins and neurotransmitter content, that IPANs are most susceptible to neurodegeneration when aged are compared to young animals6,7. Given the crucial gatekeeper role that the IPANs have in relaying information to the afferent vagus, any study of age-related changes in vagal function must be accompanied by a study of age-related functional changes in myenteric IPANs.
Background mesenteric nerve fibre discharge is diminished in aged compared to young adult humans8 and this finding has been replicated in C57BL/6 mice for which constitutive mesenteric multiunit spiking was significantly reduced in aged (18–24 mo) compared to young (3 mo) animals9. Also, we have previously reported that the background vagal afferent firing rate is reduced in aged CD-1 mice by 62% compared to young CD-1 and that intraluminal infusion of the aminosterol squalamine dilactate could return vagal firing rates to those seen young animals10. Since electrical vagal stimulation has been used clinically to treat depressive disorders11 it is possible that a diminished or altered resting vagal afferent firing rate and pattern in the elderly contributes to the significant occurrence of behavioural depression in this group12.
The class of neuron most likely responsible for the reduction in resting vagus basal firing rate with ageing is the IPAN. With respect to how IPANs signal to the afferent vagus, we have previously reported5 that, for young mice, neurotransmission is via an IPAN soma to vagal afferent terminal nicotinic synapse that we have termed the intramural sensory synapse.
The aged murine enteric nervous system displays dystrophic neurons13 and degenerating sensory neuropeptide containing nerve fibres14. Aged enteric neurons also accumulate lipofuscin in their somata14. However despite this evidence for anatomical and neurochemical changes there have been no physiological recordings made from aged IPANs or other enteric neurons. It is thus hardly possible to know how aged IPANs are functionally altered compared to young IPANs. In view of the increased human median age in the developed world and the importance of the enteric nervous system in gut-to-brain transmission it is critical to investigate an animal model of the aged enteric nervous system. It is also important to identify drugs whose ingestion might reduce the functional effects of ageing.
The constitutive firing rate of vagal fibres innervating the small intestine is lower for aged compared to young mice (see above) but vertebrate neurons encode information by firing patterns and intervals not just firing rates. Despite this, it is not known whether there exists a specific age-associated vagal firing pattern (ageing code). 15Vagal firing patterns can be described by 4 parameters, mean interspike interval (MII), burst duration (BD), gap duration (GD) and intraburst interval (IBI), that describe firing intervals with respect to action potential bursts and the overall mean interspike interval15. This raises the question whether an ageing code incorporating these parameters can be determined by comparing vagal firing patterns between old and young animals and whether the addition of luminal squalamine to the small intestine of aged mice can reduce or eliminate the ageing code. This would be important because the afferent vagus projects monosynaptically via the nucleus of the solitary tract to the hypothalamic arcuate nucleus16 that releases growth hormone releasing hormone which programs somatic ageing17, thus removal of the gut-derived vagus ageing code by squalamine might alter the functional effects of somatic ageing.
In addition, acute treatment of major depressive disorders with selective serotonin reuptake inhibitor (SSRI) antidepressants such as sertraline may reduce therapeutic efficacy in the aged12,18.To discover whether peripheral vagal mechanisms might contribute to this effect we applied intraluminal sertraline to the aged intestine to establish whether the SSRI can still facilitate afferent vagal firing as it does in young mice15. We also tested if squalamine, which evokes an antidepressant vagal code in young animals15, is more resistant to ageing in this regard than sertraline.
For the present article, mixed extracellular action potentials (multiunit responses) were recorded from the jejunal mesenteric nerve bundles, and extracellular discharge from individual single vagal fibres (single units) identified by their unique action potenial shape and amplitude15,19.
Patch clamp whole-cell recordings were made from the myenteric plexus using ex vivo segments of mouse jejunum as described in Mao et al. 20, and passive and active physiological properties of IPANs examined.
We identified the defining temporal characteristics of an ageing code for both IPANs and afferent vagal fibres when neurons from aged (18–24 mo) were compared to those from young (3 mo) animals. We also determined that the vagal ageing code was suppressed by application of squalamine.
Results
Young and old IPANs differ physiologically
We have previously reported10 that colonic migrating motor complexes for aged CD-1 mice have reduced velocity and frequency compared to similar motor complexes recorded from young mice. It is well-known that such colon and small intestine propulsive motor patterns are generated by the enteric nervous system21, pp. 81-89 and disappear when the enteric nervous system is absent22, when IPANs are selectively silenced pharmacologically23 or by point mutation-induced inhibition of protein kinase A in IPANs24.
In the current study the electrical characteristics of the IPANs of aged mice were compared with those of young ones. We identified all IPANs electrophysiologically as myenteric neurons that possessed a hump on the descending phase of their action potential and had a post-action potential slow afterhyperpolarisation (sAHP)20,25 For passive cell membrane characteristics, the resting membrane potential (Vm,) input resistance (Rin,) and leak conductance (gleak,) differed between young (green) and aged (brown) myenteric IPANs (Fig. 1a). However, neither membrane capacitance (Cm) nor action potential (AP) width at half height above baseline (AP1/2width) differed statistically between young and aged IPANs. Vm increased by 24% from -55 mV (effect size η2p = 0.46), Rin decreased by 35% from 265 MΩ (η2p = 0.31) and gleak increased by 76% from 4.1 nS (η2p = 0.50) when aged were compared to young neurons (Fig. 1a). For active membrane characteristics young IPANs showed greater excitability than young ones. The rheobase (threshold current for evoking a single action potential (AP) increased 136% from 45 pA (η2p = 0.44), average no. APs evoked by a stimulus pulse injected at twice rheobase intensity decreased by 36% from 2.8 (η2p = 0.38), and the magnitude (area under the curve) of the inhibitory slow afterhyperpolarisation (sAHPAUC) evoked by 3 APs increased by 46% from -59 mV.s (η2p = 0.35) (Fig. 1a). Representative traces of IPAN action potentials are shown in Fig. 1b (young) and Fig. 1d (aged), 1st order time differentials of the APs demonstrated that both young and aged IPANs possessed a hump on the AP descending phase indicating that Ca2+ influx contributed to the APs20,26. Fig. f & g illustrate AP firing at twice rheobase stimulus intensity for young and aged neurons, respectively. Figure 1h & i show traces of the sAHP for young (h) and aged (i) IPANs. 12 young and 8 aged IPANs were recorded from, of these 9 young and 7 aged were filed with neurobiotin marker dye and their shape recovered immunohistologically. All 16 had Dogiel type II morphology with smooth round or oval somas and long circumferentially-directed axons. Figure 1j & k show traces of young and aged IPANs revealing typical Dogiel type II morphology. In summary, the sAHP current increased with age. Ageing was also associated with hyperpolarised Vm, increased plasma membrane permeability (decreased Rin & increased gleak), and a reduction in the number of APs discharged; all of which would be associated with a larger background sAHP current. Contrariwise, cell capacitance, action potential shape and the action potential hump were unaltered by ageing.
a For this and all other bar graphs in the present paper, mean values given at base of bar graphs, open circles represent values measured from individual neurons. Resting membrane potentials (Vm) of aged are more polarized than those of young mice. Cm (membrane capacitance = measure of cell surface membrane area) was not different. Input resistance (Rin), a measure of number of open background channels = reciprocal of cell membrane leakiness)-aged IPANs had lower Rin. Background leak conductance (gleak) was lower for young than for old IPANs. Rheobase (threshold current required to evoke one action potential 50% of the time) was lower for young than old IPANs. The width of the action potential at half height of its positive amplitude (AP1/2 width) was not statistically different for young vs aged IPANs. The number of action potentials evoked at 2 x rheobase intensity (No. APs 2 x rheobase) was greater for young compared to aged IPANs. The area under the curve of the postaction potential slow afterhyperpolarisation (sAHP AUC) was greater for aged than young IPANs. b, d Representative action potential shapes for young and aged IPANs (colour-code: green indicates young, brown indicates aged). c, e First order time derivative of action potential showing that both old and young spikes have a hump (arrowhead) on their descending phase, indicating that calcium influx (which contributes significantly to AP1/2 width) did not differ notably between young and aged IPANs. f, g Example traces of action potentials evoked at 2x rheobase for young and aged IPANs. h, i Traces of post-action potential sAHP from young vs aged IPANs. Positions of truncated action potentials indicated by filled circles. j, k Examples of IPAN shapes revealed after intracellular neurobiotin dye filling. IPANs had smooth oval cell bodies with multiple long axonal processes running circumferentially within the myenteric plexus (Dogiel type II cell morphology). The full extent of these processes are not shown. Statistics: Nyoung = 12 (from 6 mice), Naged = 8 (from 4 mice). All comparisons made using unpaired t tests except for No. APs 2 x rheobase for which a Mann-Whitney test was applied. All tests were two-tailed. All error bars represent the standard error of the mean.
Aged jejunum mesenteric nerve bundles discharge multiunit spikes at a slower rate than those from young mice
Using a suction recording electrode we recorded the resting mixed (multiunit) AP extracellular spikes discharge from mesenteric nerve bundles attached to short jejunal segments. The average multiunit firing rated was 61% slower for aged compared to young mice (Fig. 2a). Representative examples in Fig. 2 of young vs aged multiunit spikes are given in panel b and c, respectively.
a Multiunit spike firing rates were statistically slower for recording made from aged compared to young mice. b Representative multiunit trace recorded from young animal. c Multiunit trace from aged animal. Statistics: a Comparison made using unpaired t test, two-tailed. Nerve fibre bundles: Nyoung = 8 and Naged = 22. Means given at base of bar graphs, error bars are standard errors.
Aged IPANs exposed to squalamine displayed a young physiological phenotype
We recorded from aged IPANs (see above) and 30 minutes after completing the first sets of physiological measurements, the Krebs buffer superfusate bathing the myenteric plexus preparation was switched to one that contained 30 μM squalamine lactate10; measurements were then repeated. Addition of squalamine had a general excitatory effect increasing the electroresponsiveness of IPANs to resemble that for young IPANs. The onset latency for depolarisation and reduction in sAHPAUC ranged from 4 to 5 min. For passive cell membrane characteristics: Vm depolarized by 21% to -54 mV (η2p = 0.61), Rin increased by 65% to 283 MΩ (η2p = 0.54), gleak decreased by 31% to 5.0 nS (η2p = 0.32). For active membrane characteristics: rheobase decreased by 45% to 58 pA (η2p = 0.34), the average number of AP fired at 2x rheobase increased by 67% to 3.0 (η2p = 0.47), and sAHPAUC decreased by 26% to -64 mV.s (η2p = 0.29) (Fig. 3a). Cm and AP1/2width were not altered by squalamine. Representative traces of IPAN action potentials in the presence of squalamine are shown in Fig. 3b-e. Figure 3b shows an AP and 3c gives the 1st order time differentials of the AP confirming that the IPAN AP possessed a hump on its descending phase indicating that Ca2+ influx contributed to the AP. Figure 3d & e illustrate the increased AP firing duration at twice rheobase stimulus intensity for an aged neurons in the presence of squalamine (cf. Fig. 1g). Figure 3e depicts the reduced sAHP in the presence of squalamine (cf. Fig. 1i).
a Squalamine depolarised Vm, increased Rin, reduced gleak decreased Rheobase, increased No. APs 2 x rheobase, and decreased sAHP AUC. Cm and AP1/2 width were not statistically different. b, c Traces of IPAN action potential (b) and first order time derivative (c) in the presence of squalamine. d Example of IPAN action potential discharge at 2 x rheobase stimulus intensity. e Representative post-action potential sAHP. Position of truncated action potential indicated by filled circle. Statistics: All experiments were paired comparing before (luminal Krebs buffer only) and after addition of squalamine to Krebs. N = 9 (from 5 mice). All comparisons made using paired t tests except for No. APs 2 x rheobase for which a Wilcoxon test was applied. All tests were two-tailed. All error bars represent the standard error of the mean.
Comparing young to aged vagal single unit resting discharge revealed an ageing code which was eliminated by adding squalamine to the lumen of aged jejunal segments
Single units were extracted from multiunit vagal nerve recordings using principle component analysis15. We measured several distinct parameters that fully describe the firing patterns observed within the 30 min recording periods used for each test sample. The parameters were: mean interspike interval (MII), average burst duration (BD), average gap duration between bursts (GD) and the average intraburst interval (IBI) (Fig. 4a). 70% of single vagal units recorded from young mice discharged ≥ 1 burst. Significantly fewer such events were detected for aged single units (Fig. 4b). When young were compared to aged mice, ageing increased MII, GD, IBI but not BD. MII increased by 427% (η2p = 0.20), GD by 38% (η2p = 0.19) and IBI by 46% (η2p = 0.22) (Fig. 4c).
a Diagram of stylised single unit discharge illustrating the 4 parameters measured to quantify individual spike (single unit) firing patterns. The parameters were: mean interspike interval (MII), burst duration (BD), gap duration (GD) and intraburst interval (IBI). b Bar graphs showing that the number of vagal fibre single units displaying more than 1 spike burst during the 30 min recording period was greater for young than old mice. c Dot plots with superimposed bar graphs (mean ± s.e.m.) showing values for all 4 single unit firing parameters. Nine young mice were compared to 19 aged ones. MII, GD and IBI reached statistical significance. d No statistical difference was discernible for any of 4 firing pattern parameters when recordings taken from the 19 young mice luminally perfused with only Krebs buffer were compared to those taken from 10 aged mice whose lumen was perfused with Krebs containing 30 μM squalamine. e The ageing code revealed by plotting fractional differences (mean ± s.e.m.) of aged vs. young mice for each of the 4 firing parameters. All of parameters except for BD contributed to the code. f Heat map of the parameter fractional differences showing that the ageing code is categorically different from the prodepressant (LPS) or antidepressant (JB-1, fluoxetine, sertraline) codes. g The ageing code was eliminated when the lumen of aged animals was perfused with Krebs with added squalamine and compared to young mice whose jejunal lumen was perfused with Krebs only. Statistics: b Contingency table analysis by two-sided Fisher’s exact test. c, d Comparisons of parameter means for young vs. aged or young vs. aged + squalamine made using Dunnett’s T3 multiple comparisons t tests.
Addition of 30 μM squalamine to the Krebs buffer perfusing the lumen reduced the sample mean differences for MII, BD, GD and IBI to statistically insignificant 8%, -4%, -5%, and 2%, respectively (Fig. 4d). Plotting fractional changes for aged compared to young mice for each of the 4 parameters revealed a unique, hitherto unknown, ageing code (Fig. 4e). The heat map in Fig. 4f demonstrates that the ageing code is categorically different for those previously calculated for prodepressant (LPS) or antidepressant (JB-1, fluoxetine or sertraline) luminal stimuli acting on the vagus15. The ageing code was absent when squalamine was present in the lumen (Fig. 4g) for which the standard error of the mean spanned the zero line for fractional differences.
The ageing code was conserved across sexes
The vagal code evoked by young vs. aged CD1 female mice was qualitatively similar for that for male mice (Fig. 5). Thus, both sexes revealed the canonical ageing code of increased MII, increased GD, and increased IBI.
a Dot plots with superimposed bar graphs (mean ± s.e.m.) showing values for all 4 single unit firing parameters. Five young mice were compared to 8 aged ones. MII, GD and IBI reached statistical significance. b No statistical difference was discernible for any of parameters when recordings taken from the 5 young mice perfused with only Krebs buffer were compared to those taken from 5 aged mice whose lumen was perfused with Krebs containing 30 μM squalamine. c The ageing code revealed by plotting fractional differences (mean ± s.e.m.) of aged vs young mice. All of parameters except for BD contributed to the code. d The ageing code was eliminated when the lumen of aged animals was perfused with squalamine. c,d, Comparisons of parameter means for young vs. aged or young vs. aged + squalamine made using Dunnett’s T3 multiple comparisons t tests.
Swiss Webster mice
Swiss Webster mice also exhibited the ageing code. We repeated the comparison between 17 young and 12 aged male mice for Swiss Webster (SW) mice and the effects of squalamine on the code to demonstrate that the ageing code is not strain specific. All protocols and the calculation of the ageing code were performed in the same manner as for CD-1 mice.
Clearly, SW mice also exhibited an ageing code when young were compared to aged mice (Supplementary Fig. 1). Additionally, as was the case for CD1 mice, addition of 10 µM intraluminal squalamine altered parameter levels for aged vagal fibres to levels seen in young mice on the ageing code disappeared in the presence of squalamine (Supplementary Fig. 1).
All of 9 young SW and 7 aged IPANs that were iontophoretically injected with Neurobiotin marker dye revealed the multipolar shapes characteristic of IPANs. Examples of SW mouse IPAN shapes revealed after intracellular Neurobiotin dye filling are shown in Supplementary Fig. 2.
Squalamine but not sertraline augmented afferent aged vagus spike firing
We have published15 that for young mice squalamine evokes a vagal code closely resembling that of sertraline. In the current study we compare the effects of 10 μM sertraline with those of 30 μM squalamine15 on the vagal code. These concentrations were the same as we have used previously15 for ex vivo vagal single unit recording and were chosen because we had shown that they activate vagal single units by ~20% above resting firing rates for young mice. When this study was conducted with preparations from aged mice, sertraline increased MII for most single units whereas squalamine decreased MII all units (Fig. 6a), with a greater proportion of single units discharging with ≥1 burst for units exposed to squalamine than to sertraline (Fig. 6b). The changes occurred due to an increase in MII for sertraline (Fig. 6c), and a decrease in MII and IBI for squalamine (Fig. 6d). These data suggest that, unlike for young mice, sertraline and squalamine have opposing effects for aged vagus single unit firing rates. There was also a reduced repertoire for complex discharge patterns involving action potential bursting when sertraline was compared to squalamine.
a Squalamine decreased MII for all aged single units tested, sertraline increased MII for more than 70% of units. b The proportion of aged single units with more than 1 burst in their firing pattern was higher for units exposed to intraluminal squalamine than for ones exposed to sertraline. c Sertraline increased MII for single units from aged mice with no statistically discernable effects on BD, GD or IBI. d Squalamine decreased MII and IBI for aged mice. Statistics: a, b Contingency table analysis by two-sided Fisher’s exact tests. c, d Comparisons of parameter means for aged vs. aged + sertraline or aged vs. aged + squalamine made using paired t tests. There were not enough single unit bursts (0 or 1) for aged mice to make statistical comparisons for GD. All error bars represent the standard error of the mean.
Representative event markers and sequential rate histograms depicting the 2 different representative single units from aged animals show that while intraluminal squalamine increased (Fig. 7a-c), sertraline decreased the firing rate (Fig. 7d-f). In contrast, sertraline increased the single-unit firing rate for a representative recording taken from a young vagal fibres (Fig. 7g-i). We have previously published that intraluminal squalamine increases the firing rate for young vagal fibres15. In register with this increase in young fibre firing rates, mucosal application of 30 μM squalamine (Fig. 7k) or 10 μM sertraline (Fig. 7i) increased the number of IPAN action potentials evoked by intracellular injection of 500 ms duration depolarising current pulses from 2.9 ± 0.2 to 5.4 ± 0.3 (P < 0.0001, paired t test, N = 11, 3 mice) for squalamine and from 2.9 ± 0.2 to 7.8 ± 0.2 (P < 0.0001, paired t test for sertraline, N = 12, 3 mice).
a Event markers showing the occurrence of an individual single unit during its 30 min recording period for intraluminal squalamine test for aged mouse. b Superimposed traces of 26 single units used to generate event markers for a. c Binned sequential rate histogram showing evolution of excitatory response to squalamine. d Single unit event markers showing occurrence of single unit during sertraline test for aged vagus. e 25 superimposed traces of single units used to generate event markers for d. f Binned sequential rate histogram showing reduction in single unit firing in response to intraluminal application of sertraline. g Event markers for single unit from young mouse. h 20 superimposed traces of single unit used for generation of event markers in g. i Histogram showing excitatory response for intraluminal sertraline in young mouse. k For young mice mucosal application of 30 μM squalamine increased the number of IPAN action potentials evoked by injection of 500 ms depolarising current pulse at 2x threshold intensity. i, Sertraline (10 μM) increased the number of action potentials evoked by injection of a 500 ms depolarising pulse.
Discussion
Our findings show IPANs of aged mice differed neurophysiologically from those of young mice. Using ex vivo patch clamp recordings from in situ myenteric IPANs we found that aged IPANs are less excitable than young ones. Aged IPANs had more hyperpolarised Vm, a smaller Rin and a greater gleak. For active membrane characteristics, aged IPANs had a greater Rheobase, a smaller No. APs 2 x Rheobase (smaller number of APs discharged at twice Rheobase stimulus intensity) and a greater sAHPAUC. Addition of squalamine to the Krebs buffer perfusing the gut lumen returned aged IPANs to a young electroresponsive phenotype.
Using extracellular AP recordings, we found that the average firing rate of jejunal mesenteric multiunit spikes was lower for aged than for young CD-1 mice confirming and extending a similar previous finding for which only a trend that did not quite reached statistical significance was reported10.
Single unit recordings revealed that APs recorded from young mouse tissue showed a greater number of bursts than those recorded using aged mice. Aged mice had a larger single unit mean interspike interval (MII), firing gap duration (GD) and within burst intraburst interval (IBI) while burst duration (BD) did not differ statistically. Plotting these parameters as fractional differences aged vs young revealed a unique sensory vagus ageing code. Addition of squalamine to the superfusate bathing the myenteric plexus abolished the ageing code changing the parameters measured from aged vagal fibres to values seen for young fibres. Interestingly, the ageing code, like the antidepressant code15, was not confined to only one mouse strain but was also present in Swiss Webster mice.
The vagus-stimulating action of sertraline, but not squalamine, was lost for aged mice. Aged vagal single units exposed to the antidepressant sertraline exhibited fewer bursts than those exposed to squalamine. Sertraline increased MII (slowing the firing rate) while having no statistical effects on the other parameters; in contrast squalamine enhanced vagal firing by decreasing MII and IBI.
Previous studies of ENS and intestinal vagal ageing have been confined only to anatomical changes, however a recent paper using 8-9 mo old hSNCAA53T constructs as a model for Parkinson’s disease showed reduced excitability for IPANs27. Colonic migrating motor complexes in aged (24 mo) mice are significantly slower than those in young (3 mo) ones10 and this is also consistent with decreased function of the aged ENS. Normal ageing has been associated with a plethora of intracellular molecular changes, including abnormal reactive oxygen species and Ca2+ levels that trigger mitochondrial dysfunction. In this regard, IPAN mitochondria influence the resting membrane potential and importantly play a role in the post-action potential uptake of Ca2+ released from intracellular stores28. Indeed, experimental impairment of mitochondria in young adult IPANs has been shown to lead to the exaggeration and prolongation of the sAHP, exactly as has been demonstrated in the present paper for aged IPANs28.
We confirmed our earlier results showing a trend that resting CD-1 mesenteric nerve multiunit firing rates were lower for aged than young mice10, and that intraluminal squalamine could restore the decreased firing rates for aged mice. Our mesenteric multiunit results were also consistent with studies that reported mesenteric nerve multiunit activity was lower for aged (64 y) than younger (47 y) humans and that there were fewer single unit bursts for aged humans.
In the present paper we reveal a canonical ageing code of increased MII, increased GD, and increased IBI for aged vs. young vagal single unit firing patterns. This code was present for both male and female CD-1 mice. Thus, if there are differences across the sexes in afferent vagal signalling, they are likely to be manifest in the central nervous system rather than at the level of the subdiaphragmatic vagus. We had previously published10 that the single unit mean interspike interval is larger for aged than for young CD-1 mice, and that addition of squalamine to the Krebs buffer perfusing the lumen of the aged jejunum returned MII to values recorded from young mice. However, no neurons in any animal nervous system seem to encode information solely by firing rate, rather firing patterns and intervals must be considered to understand how neurons encode information.
A simpler vagal code analysis has determined that for young (7-12 wks) male rats single unit spike burst frequencies increase in relation to eating29. A recent publication30 using only young 8-16 wk old mice used density-spaced clustering algorithms of spike shapes contained within cervical multiunit signals, recorded in vivo. This method revealed that injected cytokines could be identified by increases in the vagal single unit firing rate that the cytokines elicited30. Overall, and to the best of our knowledge, no analogous ageing code to the one being offered in the present paper has yet been published for any nervous system. As we have mentioned in the Introduction, some aged individuals are relatively resistant to the antidepressant effects of SSRIs, but a direct comparison between sertraline and squalamine on the firing rates of aged afferent vagal fibres also has not yet been revealed.
Why does the electrical excitability of IPANs and firing pattern of afferent vagal fibres change with old age? Some insight might be had from our current understanding of the potential mechanisms by which squalamine might be acting. We have previously shown that local administration of squalamine restores electrical activity in IPANs from Parkinson’s disease mouse models genetically engineered to accumulate aggregates of alpha-synuclein within the enteric neurons27. Normal peristalsis was restored and brain-directed vagal afferent is stimulated. In addition, orally administered squalamine successfully restored gut motility and several neurological symptoms in elderly patients with Parkinson’s disease-associated constipation in two a recently completed Phase 2b clinical trials31,32, demonstrating the translatability of the preclinical observations to humans.
In aqueous solution squalamine exists as a zwitterion with a net positive charge. As a consequence of its chemical structure it is highly amphiphilic, it is both highly water soluble and membrane active, and will bind electrostatically to membranes that contain anionic phospholipids and subsequently embed within the membrane33,34,35,36,37,38,39. Furthermore, previous studies have demonstrated that squalamine can effectively both displace proteins that are bound electrostatically to neuronal membranes and additionally, prevent their initial aggregation on the membrane surface40. For example, studies in C. elegans, engineered to express an aggregating human mutation in alpha-synuclein, develop paralysis as aggregates of alpha-synuclein accumulate within their excitable muscle cells40. Exposure of these worms to increasing concentrations of squalamine results in a proportional reduction in the number of protein aggregates and a dose-dependent increase in motility40 .Recent studies have demonstrated that misfolded proteins, defined as those that resist proteinase digestion, accumulate with ageing in all organs of the mouse41. These ageing-associated misfolded proteins could include alpha-synuclein, since numerous studies have reported that alpha-synuclein increases with age in older rats, monkeys, and humans42,43,44,45. These data are not surprising, as age is a key risk factor for many neurodegenerative disorders. Based on these observations we speculate that squalamine might improve the electrical excitability of the IPANs in the aged mouse through displacement of misfolded proteins from cellular membranes involved in neuronal electrical activity.
Numerous studies have shown that once integrated into a membrane the spatial organization of lipids within the membrane, fluidity, and tensile strength are altered37. Because squalamine electrostatically reduces the overall surface charge of the membrane, the function of membrane proteins positioned by electrostatic forces can be affected. For example, the application of squalamine to a mouse cortical neuron ex vivo activates the synaptic AMPA receptor46, and in other experimental settings inhibits the Type 3 sodium hydrogen exchanger, which regulates intracellular pH47. Thus, it is also possible that squalamine could enhance electrical excitability of the aged IPAN as a consequence of the modulation of the activity of membrane-associated proteins. The precise mechanism by which squalamine restores the electrical activity of the aged IPAN to a more youthful phenotype, however, remains to be determined.
Squalamine does have antibiotic activity and could perhaps alter gut propulsive activity either directly by stimulating IPANs (as shown here) or by lessening of Parkinsonian intestinal dysbiosis48,49. However, antibiotics may have conflicting effects on gut motility: bacitracin, neomycin, and penicillin V increase colonic propulsion50,51 while vancomycin or ampicillin decrease faecal output52. It is not clear that squalamine’s antimicrobial activity, per se, can explain its therapeutic effects on constipation in Parkinson’s disease.
In conclusion, our findings show that ageing is associated with decreased excitability of intrinsic primary afferent neurons in the enteric nervous system and a specific afferent jejunal gut-brain axis ageing code deduced from the resting firing pattern of vagal single unit fibres. The ageing code could be suppressed by intraluminal squalamine. We also showed that whilst sertraline decreased the firing rate of vagal afferent single units, squalamine retained the ability to excite vagal fibres for the aged vagus. These findings suggest targeting the vagus nerve using pharmacological or electroceutical approaches may be a productive research area for age related disorders involving the gut-brain axis.
Methods
Animals
Six to eight-week-old female and male CD-1 or male Swiss Webster (SW) mice were purchased from Charles River (Montreal, QC, Canada). Young animals (3 mo) were allowed to habituate to the animal facility for 1 week while older animals were housed until they were 18–24 mo of age when they were used for experimentation. Animals were housed 4/cage and under controlled conditions (21◦C) on a 12-h light/dark cycle (lights on at 5:00 a.m.) and fed ad libitum. All experiments were carried out in accordance with the guidelines of the Canadian Council on Animal Care and ARRIVE Guidelines and were approved by McMaster University’s Animal Research Ethics Board (Animal Utilisation Protocols: 16-08-30 & 20-05-21). Mice were euthanized by cervical dislocation and all action potential recordings performed ex vivo.
Enteric nervous system
A 2 cm segment of ileum was removed from freshly euthanized mice and the tissue was placed in a 2 ml recording petri dish whose inside base was lined with sylgard (170 silicone elastomer, Dow Corning, Midland, MI, USA) and filled with Krebs buffer of the following composition (mM): NaCl 118.1, KCl 4.8, NaHCO3 25, NaH2PO4 1.0, MgSO4 1.2, glucose 11.1, CaCl2 2.5; the buffer was continuously bubbled with carbogen (95% O2–5% CO2). Nicardipine (3 μM) (Sigma-Aldrich, Oakville, ON, Canada) was routinely added to the saline to prevent spontaneous muscle contraction. The segment was opened along a line parallel to the mesenteric attachment and pinned flat, under moderate tension, mucosa uppermost. The myenteric plexus was exposed by dissecting away the mucosa, submucosa, and circular muscle. The recording dish was then mounted on an inverted microscope (Nikon Eclipse TE 2000-S,Melville, NY, USA) and imaged via a PC computer using a Rolera-XR camera (Surrey, BC, Canada) and the tissue continuously superfused (4 ml min-1) with carbogenated Krebs warmed to 34 oC. A ganglion was prepared for patch clamping as described previously53; briefly, the selected ganglion was exposed by gravity flow for 15 min to 3 ml of 0.02% protease type XIV in Krebs saline (Sigma-Aldrich), then the upper surfaces of myenteric neurons were revealed by cleaning part of the ganglion with a fine hair until individual neuron soma became just visible. As noted previously53 there was no evidence of cell swelling after this gentle treatment.
Signals were measured in voltage recording (current clamp) mode using an Axon Instruments Multiclamp 700 A computer amplifier (Molecular Devices, San Jose, CA, USA), and a Digidata 1322 A (Axon Instruments) digitizer was used for A/D conversion. Signals were low pass, 4-point Bessel filtered at 5 kHz, and then digitized at 20 kHz. Data were stored on computer and analyzed offline using Pclamp software (Molecular Devices). Voltage or current commands were delivered to the amplifier under computer control using Clampex 9 (Molecular Devices) software. Patch pipettes were pulled on a Flaming-Brown-P97 (Sutter Instrument, Novato, CA, USA) electrode puller to produce micropipettes with resistances 4–6 MΩ. The patch pipettes were made from thick-walled borosilicate glass (Sutter Instrument) and filled with a solution of the following composition in mM: KMeSO4 110-115, NaCl 9, CaCl2 0.09, MgCl2 1.0, HEPES 10, Na3GTP 0.2, BAPTA.K4 0.2 with 0.2 % neurobiotin (Vector Laboratories, Newark, CA, US) 14 mL KOH to bring the pH to 7.3. The online program Maxchelator (Maxchelator:https://somapp.ucdmc.ucdavis.edu/pharmacology/bers/maxchelator) gives a predicted value free [Ca2+] of 0.18 μM at 34 oC54 for this intracellular solution. This value is close to resting free [Ca2+] as estimated using Ca2+-sensitive dyes in guinea pig Dogiel type II neurons (IPANs)55,56.
With the amplifier in voltage-clamp recording, about 50 hPa positive pressure was internally applied to the pipette before its tip entered the Krebs buffer superfusing the myenteric plexus preparation; the pressure was maintained until the tip was in close apposition to a neuron membrane. Only recordings with seal resistances ≥ 4 GΩ were used for analysis. After gigaseal formation, the amplifier was switched to current clamp recording and whole cell recording mode entered by further suction. During the recording period, depolarising or hyperprolarising current pulses could be injected, under computer control, via the patch pipette using Pclamp 9 Clampex software (Molecular Devices). Access resistance and cell membrane resistance, capacitance and time constants, were periodically monitored by software programmed switching to the Pclamp membrane test protocol which injects square wave pulses oscillating about the holding potential.
At the end of each recording, neurons were ionophoretically loaded with neurobiotin by passing 40 × 500 ms duration +0.1 nA current pulses via the patch pipette. The tissue was fixed in Zamboni’s fixative (2% v/v picric acid, 4% paraformaldehyde in 0.1 M Na2HPO4/NaH2PO4 buffer, pH = 7.0) overnight at 4 oC, and then cleared using 3 ×10 min washes of DMSO followed by 3 × 10 min washes with PBS. The tissue was then exposed to streptavidin-Texas Red (Vector Laboratories), diluted 1:50, to reveal neurobiotin. After final rinsing, the tissue was mounted in PBS containing 80% glycerol and 0.1% NaN3 and viewed under fluorescence epi-illumination on an inverted microscope (Nikon Eclipse TE 2000-S,Melville, NY, USA) and imaged using a Rolera-XR camera (Surrey, BC, Canada) Texas Red (596 nm & 620 nm excitation and emission peaks). Shapes of fluorescing neurons were traced using Inkscape 1.2 (Inkscape Project, available from: https://inkscape.org).
Mesenteric nerve recording
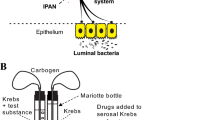
Mice were sacrificed by cervical dislocation and all action potential recordings performed ex vivo. Short (2.5 cm) segments of proximal jejunum with attached mesenteric arcade containing a single neuromuscular bundle were immediately removed and placed in a sylgard lined recording petri dish filled with Krebs buffer. The segment was emptied of contents using a syringe filled with Krebs, then both ends were cannulated with silicone tubing. The gut and mesenteric tissue were pinned to the sylgard using pins cut from 0.25 mm diameter tungsten wire and the mesenteric nerve bundle exposed by microdissection under a stereomicroscope. The preparation was then transferred to a Nikon Eclipse TE 2000 inverted microscope and the lumen gravity perfused at 1 ml/min with room temperature (22 °C) carbogenated Krebs or Krebs plus 30 μM squalamine dilactate using several Mariotte bottles57 attached to a plastic manifold. The serosal compartment was separately perfused at 5 ml/min with prewarmed (34°C) Krebs solution to which 3 µM nicardipine had been added to isolate vagal chemosensory responses by preventing active muscle contractions but not vagal responses to distension58.
The cleaned nerve was sucked into a glass-recording pipette attached to a patch-clamp electrode holder, and extracellular nerve recordings made with pClamp software using a Multi-Clamp 700B amplifier and Digidata 1440 A signal converter (Molecular Devices). The nerve bundle within the pipette was isolated from the Krebs within the recording dish by gently pressing the tip into fat tissue adherent to the uncleaned parts mesenteric arcade. Electrical signals were bandpass-filtered at 0.1–2 kHz, sampled at 20 kHz, and displayed and stored on a personal computer58.
Baseline recording with Krebs buffer in the gut lumen was performed for 15 min to verify that the resting firing rate was stationary using Pclamp software; samples with non-stationary discharge (windup or rundown) were discarded. Then recording continued for 30 min which constituted the test period for young vs aged comparisons. For experiments where squalamine or sertraline were added to the luminal perfusate, the Krebs buffer only control recording period was 30 min and this was followed by another 30 min of recording in the presence of either drug. Rundown of constitutive vagal discharge in this system is not evident until >90 min of recording58. Only one luminal test additive was applied once per animal to avoid possible signal rundown. After recording responses to luminal test substances and to allow post-hoc identification of vagal single units, we applied 0.2 ml CCK to the serosal surface of the jejunum using a handheld micropipette. Finally we distended the intestine by raising the intraluminal pressure to 14 hPa to demonstrate that the isolated single units could still respond to distension. Testing for the response of each of the isolated single units to CCK is a well-established method for identifying vagal fibres within the mesenteric nerve bundle58,59. Cholecystokinin (25–33) sulphated (AnaSpec, Fremont, CA, USA) was dissolved in dimethyl sulfoxide (DMSO) to make a 1 mM stock solution. Aliquots were diluted on the day of the experiment to a working concentration of 0.1 µM in Krebs buffer, with a final DMSO concentration ≤0.0001%.
We tested the following psychoactive agents: 10 µM sertraline hydrochloride15 (MilliporeSigma, Burlington, MA, USA). Squalamine dilactate was provided by Dr Michael Zasloff, Georgetown University (Washington, DC, United States). Squalamine dilactate powder was dissolved in 90% ethanol to make a stock solution, then aliquoted and stored at -20 °C until use. Stock solution was diluted in Krebs buffer to a working concentration of 30 µM for in vitro experiments15. These concentrations activate young adult vagal fibres by approximately the same intensity of ≈20% above baseline firing rates.
Analysis of single-unit firing patterns
Each analysed vagal single unit was discriminated from others in the multiunit recording using principal component analysis of their action potential shape, amplitude and width using the Dataview program60 for extracellular action potential analysis. Single units belonging to each vagal axon were converted into a single event point processes and displayed and used for further analysis15.
For each single unit event channel in Dataview point processes intervals vs time were displayed as using the event parameter histogram plot option and the mean interspike interval (MII) read from the descriptive statistics panel.
Event bursts were detected by the Poisson surprise method15,61. For each control or treatment event channel being measured in Dataview the Event analyse: Histograms/statistics option of the programme calculated the gap (GD) and burst (BD) durations15. For intraburst intervals (IBI) and the Krebs and treatment bursts event channels that were created by the Poisson surprise method were logically combined using the AND gate function, thus extracting only the bursts from the point process events for either the control or treatment recording periods.
Statistics and reproducibility
Descriptive statistics were calculated in GraphPad Prism ver. 8.3 (GraphPad Software, San Diego, USA) are given as mean ± standard errors. When a statistical test was performed, the P value given is the probability of the test statistic being at least as extreme as the one observed if the null hypothesis of no difference is admitted. The partial eta squared statistic η2p62, pp. 70-71 gives the effect size for differences calculated in the t-test module within GraphPad. According to Cohen’s guidelines63 for interpreting η2p, 0.01 indicates a small, 0.06 a medium and 0.14 a large effect size. Fractional changes in measured parameters, each given as mean ± standard error, were performed in GraphPad, which also calculated the propagated standard error for the fractional changes.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information files. Source data underlying figures are provided in Supplementary Data 1.
References
Berthoud, H. R., Jedrzejewska, A. & Powley, T. L. Simultaneous labeling of vagal innervation of the gut and afferent projections from the visceral forebrain with dil injected into the dorsal vagal complex in the rat. J. Comp. Neurol. 301, 65–79 (1990).
Phillips, R. J., Walter, G. C. & Powley, T. L. Age-related changes in vagal afferents innervating the gastrointestinal tract. Auton. Neurosci. 153, 90–98 (2010).
Ekblad, E., Winther, C., Ekman, R., Hakanson, R. & Sundler, F. Projections of peptide-containing neurons in rat small intestine. Neuroscience 20, 169–188 (1987).
Keast, J. R., Furness, J. B. & Costa, M. Origins of peptide and norepinephrine nerves in the mucosa of the guinea pig small intestine. Gastroenterology 86, 637–644 (1984).
Perez‐Burgos, A., Mao, Y. K., Bienenstock, J. & Kunze, W. A. The gut‐brain axis rewired: adding a functional vagal nicotinic sensory synapse. FASEB J. 28, 3064–3074 (2014).
Wade, P. R. & Cowen, T. Neurodegeneration: a key factor in the ageing gut. Neurogastroenterol. Motil. 16, 19–23 (2004).
Wade, P. R. I. Age-related changes in the enteric nervous system. Am. J. Physiol.-Gastrointest. Liver Physiol. 283, G489–G495 (2002).
Yu, Y. et al. Interplay between mast cells, enterochromaffin cells, and sensory signaling in the aging human bowel. Neurogastroenterol Motil. 28, 1465–1479 (2016).
Keating, C., Nocchi, L., Yu, Y., Donovan, J. & Grundy, D. Ageing and gastrointestinal sensory function: altered colonic mechanosensory and chemosensory function in the aged mouse. J. Physiol. 594, 4549–4564 (2016).
West, C. L. et al. Colonic Motility and Jejunal Vagal Afferent Firing Rates Are Decreased in Aged Adult Male Mice and Can Be Restored by an Aminosterol. Front Neurosci. 13, 955 (2019).
Pigato, G. et al. Vagus Nerve Stimulation in Treatment-Resistant Depression: A Case Series of Long-Term Follow-up. J. ECT 39(1), 23–27 (2022).
Tedeschini, E. et al. Efficacy of antidepressants for late-life depression: a meta-analysis and meta-regression of placebo-controlled randomized trials. J. Clin. Psychiatry 72, 1660–1668 (2011).
Gamage, P. P., Ranson, R. N., Patel, B. A., Yeoman, M. S. & Saffrey, M. J. Myenteric neuron numbers are maintained in aging mouse distal colon. Neurogastroenterol Motil, 25(7), e495–e505 (2013).
Saffrey, M. J. Cellular changes in the enteric nervous system during ageing. Dev. Biol. 382, 344–355 (2013).
West, C. L. et al. Identification of SSRI-evoked antidepressant sensory signals by decoding vagus nerve activity. Sci. Rep. 11, 21130 (2021).
Ricardo, J. A. & Koh, E. T. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res 153, 1–26 (1978).
Zhang, G. et al. Hypothalamic programming of systemic ageing involving IKK-beta, NF-kappaB and GnRH. Nature 497, 211–216 (2013).
Tham, A. et al. Efficacy and tolerability of antidepressants in people aged 65 years or older with major depressive disorder - A systematic review and a meta-analysis. J. Affect Disord. 205, 1–12 (2016).
Nullens, S. et al. In Vitro Recording of Mesenteric Afferent Nerve Activity in Mouse Jejunal and Colonic Segments. J. Vis. Exp. 116, 54576 (2016).
Mao, Y., Wang, B. & Kunze, W. Characterization of myenteric sensory neurons in the mouse small intestine. J. Neurophysiol. 96, 998–1010 (2006).
Furness, J. B. The enteric nervous system (Blackwell Publishing, Massachusetts, Oxford, Carlton, 2006).
Kunze, W. A. & Furness, J. B. The enteric nervous system and regulation of intestinal motility. Annu. Rev. Physiol. 61, 117–142 (1999).
Wang, B. et al. Lactobacillus reuteri ingestion and IK(Ca) channel blockade have similar effects on rat colon motility and myenteric neurones. Neurogastroenterol. Motil. 22, 98–107, e133 (2010).
Howe, D. G. et al. Inhibition of protein kinase A in murine enteric neurons causes lethal intestinal pseudo-obstruction. J. Neurobiol. 66, 256–272 (2006).
Bornstein, J. C., Furness, J. B. & Kunze, W. A. Electrophysiological characterization of myenteric neurons: how do classification schemes relate? J. Auton. Nerv. Syst. 48, 1–15 (1994).
Rugiero, F. et al. Analysis of whole-cell currents by patch clamp of guinea-pig myenteric neurones in intact ganglia. J. Physiol. 538, 447–463 (2002).
West, C. L. et al. Squalamine Restores the Function of the Enteric Nervous System in Mouse Models of Parkinson’s Disease. J. Parkinsons Dis. 10, 1477–1491 (2020).
Vanden Berghe, P., Kenyon, J. L. & Smith, T. K. Mitochondrial Ca2+ uptake regulates the excitability of myenteric neurons. J. Neurosci. 22, 6962–6971 (2002).
Marmerstein, J. T., McCallum, G. A. & Durand, D. M. Decoding Vagus-Nerve Activity with Carbon Nanotube Sensors in Freely Moving Rodents. Biosensors 12, 114 (2022).
Zanos, T. P. et al. Identification of cytokine-specific sensory neural signals by decoding murine vagus nerve activity. Proc. Natl. Acad. Sci. (2018).
Camilleri, M. et al. Oral ENT-01 Targets Enteric Neurons to Treat Constipation in Parkinson Disease : A Randomized Controlled Trial. Ann. Intern Med 175, 1666–1674 (2022).
Hauser, R. A. et al. Targeting neurons in the gastrointestinal tract to treat Parkinson’s disease. Clin. parkinsonism Relat. Disord. 1, 2–7 (2019).
Errico, S. et al. Making biological membrane resistant to the toxicity of misfolded protein oligomers: a lesson from trodusquemine. Nanoscale 12, 22596–22614 (2020).
Limbocker, R. et al. Squalamine and trodusquemine: two natural products for neurodegenerative diseases, from physical chemistry to the clinic. Nat. Prod. Rep. 39, 742–753 (2022).
Limbocker, R. et al. Trodusquemine displaces protein misfolded oligomers from cell membranes and abrogates their cytotoxicity through a generic mechanism. Commun. Biol. 3, 435 (2020).
Limbocker, R. et al. Squalamine and Its Derivatives Modulate the Aggregation of Amyloid-β and α-Synuclein and Suppress the Toxicity of Their Oligomers. Front Neurosci. 15, 680026 (2021).
Errico, S. et al. Quantitative Attribution of the Protective Effects of Aminosterols against Protein Aggregates to Their Chemical Structures and Ability to Modulate Biological Membranes. J. Med Chem. 66, 9519–9536 (2023).
Kreiser, R. P. et al. A Brain-Permeable Aminosterol Regulates Cell Membranes to Mitigate the Toxicity of Diverse Pore-Forming Agents. ACS Chem. Neurosci. 13, 1219–1231 (2022).
Capitini, C. et al. Studying the trafficking of labeled trodusquemine and its application as nerve marker for light-sheet and expansion microscopy. Faseb j. 36, e22655 (2022).
Perni, M. et al. A natural product inhibits the initiation of α-synuclein aggregation and suppresses its toxicity. Proc. Natl Acad. Sci. USA 114, E1009–e1017 (2017).
Cuanalo-Contreras, K. et al. Extensive accumulation of misfolded protein aggregates during natural aging and senescence. Front Aging Neurosci. 14, 1090109 (2022).
McCormack, A. L., Mak, S. K. & Di Monte, D. A. Increased α-synuclein phosphorylation and nitration in the aging primate substantia nigra. Cell Death Dis. 3, e315 (2012).
Bobela, W., Aebischer, P. & Schneider, B. L. Αlpha-Synuclein as a Mediator in the Interplay between Aging and Parkinson’s Disease. Biomolecules 5, 2675–2700 (2015).
Van Den Berge, N. et al. Ageing promotes pathological alpha-synuclein propagation and autonomic dysfunction in wild-type rats. Brain 144, 1853–1868 (2021).
Singh, Y. et al. Overexpression of human alpha-Synuclein leads to dysregulated microbiome/metabolites with ageing in a rat model of Parkinson disease. Mol. Neurodegener. 18, 44 (2023).
Sumioka, A., Yan, D. & Tomita, S. TARP phosphorylation regulates synaptic AMPA receptors through lipid bilayers. Neuron 66, 755–767 (2010).
Alexander, R. T. et al. Membrane surface charge dictates the structure and function of the epithelial Na+/H+ exchanger. Embo j. 30, 679–691 (2011).
Wallen, Z. D. et al. Metagenomics of Parkinson’s disease implicates the gut microbiome in multiple disease mechanisms. Nat. Commun. 13, 6958 (2022).
Sheng, S., Zhao, S. & Zhang, F. Insights into the roles of bacterial infection and antibiotics in Parkinson’s disease. Front Cell Infect. Microbiol 12, 939085 (2022).
Delungahawatta, T. et al. Antibiotic Driven Changes in Gut Motility Suggest Direct Modulation of Enteric Nervous System. Front Neurosci. 11, 588 (2017).
Forsythe, P., Kunze, W. & Bienenstock, J. Moody microbes or fecal phrenology: what do we know about the microbiota-gut-brain axis. in BMC Med. 14(1), 58 (2016).
Ge, X. et al. Antibiotics-induced depletion of mice microbiota induces changes in host serotonin biosynthesis and intestinal motility. J. Transl. Med 15, 13 (2017).
Kunze, W. A., Clerc, N., Furness, J. B. & Gola, M. The soma and neurites of primary afferent neurons in the guinea-pig intestine respond differentially to deformation. J. Physiol. 526, 375–385 (2000).
Bers, D. M., Patton, C. W. & Nuccitelli, R. A practical guide to the preparation of Ca2+ buffers. Methods Cell Biol. 40, 3–29 (1994).
Hillsley, K., Kenyon, J. L. & Smith, T. K. Ryanodine-sensitive stores regulate the excitability of AH neurons in the myenteric plexus of guinea-pig ileum. J. Neurophysiol. 84, 2777–2785 (2000).
Tatsumi, H., Hirai, K. & Katayama, Y. Measurement of the intracellular calcium concentration in guinea-pig myenteric neurons by using fura-2. Brain Res 451, 371–375 (1988).
McCarthy, E. L. Mariotte’s Bottle. Science 80, 100 (1934).
Perez-Burgos, A. et al. Psychoactive bacteria Lactobacillus rhamnosus (JB-1) elicits rapid frequency facilitation in vagal afferents. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G211–G220 (2013).
Richards, W., Hillsley, K., Eastwood, C. & Grundy, D. Sensitivity of vagal mucosal afferents to cholecystokinin and its role in afferent signal transduction in the rat. J. Physiol. 497, 473–481 (1996).
Heitler, W. J. DataView: A Tutorial Tool for Data Analysis. Template-based Spike Sorting and Frequency Analysis. J. Undergrad. Neurosci. Educ. 6, A1–A7 (2007).
Legendy, C. R. & Salcman, M. Bursts and recurrences of bursts in the spike trains of spontaneously active striate cortex neurons. J. Neurophysiol. 53, 926–939 (1985).
Motulsky, H. J. Analyzing Data with GraphPad Prism (Graphpad Software Inc. www.graphpad.com, San Diego, CA, 1999).
Cohen, J. Statistical power analysis for the behavioral sciences (Routledge, Abingdon. England, 1988).
Acknowledgements
This work was supported by grants RGPIN-2019-05982 & RGPIN-2021-03816 from the Natural Sciences and Engineering Research Council of Canada Discovery Grant Program awarded to WK. It was also supported by a Clifton W. Sherman Scholarship and a Queen Elizabeth II Graduate Scholarship in Science & Technology to CW.
Author information
Authors and Affiliations
Contributions
K.M.N., C.W., Y.M. performed the experiments. W.K. designed the study. and wrote the initial manuscript draft. K.M., A.S., D.B. & M.Z. contributed to study design and helped with the initial draft. P.F., M.A., H.H., E.I. helped with revisions and drafting. This paper is dedicated to the memory of John Bienenstock, Distinguished University Professor of Pathology and Molecular Medicine at McMaster University. John was the Founding Director of the Brain-Body Institute and a mentor to innumerable graduate students, post-doctoral fellows and visiting scientists. He was a personal friend and the inspiration behind the work described in the present paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Ibrahim Javed, Wenfei Han and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Karli Montague-Cardoso and George Inglis. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
McVey Neufeld, KA., Mao, YK., West, C.L. et al. Squalamine reverses age-associated changes of firing patterns of myenteric sensory neurons and vagal fibres. Commun Biol 7, 80 (2024). https://doi.org/10.1038/s42003-023-05623-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-023-05623-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.