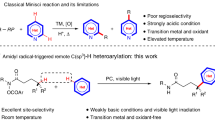
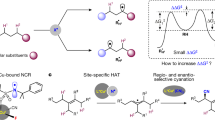
Abstract
Homolytic aromatic substitution is an enabling strategy for arene C–H functionalization with numerous important synthetic applications. However, despite notable advances, exerting control over site selectivity remains a formidable challenge. Here we report a unified highly site-selective arene C–H amination of various electronically distinct arenes using an iron-aminyl radical. Unlike the conventionally employed free nitrogen-centred radicals, the metal-supported aminyl radical possesses a general attractive interaction with diverse inherent functional groups in arene substrates, engendering extraordinary regioselectivity through substrate chelation. Importantly, this approach has the ability to override the inherent influences of polar and steric effects that typically dictate regiochemical control in homolytic aromatic substitution, leading to high site selectivity. The broad scope of applicable directing groups renders this method a powerful tool to streamline the synthesis of various important aniline building blocks and pharmaceuticals, and allows for the late-stage C–H amination of structurally diverse drugs and other bioactive molecules.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Experimental procedures and characterization data along with computational information are included in the Supplementary Information. All other data are available from the corresponding authors upon reasonable request.
References
Mortier, J. (ed.) Arene Chemistry: Reaction Mechanisms and Methods for Aromatic Compounds (Wiley, 2015).
Ruffoni, A., Mykura, R., Bietti, M. & Leonori, D. The interplay of polar effects in controlling the selectivity of radical reactions. Nat. Synth. 1, 682–695 (2022).
Bowman, W. R. & Storey, J. M. D. Synthesis using aromatic homolytic substitution—recent advances. Chem. Soc. Rev. 36, 1803–1822 (2007).
Proctor, R. S. J. & Phipps, R. J. Recent advances in Minisci-type reactions. Angew. Chem. Int. Ed. 58, 13666–13699 (2019).
Boursalian, G. B., Ham, W. S., Mazzotti, A. R. & Ritter, T. Charge-transfer-directed radical substitution enables para-selective C–H functionalization. Nat. Chem. 8, 810–815 (2016).
Ruffoni, A. et al. Practical and regioselective amination of arenes using alkyl amines. Nat. Chem. 11, 426–433 (2019).
Fan, W.-T., Li, Y., Wang, D., Ji, S.-J. & Zhao, Y. Iron-catalyzed highly para-selective difluoromethylation of arenes. J. Am. Chem. Soc. 142, 20524–20530 (2020).
Choi, J., Laudadio, G., Godineau, E. & Baran, P. S. Practical and regioselective synthesis of C-4-alkylated pyridines. J. Am. Chem. Soc. 143, 11927–11933 (2021).
Ricci, A. (ed.) Amino Group Chemistry: from Synthesis to the Life Sciences (Wiley-VCH, 2008).
Schaefer, T. et al. 4H-Imidazo[1,2-a]imidazole derivatives for electronic applications. World Intellectual Property Organization WO2013068376A1 (2013).
Zavialov, K. V. et al. Preparation of substituted pyrrolopyridines and pyrrolopyrimidines as epidermal growth factor receptor inhibitors. World Intellectual Property Organization WO2022146201A1 (2022).
Mellini, P. et al. Identification of diketopiperazine-containing 2‑anilinobenzamides as potent sirtuin 2 (SIRT2)-selective inhibitors targeting the “selectivity pocket”, substrate-binding site, and NAD+‑binding site. J. Med. Chem. 62, 5844–5862 (2019).
Weintraub, P. M. et al. Preparation of substituted pyridinones and pyridines as inhibitors of poly(ADP-ribose) polymerases (PARP). World Intellectual Property Organization WO2005097750A1 (2005).
Alonso, C. et al. Novel topoisomerase I inhibitors. Syntheses and biological evaluation of phosphorus substituted quinoline derivates with antiproliferative activity. Eur. J. Med. Chem. 149, 225–237 (2018).
Tejería, A. et al. Antileishmanial activity of new hybrid tetrahydroquinoline and quinoline derivatives with phosphorus substituents. Eur. J. Med. Chem. 162, 18–31 (2019).
Shen, Q. & Hartwig, J. F. Palladium-catalyzed coupling of ammonia and lithium amide with aryl halides. J. Am. Chem. Soc. 128, 10028–10029 (2006).
Surry, D. S. & Buchwald, S. L. Selective palladium-catalyzed arylation of ammonia: synthesis of anilines as well as symmetrical and unsymmetrical di- and triarylamines. J. Am. Chem. Soc. 129, 10354–10355 (2007).
Kim, J. & Chang, S. Ammonium salts as an inexpensive and convenient nitrogen source in the Cu-catalyzed amination of aryl halides at room temperature. Chem. Commun. 2008, 3052−3054 (2008).
Jiao, J., Murakami, K. & Itami, K. Catalytic methods for aromatic C−H amination: an ideal strategy for nitrogen-based functional molecules. ACS Catal. 6, 610–633 (2016).
Park, Y., Kim, Y. & Chang, S. Transition metal-catalyzed C−H amination: scope, mechanism, and applications. Chem. Rev. 117, 9247–9301 (2017).
Morofuji, T., Shimizu, A. & Yoshida, J.-I. Electrochemical C–H amination: synthesis of aromatic primary amines via N-arylpyridinium ions. J. Am. Chem. Soc. 135, 5000–5003 (2013).
Ham, W. S., Hillenbrand, J., Jacq, J., Genicot, C. & Ritter, T. Divergent late-stage (hetero)aryl C−H amination by the pyridinium radical cation. Angew. Chem. Int. Ed. 58, 532–536 (2019).
Rössler, S. L. et al. Pyridyl radical cation for C−H amination of arenes. Angew. Chem. Int. Ed. 58, 526–531 (2019).
Zheng, Y.-W. et al. Photocatalytic hydrogen-evolution cross-couplings: benzene C–H amination and hydroxylation. J. Am. Chem. Soc. 138, 10080–10083 (2016).
Romero, N. A., Margrey, K. A., Tay, N. E. & Nicewicz, D. A. Site-selective arene C–H amination via photoredox catalysis. Science 349, 1326–1330 (2015).
Paudyal, M. P. et al. Dirhodium-catalyzed C−H arene amination using hydroxylamines. Science 353, 1144–1147 (2016).
Wang, T. et al. A metal-free direct arene C−H amination. Adv. Synth. Catal. 363, 2783–2795 (2021).
Kim, H., Heo, J., Kim, J., Baik, M.-H. & Chang, S. Copper-mediated amination of aryl C–H bonds with the direct use of aqueous ammonia via a disproportionation pathway. J. Am. Chem. Soc. 140, 14350–14356 (2018).
Yu, L. et al. Ammonia as ultimate amino source in synthesis of primary amines via nickel-promoted C–H bond amination. Org. Lett. 21, 5634–5638 (2019).
Anugu, R. R., Munnuri, S. & Falck, J. R. Picolinate-directed arene meta-C−H amination via FeCl3 catalysis. J. Am. Chem. Soc. 142, 5266–5271 (2020).
Xu, L.-L. et al. Copper mediated C–H amination with oximes: en route to primary anilines. Chem. Sci. 9, 5160–5164 (2018).
Li, Z., Yu, H. & Bolm, C. Dibenzothiophene sulfoximine as an NH3 surrogate in the synthesis of primary amines by copper-catalyzed C−X and C−H bond amination. Angew. Chem. Int. Ed. 56, 9532–9535 (2017).
Matsubara, T., Asako, S., Ilies, L. & Nakamura, E. Synthesis of anthranilic acid derivatives through iron-catalyzed ortho amination of aromatic carboxamides with N‑chloroamines. J. Am. Chem. Soc. 136, 646–649 (2014).
Peng, J., Chen, M., Xie, Z., Luo, S. & Zhu, Q. Copper-mediated C(sp2)–H amination using TMSN3 as a nitrogen source: redox-neutral access to primary anilines. Org. Chem. Front. 1, 777–781 (2014).
Tezuka, N. et al. Direct hydroxylation and amination of arenes via deprotonative cupration. J. Am. Chem. Soc. 138, 9166–9171 (2016).
Pratley, C., Fenner, S. & Murphy, J. A. Nitrogen-centered radicals in functionalization of sp2 systems: generation, reactivity, and applications in synthesis. Chem. Rev. 122, 8181–8260 (2022).
Minisci, F., Galli, R. & Cecere, M. Homolytic amination of aromatic compounds by redox systems. Reactivity and orientation. Tetrahedron Lett. 6, 4663–4667 (1965).
Legnani, L., Cerai, G. P. & Morandi, B. Direct and practical synthesis of primary anilines through iron-catalyzed C−H bond amination. ACS Catal. 6, 8162–8165 (2016).
Liu, J. et al. Iron-catalyzed amination of (hetero)arenes with a redox-active aminating reagent under mild conditions. Chem. Eur. J. 23, 563–567 (2017).
D’Amato, E. M., Börgel, J. & Ritter, T. Aromatic C–H amination in hexafluoroisopropanol. Chem. Sci. 10, 2424–2428 (2019).
See, Y. Y. & Sanford, M. S. C−H amination of arenes with hydroxylamine. Org. Lett. 22, 2931–2934 (2020).
Gillespie, J. E., Morrill, C. & Phipps, R. J. Regioselective radical arene amination for the concise synthesis of ortho-phenylenediamines. J. Am. Chem. Soc. 143, 9355–9360 (2021).
Morrill, C., Gillespie, J. E. & Phipps, R. J. An aminative rearrangement of O-(arenesulfonyl)hydroxylamines: facile access to ortho-sulfonyl anilines. Angew. Chem. Int. Ed. 61, e202204025 (2022).
Gillespie, J. E., Lam, N. & Phipps, R. J. Ortho-selective amination of arene carboxylic acids via rearrangement of acyl O-hydroxylamines. Chem. Sci. 14, 10103–10111 (2023).
Qiu, X. et al. Cleaving arene rings for acyclic alkenylnitrile synthesis. Nature 597, 64–69 (2021).
Olivos Suarez, A. I., Lyaskovskyy, V., Reek, J. N. H., van der Vlugt, J. I. & de Bruin, B. Complexes with nitrogen-centered radical ligands: classification, spectroscopic features, reactivity, and catalytic applications. Angew. Chem. Int. Ed. 52, 12510–12529 (2013).
Hennessy, E. T. & Betley, T. A. Complex N-heterocycle synthesis via iron-catalyzed, direct C–H bond amination. Science 340, 591–595 (2013).
Li, J. et al. Site-specific allylic C–H bond functionalization with a copper-bound N-centred radical. Nature 574, 516–521 (2019).
Nakafuku, K. M. et al. Enantioselective radical C–H amination for the synthesis of β-amino alcohols. Nat. Chem. 12, 697–704 (2020).
Xu, P., Xie, J.-J., Wang, D.-S. & Zhang, X. P. Metalloradical approach for concurrent control in intermolecular radical allylic C−H amination. Nat. Chem. 15, 498–507 (2023).
Wiese, S. et al. Catalytic C–H amination with unactivated amines through copper(II) amides. Angew. Chem. Int. Ed. 49, 8850–8855 (2010).
Gephart, R. T. III et al. Catalytic C–H amination with aromatic amines. Angew. Chem. Int. Ed. 51, 6488–6492 (2012).
Sambiagio, C. et al. A comprehensive overview of directing groups applied in metal-catalysed C–H functionalisation chemistry. Chem. Soc. Rev. 47, 6603–6743 (2018).
Chatterjee, S. et al. A combined spectroscopic and computational study on the mechanism of iron-catalyzed aminofunctionalization of olefins using hydroxylamine derived N−O reagent as the “amino” source and “oxidant”. J. Am. Chem. Soc. 144, 2637–2656 (2022).
Zhou, Y. et al. Mechanism and reaction channels of iron-catalyzed primary amination of alkenes by hydroxylamine reagents. ACS Catal. 13, 1863–1874 (2023).
Fürstner, A. Iron catalysis in organic synthesis: a critical assessment of what it takes to make this base metal a multitasking champion. ACS Cent. Sci. 2, 778–789 (2016).
Ndubaku, C. O. et al. Pyrazolopyrimidinone compounds and uses thereof. World Intellectual Property Organization WO2019055750A1 (2019).
Liu, Y.-J. et al. Overcoming the limitations of directed C–H functionalizations of heterocycles. Nature 515, 389–393 (2014).
Acknowledgements
We acknowledge the National Natural Science Foundation of China (nos 22101140, 22371143 and 22188101 to F.W. and no. 22073067 to Y.D.), the Fundamental Research Funds for the Central Universities (no. 63223009 to F.W.) and the Haihe Laboratory of Sustainable Chemical Transformations, Frontiers Science Center for New Organic Matter at Nankai University (no. 63181206 to F.W.) for financial support. We thank S.-Y. Xin and J.-K. Cheng from the F.W. group for the experimental assistance. We acknowledge S. S. Stahl (University of Wisconsin–Madison), J. A. Buss (University of Michigan) and D. Wang (Marquette University) for the manuscript proofreading and helpful discussions.
Author information
Authors and Affiliations
Contributions
C.-R.M. and G.-W.H. carried out experimental work with support from Z.-L.W. and Z.-H.L.; H.X. and Y.D. performed density functional theory calculations. C.-R.M. and Y.Y. carried out electron paramagnetic resonance measurements. J.L. and G.L. carried out high-resolution mass spectrometry measurements. F.W. handled the project administration. Y.D. and F.W. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks John Falck, Anthony J. Fernandes and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–25, Tables 1–10, Methods and References.
Supplementary Data 1
Cartesian coordinates of optimized structures.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, CR., Huang, GW., Xu, H. et al. Site-selective arene C–H amination with iron-aminyl radical. Nat Catal (2024). https://doi.org/10.1038/s41929-024-01140-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41929-024-01140-5