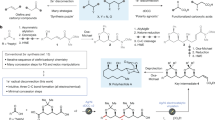
Abstract
Construction of C‒C bonds in medicinal chemistry frequently draws on the reductive coupling of organic halides with ketones or aldehydes. Catalytic C(sp3)‒C(sp3) bond formation, however, is constrained by the competitive side reactivity of radical intermediates following sp3 organic halide activation. Here, an alternative model deploys catalytic Ag surfaces for reductive fragment-based electrophile coupling compatible with sp3 organic halides. We use in situ spectroscopy, electrochemical analyses and simulation to uncover the catalytic interfacial structure and guide reaction development. Specifically, Mg(OAc)2 outcompetes the interaction between Ag and the aldehyde, thereby tuning the Ag surface for selective product formation. Data are consistent with an increased population of Mg-bound aldehyde facilitating the addition of a carbon-centred radical (product of Ag-electrocatalysed organic halide reduction) to the carbonyl. Electron transfer from Ag to the resultant alkoxy radical yields the desired alcohol. Molecular interfacial tuning at reusable catalytic electrodes will accelerate development of sustainable organic synthetic methods.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are included in the article (and its Supplementary Information) or available from the corresponding author on reasonable request.
References
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Huffman, B. J. et al. Electronic complementarity permits hindered butenolide heterodimerization and discovery of novel cGAS/STING pathway antagonists. Nat. Chem. 12, 310–317 (2020).
Wei, W., Cherukupalli, S., Jing, L., Liu, X. & Zhan, P. F. sp3: a new parameter for drug-likeness. Drug Discov. Today 25, 1839–1845 (2020).
Rappoport, Z. & Marek, I. (eds) The Chemistry of Organomagnesium Compounds (Wiley, 2008).
Wu, G. & Huang, M. Organolithium reagents in pharmaceutical asymmetric processes. Chem. Rev. 106, 2596–2616 (2006).
Schneider, N., Lowe, D. M., Sayle, R. A., Tarselli, M. A. & Landrum, G. A. Big data from pharmaceutical patents: a computational analysis of medicinal chemists bread and butter. J. Med. Chem. 59, 4385–4402 (2016).
Nicolaou, K. C., Ellery, S. P. & Chen, J. Samarium diiodide mediated reactions in total synthesis. Angew. Chem. Int. Ed. Engl. 48, 7140–7165 (2009).
Szostak, M., Fazakerley, N. J., Parmar, D. & Procter, D. J. Cross-coupling reactions using samarium(II) iodide. Chem. Rev. 114, 5959–6039 (2014).
Gil, A., Albericio, F. & Álvarez, M. Role of the Nozaki-Hiyama-Takai-Kishi reaction in the synthesis of natural products. Chem. Rev. 117, 8420–8446 (2017).
Garcia, K. J., Gilbert, M. M. & Weix, D. J. Nickel-catalyzed addition of aryl bromides to aldehydes to form hindered secondary alcohols. J. Am. Chem. Soc. 141, 1823–1827 (2019).
Swyka, R. A., Zhang, W., Richardson, J., Ruble, J. C. & Krische, M. J. Rhodium-catalyzed aldehyde arylation via formate-mediated transfer hydrogenation: beyond metallic reductants in Grignard/Nozaki-Hiyami-Kishi-type addition. J. Am. Chem. Soc. 141, 1828–1832 (2019).
Kadam, A. et al. Comparative performance evaluation and systematic screening of solvents in a range of Grignard reactions. Green. Chem. 15, 1880–1888 (2013).
Curran, D. P., Diederichsen, U. & Palovich, M. Radical cyclizations of acylgermanes. New reagent equivalents of the carbonyl radical acceptor synthon. J. Am. Chem. Soc. 119, 4797–4804 (1997).
Wilsey, S., Dowd, P. & Houk, K. N. Effect of alkyl substituents and ring size on alkoxy radical cleavage reactions. J. Org. Chem. 64, 8801–8811 (1999).
Salamone, M. & Bietti, M. Reaction pathways of alkoxyl radicals. The role of solvent effects on C–C bond fragmentation and hydrogen atom transfer reactions. Synlett. 25, 1803–1816 (2014).
Crabtree, R. H. in The Organometallic Chemistry of the Transition Metals (ed. Crabtree, R. H.) 185–203 (Wiley, 2014).
Zhang, W. & Lin, S. Electroreductive carbofunctionalization of alkenes with alkyl bromides via a radical-polar crossover mechanism. J. Am. Chem. Soc. 142, 20661–20670 (2020).
Zhang, W. et al. Electrochemically driven cross-electrophile coupling of alkyl halides. Nature 604, 292–297 (2022).
Wu, H. et al. Cathodic carbonyl alkylation of aryl ketones or aldehydes with unactivated alkyl halides. Org. Lett. 24, 9342–9347 (2022).
Roth, H., Romero, N. & Nicewicz, D. Experimental and calculated electrochemical potentials of common organic molecules for applications to single-electron redox chemistry. Synlett. 27, 714–723 (2015).
Gao, Y. et al. Electrochemical Nozaki-Hiyama-Kishi coupling: scope, applications, and mechanism. J. Am. Chem. Soc. 143, 9478–9488 (2021).
Rondinini, S., Mussini, P. R., Muttini, P. & Sello, G. Silver as a powerful electrocatalyst for organic halide reduction: the critical role of molecular structure. Electrochim. Acta 46, 3245–3258 (2001).
Isse, A. A., De Giusti, A., Gennaro, A., Falciola, L. & Mussini, P. R. Electrochemical reduction of benzyl halides at a silver electrode. Electrochim. Acta 51, 4956–4964 (2006).
Isse, A. A., Gottardello, S., Durante, C. & Gennaro, A. Dissociative electron transfer to organic chlorides: electrocatalysis at metal cathodes. Phys. Chem. Chem. Phys. 10, 2409–2416 (2008).
Huang, Y. F. et al. Bridging the gap between electrochemical and organometallic activation: benzyl chloride reduction at silver cathodes. J. Am. Chem. Soc. 132, 17199–17210 (2010).
Strawsine, L. M., Mubarak, M. S. & Peters, D. G. Use of silver cathodes to promote the direct reduction and intramolecular cyclization of ω-halo-1-phenyl-1-alkynes in dimethylformamide. J. Electrochem. Soc. 160, G3030–G3037 (2013).
Klymenko, O. V. et al. Uncovering the missing link between molecular electrochemistry and electrocatalysis: mechanism of the reduction of benzyl chloride at silver cathodes. Chem. Electro. Chem. 1, 227–240 (2014).
Strawsine, L. M., Sengupta, A., Raghavachari, K. & Peters, D. G. Direct reduction of alkyl monohalides at silver in dimethylformamide: effects of position and identity of the halogen. Chem. Electro. Chem. 2, 726–736 (2015).
Hartwig, J. F. in Organotransition Metal Chemistry: From Bonding to Catalysis (ed. Hartwig, J. F.) 301–320 (Univ. Science Books, 2010).
Diccianni, J. B., Katigbak, J., Hu, C. & Diao, T. Mechanistic characterization of (xantphos)Ni(I)-mediated alkyl bromide activation: oxidative addition, electron transfer, or halogen-atom abstraction. J. Am. Chem. Soc. 141, 1788–1796 (2019).
Sandford, C., Fries, L. R., Ball, T. E., Minteer, S. D. & Sigman, M. S. Mechanistic studies into the oxidative addition of Co(I) complexes: combining electroanalytical techniques with parameterization. J. Am. Chem. Soc. 141, 18877–18889 (2019).
Niu, D. F. et al. Electrocatalytic carboxylation of aliphatic halides at silver cathode in acetonitrile. Tetrahedron 64, 10517–10520 (2008).
Medvedev, J. J., Medvedeva, X. V., Engelhardt, H. & Klinkova, A. Relative activity of metal cathodes towards electroorganic coupling of CO2 with benzylic halides. Electrochim. Acta 387, 138528 (2021).
Corbin, N., Yang, D. T., Lazouski, N., Steinberg, K. & Manthiram, K. Suppressing carboxylate nucleophilicity with inorganic salts enables selective electrocarboxylation without sacrificial anodes. Chem. Sci. 12, 12365–12376 (2021).
Harwood, S. J. et al. Modular terpene synthesis enabled by mild electrochemical couplings. Science 375, 745–752 (2022).
Palkowitz, M. D. et al. Overcoming limitations in decarboxylative arylation via Ag-Ni electrocatalysis. J. Am. Chem. Soc. 144, 17709–17720 (2022).
Foresti, M. L., Innocenti, M., Forni, F. & Guidelli, R. Electrosorption valency and partial charge transfer in halide and sulfide adsorption on Ag(111). Langmuir 14, 7008–7016 (1998).
Isse, A. A. & Gennaro, A. Electrocatalytic carboxylation of benzyl chlorides at silver cathodes in acetonitrile. Chem. Commun. 23, 2798–2799 (2002).
Isse, A. A. et al. Electrocatalysis and electron transfer mechanisms in the reduction of organic halides at Ag. J. Appl. Electrochem. 39, 2217–2225 (2009).
Isse, A. A., Mussini, P. R. & Gennaro, A. New insights into electrocatalysis and dissociative electron transfer mechanisms: the case of aromatic bromides. J. Phys. Chem. C. 113, 14983–14992 (2009).
Syroeshkin, M. A. et al. Electrochemical behavior of N-oxyphthalimides: cascades initiating self-sustaining catalytic reductive N—O bond cleavage. J. Phys. Org. Chem. 30, e3744 (2017).
Douch, J. & Mousset, G. Electrochemical reduction of carbonyl compounds in non-aqueous solvent in the presence of EuCl3·6H2O. Can. J. Chem. 65, 549–556 (1987).
Ischay, M. A., Anzovino, M. E., Du, J. & Yoon, T. P. Efficient visible light photocatalysis of [2+2] enone cycloadditions. J. Am. Chem. Soc. 130, 12886–12887 (2008).
Fournier, F. & Fournier, M. Transferts d’électrons assistés par les métaux de transition:influence de la nature du cation métallique sur la réduction de composés carbonylés en milieu aprotique. Can. J. Chem. 64, 881–890 (2011).
Andrieux, C. P., Grzeszczuk, M. & Savéant, J.-M. Electrochemical generation and detection of transient intermediates: dimerizing species. J. Electroanal. Chem. Interfacial Electrochem. 318, 369–372 (1991).
Vasilenko, V., Blasius, C. K., Wadepohl, H., Gade, L. H. & Li, R. Borohydride intermediates pave the way for magnesium-catalysed enantioselective ketone reduction. Chem. Commun. 56, 1203–1206 (2020).
Reetz, M. T., Harmat, N. & Mahrwald, R. Ligand effects in Grignard additions. Angew. Chem. Int. Ed. Engl. 31, 342–344 (1992).
Fournier, V., Marcus, P. & Olefjord, I. Oxidation of magnesium. Surf. Interface Anal. 34, 494–497 (2002).
Hou, S. et al. Solvation sheath reorganization enables divalent metal batteries with fast interfacial charge transfer kinetics. Science 374, 172–178 (2021).
Osawa, M. Dynamic processes in electrochemical reactions studied by surface-enhanced infrared absorption spectroscopy (SEIRAS). Bull. Chem. Soc. Jpn 70, 2861–2880 (1997).
Osawa, M. Diffraction and Spectroscopic Methods in Electrochemistry (Advances in Electrochemical Science and Engineering (eds Alkire, R. C. et al.) 269–314 (Wiley-VCH, 2006).
Miyake, H., Ye, S. & Osawa, M. Electroless deposition of gold thin films on silicon for surface-enhanced infrared spectroelectrochemistry. Electrochem. Commun. 4, 973–977 (2002).
Yaguchi, M., Uchida, T., Motobayashi, K. & Osawa, M. Speciation of adsorbed phosphate at gold electrodes: a combined surface-enhanced infrared absorption spectroscopy and DFT study. J. Phys. Chem. Lett. 7, 3097–3102 (2016).
Ros, R., Leenarda, M., Boschi, T. & Roulet, R. Cyanobenzylpalladium(H) complexes. Synthesis and spectroscopic properties. Inorg. Chim. Acta 25, 61–64 (1977).
Delley, M. F., Nichols, E. M. & Mayer, J. M. Interfacial acid-base equilibria and electric fields concurrently probed by in situ surface-enhanced infrared spectroscopy. J. Am. Chem. Soc. 143, 10778–10792 (2021).
Kuş, N., Sharma, A., Reva, I., Lapinski, L. & Fausto, R. Thermal and photoinduced control of relative populations of 4-methoxybenzaldehyde (p-anisaldehyde) conformers. J. Phys. Chem. A 114, 7716–7724 (2010).
Gunasekaran, S., Seshadri, S., Muthu, S., Kumaresan, S. & Arunbalaji, R. Vibrational spectroscopy investigation using ab initio and density functional theory on p-anisaldehyde. Spectrochim. Acta A 70, 550–556 (2008).
Fathima, A. A., Umadevi, M. & Ramakrishnan, V. Changes in spectral features with varying mole fractions of anisaldehyde in binary mixtures. J. Raman Spectrosc. 38, 271–276 (2007).
Bron, M. et al. Bridging the pressure and materials gap: in-depth characterisation and reaction studies of silver-catalysed acrolein hydrogenation. J. Catal. 234, 37–47 (2005).
Wang, K. & Yang, B. Theoretical understanding on the selectivity of acrolein hydrogenation over silver surfaces: the non-Horiuti–Polanyi mechanism is the key. Catal. Sci. Technol. 7, 4024–4033 (2017).
Wang, A. et al. In situ identification of intermediates of benzyl chloride reduction at a silver electrode by SERS coupled with DFT calculations. J. Am. Chem. Soc. 132, 9534–9536 (2010).
Gao, P. & Weaver, M. J. Surface-enhanced Raman spectroscopy as a probe of adsorbate-surface bonding: benzene and monosubstituted benzenes adsorbed at gold electrodes. J. Phys. Chem. 89, 5040–5046 (1985).
Cai, W. Bin et al. Orientational phase transition in a pyridine adlayer on gold(111) in aqueous solution studied by in situ infrared spectroscopy and scanning tunneling microscopy. Langmuir 14, 6992–6998 (1998).
Pang, S. F., Wu, C. Q., Zhang, Q. N. & Zhang, Y. H. The structural evolution of magnesium acetate complex in aerosols by FTIR–ATR spectra. J. Mol. Struct. 1087, 46–50 (2015).
Revunova, K. & Nikonov, G. I. Main group catalysed reduction of unsaturated bonds. Dalt. Trans. 44, 840–866 (2014).
Falconnet, A., Magre, M., Maity, B., Cavallo, L. & Rueping, M. Asymmetric magnesium‐catalyzed hydroboration by metal‐ligand cooperative catalysis. Angew. Chem. Int. Ed. 58, 17567–17571 (2019).
Chaussard, J. et al. Use of sacrificial anodes in electrochemical functionalization of organic halides. Synthesis 1990, 369–381 (1990).
Klein, M. & Waldvogel, S. R. Counter electrode reactions—important stumbling blocks on the way to a working electro-organic synthesis. Angew. Chem. Int. Ed. 61, e202204140 (2022).
Novaes, L. F. T. et al. Electrocatalysis as an enabling technology for organic synthesis. Chem. Soc. Rev. 50, 7941–8002 (2021).
Boymond, L., Rottländer, M., Rard Cahiez, G. Â. & Knochel, P. Preparation of highly functionalized Grignard reagents by an iodine—magnesium exchange reaction and its application in solid-phase synthesis. Angew. Chem. Int. Ed. 37, 1701–1703 (1998).
Lee, J. S., Velarde-Ortiz, R., Guijarro, A., Wurst, J. R. & Rieke, R. D. Low-temperature formation of functionalized Grignard reagents from direct oxidative addition of active magnesium to aryl bromides. J. Org. Chem. 65, 5428–5430 (2000).
Shimizu, K. I., Sato, R. & Satsuma, A. Direct C-C cross-coupling of secondary and primary alcohols catalyzed by a γ-alumina-supported silver subnanocluster. Angew. Chem. Int. Ed. 48, 3982–3986 (2009).
Varshney, S., Bar-Ziv, R. & Zidki, T. On the remarkable performance of silver-based alloy nanoparticles in 4-nitrophenol catalytic reduction. Chem. Cat. Chem. 12, 4680–4688 (2020).
Savéant, J.-M. & Costentin, C. Elements of Molecular and Biomolecular Electrochemistry: An Electrochemical Approach to Electron Transfer Chemistry (Wiley, 2019).
Wuttig, A., Ryu, J. & Surendranath, Y. Electrolyte competition controls surface binding of CO intermediates to CO2 reduction catalysts. J. Phys. Chem. C. 125, 17042–17050 (2021).
Huo, S. J., Xue, X. K., Li, Q. X., Xu, S. F. & Cai, W. B. Seeded-growth approach to fabrication of silver nanoparticle films on silicon for electrochemical ATR surface-enhanced IR absorption spectroscopy. J. Phys. Chem. B 110, 25721–25728 (2006).
Savéant, J.-M. & Vianello, E. Potential-sweep voltammetry: theoretical analysis of monomerization and dimerization mechanisms. Electrochim. Acta 12, 1545–1561 (1967).
Savéant, J.-M. & Costentin, C. in Elements of Molecular and Bimolecular Electrochemistry (eds Savéant, J.-M. & Costentin, C.) 81–181 (Wiley, 2019).
Tsierkezos, N. G. Investigation of the electrochemical reduction of benzophenone in aprotic solvents using the method of cyclic voltammetry. J. Solut. Chem. 36, 1301–1310 (2007).
Wang, R. et al. Catalytic reduction of O2 by pyrazine derivatives. J. Phys. Chem. A 108, 1891–1899 (2004).
Marouani, S., Louati, A. & Gross, M. Influence du solvant sur la reduction electrochimique du nitrate et de l’acetate d’uranyle. Electrochim. Acta 33, 147–155 (1988).
Savéant, J.-M. & Vianello, E. Étude de la polarisation chimique en régime de variation linéaire du potentiel. Cas d’une désactivation spontanée, rapide et irréversible du produit de la réduction. C. R. Acad. Sci. 256, 2597–2600 (1963).
Savéant, J.-M. & Vianello, E. Potential-sweep voltammetry: general theory of chemical polarization. Electrochim. Acta 12, 629–646 (1967).
Hall, A. S., Yoon, Y., Wuttig, A. & Surendranath, Y. Mesostructure-induced selectivity in CO2 reduction catalysis. J. Am. Chem. Soc. 137, 14834–14837 (2015).
Visscher, W. Cyclic voltammetry on lead electrodes in sulphuric acid solution. J. Power Sources 1, 257–266 (1976).
Acknowledgements
We thank the research laboratories of J. Anderson, G. Dong, S. Snyder, V. Rawal, C. Amanchukwu and M. Hopkins for sharing their chemical inventories. We thank A. Tokmakoff for helpful discussions on spectroscopic data. We thank A. Filatov, K. Glusac and B. Behera for assistance with XPS data collection at the University of Chicago and the University of Illinois at Chicago, respectively. We thank J. Kurutz for assistance with NMR data collection at the University of Chicago. This research made use of the University of Chicago Mass Spectrometry Facility (National Science Foundation instrumentation grant no. CHE-1048528). This research was partially supported by the University of Chicago startup funds and a James R. Norris, Jr. fellowship to S.K. (University of Chicago, Department of Chemistry). Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number 1R35GM150845.
Author information
Authors and Affiliations
Contributions
A.W. coordinated and conceived the research. A.W., Q.-C.C. and S.K. designed the experiments. A.W., Q.-C.C., S.K. and R.M. performed the experiments. A.W., Q.-C.C. and S.K. analysed data. A.W., Q.-C.C. and S.K. wrote the paper with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Angel Cuesta and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Tables 1–6, Figs. 1–143 and references.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, QC., Kress, S., Molinelli, R. et al. Interfacial tuning of electrocatalytic Ag surfaces for fragment-based electrophile coupling. Nat Catal 7, 120–131 (2024). https://doi.org/10.1038/s41929-023-01073-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-01073-5
This article is cited by
-
Understanding organic electrosynthesis
Nature Catalysis (2024)