Abstract
Lung cancer is still the leading cause of cancer-related mortality. Over the past two decades, the management of non-small cell lung cancer (NSCLC) has undergone a significant revolution. Since the first identification of activating mutations in the epidermal growth factor receptor (EGFR) gene in 2004, several genetic aberrations, such as anaplastic lymphoma kinase rearrangements (ALK), neurotrophic tropomyosin receptor kinase (NTRK) and hepatocyte growth factor receptor (MET), have been found. With the development of gene sequencing technology, the development of targeted drugs for rare mutations, such as multikinase inhibitors, has provided new strategies for treating lung cancer patients with rare mutations. Patients who harbor this type of oncologic driver might acquire a greater survival benefit from the use of targeted therapy than from the use of chemotherapy and immunotherapy. To date, more new agents and regimens can achieve satisfactory results in patients with NSCLC. In this review, we focus on recent advances and highlight the new approval of molecular targeted therapy for NSCLC patients with rare oncologic drivers.
Similar content being viewed by others
Introduction
Lung cancer is a widespread form of cancer that affects many individuals around the world. While the treatment of advanced lung cancer has undergone significant improvements in the past two decades, the global cancer statistics continue to reveal a grim reality. Over 1.8 million patients succumb to lung cancer each year, with incidence rates continuing to rise in developing countries. In both the USA and China, lung cancer will remain the leading cause of cancer-related mortality in 2022. This troubling outcome is largely attributed to the fact that more than half of all lung cancer patients are diagnosed with advanced stage lung cancer. Non-small cell lung cancer (NSCLC) is the most prevalent form of lung cancer, accounting for approximately 85% of newly diagnosed lung cancer cases1,2.
The median overall survival (OS) is approximately 4–5 months for metastatic NSCLC patients who only receive supportive care. However, when patients received supportive care combined with induction platinum-based chemotherapy (cisplatin/paclitaxel; carboplatin/paclitaxel; cisplatin/docetaxel; carboplatin/paclitaxel), the median OS improved to 8–12 months. Over the past few decades, several trials have compared different chemotherapy regimens, but OS has improved slightly3,4. Researchers believe that research on the efficacy of chemotherapy has stagnated.
In 2002, a phase III trial compared four regimens (cisplatin/paclitaxel; cisplatin/gemcitabine; cisplatin/docetaxel; carboplatin/paclitaxel) that were platinum-based doublets in first-line metastatic NSCLC, but the trial demonstrated that there was no difference in these regimens5. When researchers first identified activating mutations of epidermal growth factor receptor (EGFR) in 2004, gefitinib also demonstrated a significantly greater survival benefit than chemotherapy in EGFR-positive patients simultaneously6,7. Therefore, the development of targeted therapy and the identification of specific driver mutations are major advances in the treatment of metastatic NSCLC. Moreover, a phase III trial compared gefitinib and platinum-based doublets, and the results indicated that targeted therapy is superior to carboplatin-paclitaxel as an initial treatment for EGFR-positive NSCLC patients8. Due to the discovery of biomarkers and the effectiveness of targeted therapy, the existing histopathological classification of lung cancer has been completely reconstructed. For instance, in stages III and IV, the use of gefitinib has less toxicity in patient with EGFR-positive status than dose chemotherapy9. This also warrants a separate listing in the National Comprehensive Cancer Network (NCCN) guidelines of targeted therapy as the first-line treatment for patients with driver gene-positive status among those receiving palliative care10. Additionally, researchers have paid more attention to this type of rare subgroup. Biomarkers of anaplastic lymphoma kinase rearrangement (ALK), recombinant C-Ros Oncogene 1 (ROS1), v-raf murine sarcoma viral oncogene homolog B1 (BRAF), and human epidermal growth factor receptor 2 (HER-2), biomarkers have been discovered in recent decades11,12,13,14. Due to the incidence rate of some targets in lung cancer (≤ 5%), these new potential oncogenic drivers are divided into rare subgroups. In addition, corresponding agents have been developed simultaneously. Some clinical trials have shown that the response rate and OS are significantly increased in patients who receive targeted therapy15,16,17.
In the past decade, tremendous changes have taken place in the treatment of advanced NSCLC. Molecular profiles can be divided into different subgroups to help determine treatment plans, and enable the patient to receive personalized treatment. Patients who harbor oncologic driver mutations can receive FDA-approved targeted therapy as first-line therapy. However, most of these novel oncologic drivers do not have clear therapeutic algorithms; in some cases, some agents that can be used for the desired indication have not been approved by the FDA. Whereas, the relatively low frequency of these genetic aberrations makes it difficult to conduct large-scale randomized clinical trials. Furthermore, some oncologic driver mutations that can cause drug resistance, such as the EGFR T790M mutation and G810 solvent-front rearranged during transfection (RET) mutants, also cause difficulties for researchers18,19.
In this review, we summarize some novel and emerging actionable oncogenic drivers in NSCLC and focus on the main clinical challenges in patients who harbor rare oncologic drivers. These new agents and regimens can provide patients more choices and greater survival benefits.
Epidermal growth factor receptor (EGFR) exon 20 insertion
The EGFR gene is expressed in 12% of white NSCLC patients and more than 50% of Asian NSCLC patients20,21,22,23. Since researchers identified the EGFR gene and developed gefitinib in 2004, three generations of EGFR inhibitors have been developed. These EGFR inhibitors have changed therapeutic algorithms and provided patients with significant survival benefits. EGFR exon 20 insertion (ex20ins) mutations are the third most common EGFR mutation subtype, and they are found in nearly 4% of EGFR-mutant NSCLC patients. It mainly occurs in Asians, women, nonsmokers and populations with adenocarcinoma. However, it is different from other common EGFR-mutant NSCLCs because it is not sensitive to first- or second-generation EGFR-tyrosine kinase inhibitors (EGFR-TKIs)24,25,26.
Previous studies have indicated that alterations in the drug-binding pocket of exon 20 lead to reduced drug action. 3D modeling revealed that EGFR ex20ins have two structures: a C-helix domain and a loop following the C-helix domain. In this structure, EGFR ex20ins mutations result in a shift of the phosphate-binding loop (P-loop) into the drug-binding pocket and increased affinity for ATP. This change can reduce the binding of first-generation inhibitors and lead to drug resistance27,28. Therefore, other agents, such as osimertinib, poziotinib and mobocertinib, have been designed to treat patients with the EGFR ex20ins mutation and have showed promising results in recently updated data (Table 1).
Osimertinib
Osimertinib, a third-generation EGFR-TKI, was approved by the Food and Drug Administration (FDA) as the first agent for acquired drug resistance after EGFR-TKI treatment. Some studies have shown that the IC50 of osimertinib for EGFR ex20ins is 10-100-fold greater than that for other EGFR mutations29,30. Interestingly, a retrospective Chinese study enrolled 6 patients with ex20ins + NSCLC who received osimertinib 80 mg once daily, and showed promising results. However, more studies have reported disappointing evidence of the efficacy of osimertinib 80 mg once daily25,31,32,33. In contrast, trials with high doses of osimertinib (160 mg) have shown promising antitumor activity. A phase II (NCT03191149) study of patients who received osimertinib 160 mg per day and showed an overall response rate (ORR) of 25% and a DCR of 85%. The median progression-free survival (mPFS) and median duration of response (DOR) were 9.7 months and 5.7 months, respectively34. Another study (POSITION20) also obtained similar results35. These studies showed that osimertinib cannot be routinely recommended (80 mg daily) for patients with NSCLC harboring ex20ins. A higher dosing scheme study should be developed.
Poziotinib
Poziotinib is an oral EGFR inhibitor that can inhibit EGFR mutations and HER-2 alternations. Researchers have demonstrated that poziotinib has better effects than other TKIs in vitro with EGFR ex20ins. Furthermore, 3D modeling also indicated that poziotinib has small terminal and substituent linkers that make it more flexible and reasonable than other EGFR-TKIs for binding the ex20ins receptor36. Several corresponding studies also showed a similar result to that of osimertinib, with an ORR of nearly 30%, DCR of approximately 85% and a manageable safety profile37,38. Interestingly, researchers have also classified loops following the C-helix domain as near- and far-loop insertions and found that the sensitivity of pozidinib is highly dependent on the insertion position of the insertion in the loop following the C-helix domain. The results indicated that near-loop insertions are more sensitive than far ring insertions, with ORRs of 46% and 0%, respectively38. Unfortunately, a phase 2 clinical trial (ZENITH20; NCT03318939) showed an ORR of 14.8% and a DCR of 68.7%. However, due to the primary endpoint was not met and there was a high incidence of grade 3 side effects, especially rash (28%) and diarrhea (26%)39. Moreover, in the ZENITH20-2 trial, rash and diarrhea were the predominant grade 3 treatment-related adverse events (TRAEs), occurring in 78.9% of all patients, and leading to the discontinuation of poziotinib in 13% of cases37. The management of adverse effects has proven to be challenging, resulting in a significant proportion of patients receiving suboptimal doses and durations of therapy. At present, this study has terminated.
Mobocertinib (TAK-788; AP32788)
Mobocertinib, a novel irreversible EGFR-TKI, can selectively inhibit the EGFR and HER-2 ex20ins mutations. A corresponding phase II study (EXCLAIM, NCT02716116) showed relatively promising results (ORR: 43%, mDOR: 14 months; mPFS: 7.3 months)40. Subsequently, an extension cohort in which patients who previously received platinum-based chemotherapy and who were evaluated for morbidity had an ORR of 28% and DCR of 78%, and side effects were well tolerated41. In July 2021, based on the ORR and long-term survival benefit of by the EXCLAIM trial, mobocertinib was accepted by the Center for Drug Evaluation of the National Medical Products Administration of China, and the FDA accelerated the approval of the application of mobocertinib for EGFR ex20ins-positive NSCLC patients who had previously received platinum-based chemotherapy in September 202127,42. Additionally, an ongoing phase 3 randomized trial (EXCLAM-2; NCT04129502) evaluated the efficacy of combination treatment with mobiocertinib and platinum-doublet chemotherapy as first-line treatments among patients with treatment-naïve advanced NSCLC whose tumors harbor EGFR ex20ins mutations. This study is the first clinical study to prospectively evaluate the efficacy of EGFR ex20ins targeted therapy compared with that of the current first-line standard therapy, and the results are eagerly anticipated. A phase III randomized study (EXCLAIM-2; NCT04129502) did not indicate a promising outcome with mobocertinib monotherapy in comparison to chemotherapy as the first-line treatment choice for this patient group. As a result, an announcement was made regarding the voluntary withdrawal of mobocertinib in the United States, which had previously received accelerated approval43.
Zipalertinib (CLN-081; TAS6417)
Zipalertinib is a novel irreversible EGFR-TKI that can inhibit EGFR ex20ins. In the 2022 American Society of Clinical Oncology (ASCO) annual meeting, a relevant study (NCT04036682) showed significant antitumor activity, and the results indicated that 66 (94%) patients achieved partial response (PR) or stable disease (SD). Moreover, two patients had observable brain metastasis regression, which makes researchers exciting44. The FDA has recently granted breakthrough therapy designation for the treatment of locally advanced non-small cell lung cancer (NSCLC) in patients with ex20ins mutations who have previously undergone platinum-based chemotherapy45.
Amivantamab (JNJ-372)
Amivantamab is a bispecific monoclonal antibody that can protect against the oncologic drivers of EGFR and MET46. Preclinical studies have demonstrated that amivantamab has more antitumor activity than other EGFR agents and can suppress cell lines harboring EGFR ex20ins mutations47,48. Due to these promising preclinical findings, the CHRYSALIS trial (NCT02609776) exhibited favorable antitumor activity (ORR: 40%, mOS: 22.8 months)49. On May 21, 2021, the FDA approved amivantamab for treating patients with locally advanced or metastatic NSCLC harboring the EGFR ex20ins mutation whose disease progressed on or after platinum-based chemotherapy50,51. Based on the breakthrough effect of amivantamab, in March 2020, amivantamab was awarded FDA breakthrough therapy designation, marking the first instance of such recognition for an ex20ins targeted therapy. Subsequently, in May 2021, another milestone was achieved by becoming the first ex20ins targeted therapy to receive FDA approval50. Besides, another study of amivantamab plus chemotherapy versus chemotherapy alone as a first-line treatment in patients with ex20ins also showed promising results (PAPILLON; NCT04538664). In this study, the combination arm exhibited a markedly greater response rate than did the chemotherapy arm (ORR: 73% vs 43%). Additionally, the combination arm also showed a faster response time to respond and durable efficacy. It should be mentioned that it also has a great effect on brain metastases. In terestingly, although the incidence of side effects in the amivantamab group was greater than that in the chemotherapy group, there were no differences between the two groups in terms of serious side effects52.
Sunvozertinib (DZD9008)
Sunvozertinib (DZD9008) is an oral, irreversible fourth-generation tyrosine kinase inhibitor (TKI)53. The phase 2 trial WU-KONG6 enrolled 97 Chinese patients with EGFR Ex20Ins in the post-platinum setting. It showed a notable ORR of 60.8%, and achieved a DCR of 87.6%54. Moreover, the results from phase 1/2 WU-KONG1 (NCT03974022) and phase 2 WUKONG15 (NCT05559645) trials, also showed a significant result, with ORRs of up to 68.4% and 77.8% for doses of 200 mg and 300 mg, respectively55. An ongoing phase 3 study, WU-KONG28, compared the efficacy of sunvozertinib (300 mg) with that of platinum-pemetrexed in the first-line treatment of patients with ex20ins mutations. Despite ongoing investigations, the breakthrough therapy designation by the US FDA has already been granted based on promising outcomes in patients with a history of platinum-based therapy56.
Furmonertinib (Alflutinib/AST2818)
Furmonertinib is a fourth-generation EGFR-TKI that has a similar structure to that of osimertinib27. The phase 1b FAVOUR 1 trial (NCT04858958) has currently enrolled 79 patients in three arms: the treatment-naive group (n = 30) received a daily dose of 240 mg, and the previously treated group received 240 mg (n = 24) and 160 mg (n = 25). The ORRs were 69.0%, 50.0%, and 40.9% respectively, with DCRs of 96.6%, 95.5% and 90.9% observed in all subgroups. The mPFS was 10.7 months for the treatment-naïve group and 7.0 months and 5.8 months for the previously treated groups receiving 240 mg and 160 mg, respectively. Of interest, an antitumor response was observed in patients with both near and far-loop EGFR Exon20ins mutations57. Although it has a similar structure to osimertinib, compared to POSITION20, the efficacy of furmonertinib is better than that of osimertinib. Moreover, further studies are required to comprehensively evaluate the efficacy of this medication in this patient subgroup. Multiple ongoing trials in China (NCT05466149) and the United States (NCT05364073, FURMO-002) aim to address this need58.
Another clinical trial
Recently, updared data on osimertinib, poziotinib, CLN-081, JNJ-372, mobocertinib, sunvozertinib and furmonertinib are promising. Other agents also have also been developed, and corresponding trials are ongoing. Tuxobertinib (BDTX-189) is an EGFR-TKI that was proven by a preclinical study, and a relevant phase II study is ongoing (NCT04209465). Other related TKIs are currently under evaluation in clinical trials, including FWD1509 (NCT05068024), and JMT101 (NCT04448379), are currently under evaluation in clinical trials27.
Mesenchymal-epithelial transition (MET)
MET oncologic drivers, also known as hepatocyte growth factor receptor (HGFR), are present in approximately 5% of patients with nonsquamous NSCLC, which is comparable to the frequency of ALK fusion59. It is located on human chromosome 7q21-q31 with 21 exons and 20 introns and encodes receptor tyrosine kinase for hepatocyte growth factor (HGF). Then, downstream signaling pathways, including RAS/ERK/MAPK and PI3K/AKT pathways, are activated when HGF specifically binds to the MET receptor. To date, several aberrations in Met have been found, including protein overexpression (15–70%), amplification (2–5%) and MET exon 14 (METex14) skipping mutations (3–4%)59,60. Additionally, aberrant MET occurs in 10–20% of NSCLC patients with EGFR mutations who previously received EGFR-TKIs.
In 2011, an NSCLC patient with MET amplification received crizotinib and achieved a rapid and durable response61. To date, there are two types of MET-related TKIs: nonselective MET-TKIs and selective MET-TKIs. Among the nonselective MET-TKIs, crizotinib was the first nonselective MET inhibitor to obtain FDA approval. A related study (Profile 1001, NCT00585195) showed a promising result in patients with METex14 mutations and a high gene copy number (GCN) in MET amplification. Similar results were also shown in other trials, such as AcSè (NCT02034981) and METROS (NCT02499614)62,63,64,65. Other agents, such as capmatinib, tepotinib, and savolitinib, have also provided favorable data in patients harboring METex14 skipping mutations and MET amplification with high GCN (Tables 2, 3)66. With the rapid expansion of data on MET inhibitors, more agents and clinical trials have been developed in recent years. Besides, most of this evidence is based on phase I and II studies, so phase III studies are warranted to confirm the efficacy and safety of MET inhibitors in various cancers.
Capmatinib
Capmatinib is a small molecule highly selective MET-related TKI that belongs to MET-class Ib inhibitors67. A previous study showed that capmatinib plus chemotherapy can achieve favorable results in EGFR-mutated, MET-amplified NSCLC68. Then, the GEOMETRY mono-1 study (NCT02414139) and another study (NCT01324479) showed that capmatinib has a meaningful benefit in patients with MET-dysregulated NSCLC10,49. In particular, in the high copy number subgroup and METex14 subgroup, these cohorts showed a high ORR (75%) or DCR (100%) and a considerable survival benefit69,70. According to these trials, capmatinib was approved by the FDA for first- and subsequent-line treatment of patients with MET-dysregulated advanced NSCLC. Moreover, because of the successful results of the GEOMETRY mono-1 study, the next phase III GeoMETry-III trial (NCT04427072) is currently evaluating capmatinib compared to docetaxel in pretreated NSCLC patients. Moreover, capmatinib has antitumor activity in crizotinib-pretreated patients with MET-altered NSCLC, although it has modest efficacy (n = 15; ORR: 10%; DCR: 80%)71. In this study, patients did not respond well to capmatinib despite disease stabilization. Researchers should focus on class II MET TKIs (merestinib and glesatinib), which do not rely on interactions with the activation loop, and some preclinical trials have also proven this scenario (Tables 2, 3)72.
Tepotinib
Tepotinib is a highly selective, type I MET-TKI for the treatment of non-small cell lung cancer harboring MET alterations. It was approved by the Japanese Ministry of Health, Labor and Welfare and the FDA in March 2020 and February 2021, respectively73. Recently, updated data from the phase II VISION trial (NCT02864992) showed promising results in 152 NSCLC patients with a METex14 skipping mutation. Both the treatment-naïve cohort (n = 69) and previously treated cohort (n = 83) had similar ORRs (44.9% vs 44.6%) and DCRs (68.1% vs 72.3%). Additionally, 13 of 15 patients with central nervous system (CNS) metastases achieved intracranial disease control, which is indicative of strong blood‒brain barrier penetration ability74. Furthermore, tepotinib has shown antitumor activity against EGFR-mutated NSCLC patients with MET amplification and high MET expression. The INSIGHT trial (NCT01982955) included 18 eligible patients and demonstrated that the tepotinib plus gefitinib group had significantly better results than the chemotherapy group in patients with high MET overexpression and MET amplification75. It promoted the emergence of the INSIGHT 2 clinical trial (NCT03940703), which demonstrated that tepotinib plus osimertinib promoted in MET-amplified NSCLC after patients experienced osimertinib resistance (Tables 2, 3)76.
Savolitinib
Savolitinib is an oral type Ib MET-TKI that has received approval in China for the treatment of metastatic NSCLC with METex14 mutation in patients who have progressed after or who are unable to tolerate platinum-based chemotherapy in June 202177. A phase II clinical trial (NCT02897479) enrolled 70 patients with positive pulmonary sarcomatoid carcinoma or other NSCLC subtypes and showed satisfactory results. Independent review center (IRC) reported an ORR of 49.2% and a DCR of 82.9%78. Furthermore, savolitinib has demonstrated efficacy in overcoming acquired MET-mediated osimertinib resistance. In the TATTON study (NCT02143466), which enrolled 144 patients in part B and 42 patients in part D, updated data revealed an ORR of 33–67% and 62% and an mPFS of 5.5–11.1 and 9.0 months, respectively79. These studies have demonstrated that savolitinib has promising antitumor activity and a durable response in patients harboring MET-related dysregulation (Tables 2, 3).
Amivantamab (JNJ-372)
Amivantamab, a bispecific antibody targeting EGFR and MET, has been shown to disrupt EGFR and MET signaling functions by ligand blocking and receptor degradation80. Previously, at the 2022 ASCO annual meeting, researchers reported data from the ongoing phase I CHRYSALIS (NCT02609776) study. In the MET-2 cohort, 43 patients with NSCLC harboring METex14 mutations had a favorable outcomes, with an ORR of 33% and a clinical benefit rate of 58.3%. The mPFS was 6.7 months (Table 2)81.
Other agents for MET alteration
Although MET-selective TKIs, such as capmatinib, tepotinib, and savolitinib, have become the new standard of care for treating NSCLC, the combination of MET-TKIs and EGFR-TKIs (osimertinib plus savolitinib, tepotinib plus gefitinib) may be a potential solution for preventing MET-driven EGFR-TKI resistance. Other drugs have been developed and shown promising results in both preclinical and updated data. Glumetinib (SCC244), a highly selective class II MET inhibitor, has shown promising effects in patients with MET alterations. An ongoing clinical trial (GLORY; NCT04270591) has reported an ORR of 60.9%, an mDOR of 8.2 months, and an mPFS of 7.6 months by a blinded independent review committee (BIRC). These data show the excellent efficacy of glumetinib in NSCLC patients harboring METex14 mutations. Furthermore, it also exhibited intracranial antitumor activity with a median intracranial tumor shrinkage of 57%82. Cabozantinib is a type of TKI that targets multiple receptors, such as VEGFR, MET, and AXL. However, a previous study (NCI 9303 II; NCT01866410) showed that the combination of cabozantinib and erlotinib had limited effects on in patients with MET amplification. Another clinical trial (NCT03911193) is currently underway, that includes patients with MET alterations83. Merestinib (LY2801653) is a multikinase inhibitor that can inhibit MET, ROS1, and AXL, and an ongoing phase II clinical trial (NCT02920996) involving NSCLC patients harboring METex14 skipping mutations84. Additionally, S49076 is a multikinase inhibitor that can inhibit MET, AXL, and FGFR1-3 oncologic drivers, and a relevant pivotal phase I/II study (EudraCT: 2015-00264631) is ongoing. Recent results have shown that 2 patients harboring MET dysregulation responded to treatment with S4907685. In addition, other agents, such as glesatinib (MGCD265), vebrreltinib (APL-101) and telisotuzumab vedotin (Teliso-V), have also been developed in recent years, and relevant clinical trials are currently underway (Table 2)86,87.
V-Raf murine sarcoma viral oncogene homolog (BRAF) V600E
The V-Raf murine sarcoma viral oncogene homolog (BRAF) gene, located on chromosome 7, has been identified as a well-known oncogene. BRAF mutations can be found in various tumors, and the most common tumor is melanoma. Approximately 2–4% of NSCLC patients have BRAF gene mutations, with the BRAF V600E mutation being detected in 50% of these cases patients60,88,89. Mutation of the BRAF V600E protein has been found to affect an enzyme that plays a crucial role in the RAS/RAF/MEK/ERK (MAPK/ERK) signaling pathway, which is essential for the proper functioning of cells and is involved in a range of biological processes such as cell growth, differentiation, and survival90. Initially, targeted therapy for the BRAF V600e mutation relied on vemurafenib or dabrafenib. However, these treatments did not yield favorable results, with response rates of only 42% and 33%, respectively91,92. Trametinib, a MEK protein inhibitor regulated by BRAF, combined with dabrafenib can be much more effective in NSCLC with the BRAF V600E mutation (NCT01336634). Recently, researchers have updated this clinical trial, and research has revealed that the ORR can increase to 63–69%, regardless of previous treatment. The mPFS was 10.8 months, and the 5-year survival rate was 22% in treatment-naïve patients. In patients in the previous system treatment group, the mPFS was 10.2 months, and the 5-year survival rate was 32%93. Another clinical trial also showed favorable results, and the ORRs of patients receiving dabrafenib plus trametinib (D + T) as a second-line or above or as a first-line therapy were 73.8% and 82.9%, respectively94. Trametinib alone or in combination with other therapies has shown excellent potential and could become a new standard treatment for BRAF V600E-positive advanced NSCLC.
Rearrangement during transfection (RET)
The RET gene is a well-known proto-oncogene and was mapped to the long arm of 10q11.2 chromosome 10. The RET protein is a membrane tyrosine kinase receptor. RET mutation and RET fusion/rearrangements are more likely to occur in thyroid cancer than in lung cancer. Nearly 2% of lung adenocarcinoma patients have RET fusion/rearrangement. To date, more than 15 kinds of RET fusions or rearrangements have been discovered in NSCLC. The most common RET fusion gene in NSCLC is kinesin family member 5B (KIF5B)-RET (70–90%), and coil-coil domain containing 6 (CCDC6)-RET (10–25%) is the second most common fusion gene95.
First-generation RET inhibitors
First-generation RET inhibitors are also known as multikinase inhibitors (MKIs). Multiple trials have demonstrated that MKIs, such as vandetanib and cabozantinib, have favorable efficacy in patients with RET-altered NSCLC, and these two agents have been approved by the FDA to treat RET-altered NSCLC96,97. However, some disadvantages, such as adverse events and drug resistance, affect therapeutic efficacy and limit clinical application. Therefore, a second-generation RET inhibitor, a selective RET inhibitor, was developed. Selpercatinib (LOXO-292) and pralsetinib (BLU-667) are representative selective RET inhibitors.
Second-generation RET inhibitors
Both selpercatinib (LOXO-292) and pralsetinib (BLU-667) are selective RET inhibitors and corresponding pivotal clinical trials have shown significant results. Based on these trials, the RET oncologic driver has demonstrated its potential and might become a well-known oncologic driver as well as ALK and ROS1 (Table 4).
The potency of pralsetinib was ≥ tenfold greater than that of MKIs approved by the FDA. In September 2020, the FDA granted accelerated approval to pralsetinib for adult patients with metastatic RET fusion-positive NSCLC. The ARROW study (NCT03037385), a pivotal clinical trial of pralsetinib, recently enrolled 233 patients who were diagnosed with RET fusion-positive NSCLC. The cohort included 27 patients who were newly diagnosed with NSCLC and 87 who had previously received platinum-based chemotherapy, and the ORRs were 70% and 61%, respectively98. Another clinical trial (NCT04222972) also showed a similar effect after treatment with pralsetinib. Interestingly, pralsetinib treatment resulted in tumor shrinkage in 97% of patients. Additionally, the observed 70% intracranial response rate is particularly surprising. These results demonstrate that pralsetinib is highly effective in treating primary tumors and metastases, including intracranial tumors99.
In May 2020, selpercatinib, a new agent that was used to treat RET-altered NSCLC, was approved by the FDA. The LIBRETTO-001 clinical trial (NCT03157128) revealed a striking survival benefit for NSCLC patients who were RET fusion-positive. Until 2022, the ORR was 61% in 247 patients who had previously been treated with chemotherapy. The median DOR was 28.6 months, and the median PFS was 24.9 months. Moreover, 69 treatment-naïve patients had a better ORR (84%) than those previously treated with chemotherapy, and the updated data reported a median DOR of 20.2 months and a median PFS of 22.0 months. Interestingly, selpercatinib can also cross the blood‒brain barrier and respond to intracranial masses. The intracranial ORR was 85%, and 27% of the patients achieved CR according to the updated data100. Another study also demonstrated similar results in patients with NSCLC who had brain metastases101. These striking results indicate that selpercatinib is a good therapeutic option for patients with RET-altered NSCLC.
Third-generation inhibitors
Two patients were treated with selpercatinib, and disease progression was observed. This is because the RET G810R/S/C solvent front mutation has been acquired and caused drug resistance18. Therefore, researchers are currently developing third-generation inhibitors to inhibit additional RET mutations that confer resistance to MKIs and second-generation RET inhibitors (Table 4).
TPX-0046 is a famous third-generation inhibitor, and it has strong potency against solvent-front RET G810 mutants. It has a smaller and more rigid macrocyclic structure than second-generation inhibitors, and this structure can generate a compact type I inhibitor and maintain antitumor activity without drug resistance102. Sword-1 (NCT04161391) is a pivotal clinical trial for TPX-0046, and it received favorable data were obtained in the initial study. As of March 2021, preliminary clinical data were collected from 14 evaluable patients with NSCLC and medullary thyroid carcinoma (MTC), which included 5 TKI-naïve patients (3 with NSCLC and 2 with MTC) and 9 previously TKI-treated patients (4 with NSCLC and 2 with MTC). Interestingly, 4 patients in the TKI-naïve group experienced tumor regression, and 3 patients in the TKI-pretreated group experienced tumor shrinkage. Moreover, the majority of treatment-related adverse events (TRAEs) were grade 1 or 2, and no grade 4 or 5 TRAEs were found103.
Zeteletinib (BOS-172738; DS-5010) is a small molecule RET inhibitor that shows favorable potency for various RET mutations, such as the M918T, V840L and V840M gatekeeper mutations. An ongoing phase I clinical trial (NCT03780517) is pivotal study for zeteletinib, and it exhibited favorable safety and ORR. As of December 2020, the study enrolled 30 NSCLC patients and had an NSCLC ORR of 33% (n = 10/33). Zeteletinib also showed safety for long-term administration. Most patients experienced grade 1–2 adverse events that were deemed unrelated to zeteletinib104.
Human epidermal growth factor receptor 2 (HER-2)
Human epidermal growth factor receptor 2 (HER-2), also referred to as ErbB2, is a well-known proto-oncogene located on chromosome 17 (17q21). Intriguingly, it is a member of the EGFR family of receptor tyrosine kinases, and is composed of three segments: an extracellular ligand binding domain, an α-helical transmembrane segment, and an intracellular tyrosine kinase domain. Unlike other members of the EGFR family, no natural ligand has been identified for HER-2. Ligand binding to other receptors in the family promotes receptor dimerization, leading to the activation of downstream signaling pathways, such as the PI3K/Akt and Ras/MAPK pathways. In HER-2-altered cancer cells, this constitutive activation results in uncontrolled cell growth105.
HER-2 alterations are frequently observed in various cancer types106,107,108. However, HER-2 aberrations can be identified in small subsets of NSCLC patients. Three HER-2-activating mechanisms have been found in NSCLC: mutation (occurring in 1–4% of cases), amplification ( occurring in 2–5% of cases) and overexpression (occurring in 10–15% of cases)109. Among HER-2 mutations, exon 20 insertions are the most frequent HER-2 mutations, accounting for 96% of HER-2 mutations. It is easily and predominantly found in patients who are young and nonsmokers, and there is no correlation between race, sex, lymph node involvement and tumor stage. Additionally, HER-2 mutations are an independent poor prognostic factor110. The ability of amplification and overexpression of HER-2 to distinguish between amplification and overexpression is still unclear. In addition, a meta-analysis demonstrated that HER-2 overexpression might be an independent prognostic factor in patients with NSCLC110,111,112. Currently, practitioners use immunohistochemistry (IHC), which categorizes the staining intensity on a scale from 0 to 3 + to determine the Her-2 amplification and overexpression. This system defines IHC 0–1 + as HER2 negative, IHC 2 + as weak to moderate, and IHC 3 + as strong when staining occurs in 10% of tumor cells. Furthermore, there is a significant relationship between HER-2 protein expression, assessed through IHC, and HER-2 gene copy number, as determined by FISH, with many patients showing polysomy rather than true amplification. Consequently, HER-2 2 + or 3 + expression should prompt the addition of FISH analysis to differentiate between these possibilities113,114,115. In addition, many prospective studies have failed to identify an association between the response of patients with an aberrant HER-2 gene and conventional chemotherapy112. These studies urge researchers to develop new agents to treat these types of patients, such as small molecule TKIs and anti-HER-2 antibodies, and bring new hope to patient with this currently incurable disease (Table 5).
Small molecule tyrosine kinase inhibitors
Nonselective HER-2 tyrosine kinase inhibitors, including afatinib, dacomitinib, and neratinib, have shown limited results in clinical trials. Afatinib, a pan-Her TKI, was first evaluated for efficacy in patients with HER-2 mutation-positive solid tumors, including NSCLC. However, in the NICHE clinical trial (NCT02369484), the result was unsatisfactory in a clinical trial (ORR = 7.7%). Only one patient with HER-2-mutant NSCLC achieved a PR116. Another nonselective pan-Her TKI (dacomitinib, neratinib) also showed unsatisfactory results117,118,119. These clinical trials have led researchers and practitioners to pay more attention to selective HER-2 TKIs (poziotinib, pyrotinib, tarloxotinib, etc.) and design related clinical trials that have shown favorable results.
Recently, novel, more selective pan-HER-2 TKIs, such as poziotinib, pyrotinib, tarloxotinib and mobocertinib, have been developed with the objective of improving outcomes in patients with NSCLC with HER-2 mutations. Each agent has shown promising antitumor activity in clinical trials.
Poziotinib, a novel, oral, irreversible pan-HER-2 TKI. It has a smaller size and more flexible structure than afatinib, making it more effective than other pan-HER-2 TKIs. Two clinical trials (NCT03318939; NCT03066206) involving participants with HER-2 mutations who previously received therapy reported highly similar results, with an ORR of nearly 27% and mPFS of 5.5 months120,121.
Pyrotinib is another oral, irreversible pan-HER-2 TKI used to treat NSCLC. A previous clinical trial demonstrated that pyrotinib has promising antitumor activity in patients with NSCLC harboring HER-2 exon 20 mutations122. Recently, a single-arm trial including 27 patients with HER-2 amplification showed promising results with an ORR of 22.2% and an mPFS of 6.3 months. Furthermore, pyrotinib can cross the brain‒blood barrier, and patients with brain metastases have an ORR of 40%123. However, because the primary endpoint was not met and there was a high incidence rate of grade 3 TRAEs, ZENITH20 trail was terminated.
Other small molecule TKIs, such as tarloxotinib, a pan-HER kinase inhibitor, also showed dramatic treatment efficacy in a patient with the HER-2 exon 20 p. A775_G776insYVMA mutation124. In addition, a phase II study (NCT03805841) is ongoing and enrolled patients with NSCLC who were pretreated with chemotherapy and harbor EGFR exon 20 insertions or HER-2 mutations. However, this clinical trial showed a limited effect. Of the nine assessable patients with NSCLC harboring HER-2 mutations, two patients achieved a PR (ORR of 22%), four patients exhibited SD (DCR of 67%), and most side effects were grade 1–2 (Table 5)125. Mobocertinib (TAK-788/AP3278), a next-generation small-molecule oral TKI, is designed to selectively target both EGFR insertions and HER-2 mutations. An ongoing phase I/II study (NCT02716116) is recruiting patients with NSCLC harboring HER-2 exon 20 alterations126.
Monoclonal antibodies and antibody‒drug conjugates against HER-2
Recently, monoclonal antibodies, such as trastuzumab, and antibody‒drug conjugates (ADCs), which is class of targeted cancer therapies that combine the specificity of monoclonal antibodies (mAbs) with the cytotoxic effects of chemotherapy drugs such as trastuzumab-emtansine and trastuzumab-deruxtecan, which target the HER-2 receptor have shown some results in clinical trials112,127.
Trastuzumab is a monoclonal immunoglobulin G1 humanized murine antibody that targets the HER-2 receptor and inhibits its dimerization. It can induce internalization and/or degradation of the receptor, eventually impeding downstream pathways. A previous study demonstrated that monotherapy with trastuzumab in patients with NSCLC harboring HER-2 gene aberrations has a limited survival benefit128,129. In addition, although trastuzumab combined with chemotherapy has shown a promising effects in previous studies, it can be realized that chemotherapy, not trastuzumab, provides survival benefits to patients130. More recently, a phase II study (IFCT 1703-R2D2, NCT03845270) also showed the efficacy of the combination of trastuzumab, pertuzumab, and docetaxel in patients with advanced NSCLC harboring HER-2 mutations who progressed after ≥ 1 platinum-based treatment, and this combination exhibited a feasible result. Forty-five patients were enrolled and showed the ORR was 29%131. Another clinical trial (DRUP, NCT02925234; MyPathway, NCT02091141) also showed limited results in patients with HER-2 alternations who were treated with trastuzumab plus pertuzumab128,132. Therefore, trastuzumab shows disappointing results regardless of whether it is used as monotherapy or in combination with chemotherapy.
Trastuzumab-emtansine (T-DM1) is a novel ADC composed of trastuzumab that targets the HER-2 receptor. Past research has shown favorable results in patients harboring HER-2 mutations133. In a recent update, 22 eligible patients were included in this study; the ORR was 38.1%, and the DCR was 52.4%. Moreover, the drug was well tolerated and had manageable side effects134. In patients harboring overexpression or amplification, T-DM1 presented a limited result. ORR was found to be 20% in the IHC 3 + group and 0% in the IHC 2 + group135.
Trastuzumab-deruxtecan (T-DXd; DS-8201a) is also a novel ADC similar to T-DM1. Recently, updated results from DESTINY-Lung01 (NCT03505710) showed a significant result in 91 patients with HER-2 mutations who received T-DXd. The results showed an ORR of 55%, an mDOR of 9.3 months, and an mPFS of 17.8 months. Safety is generally acceptable, but it is important to monitor for interstitial pneumonia (ILD)136. Another clinical trial also showed significant results137. In the HER-2-overexpressing NSCLC cohort. Although the results were not as impressive as those in cohort 2, they were still promising. The ORR was 24.5%, the mDoR was 6 months, and the mPFS was 5.4 months. Interestingly, the response rates were not based on HER-2 IHC expression levels, with an ORR of 20.0% versus 25.6% in IHC3 + and IHC2 + patients, respectively138. In August 2022, the FDA granted accelerated approval to T-DXd for patients with unresectable or metastatic NSCLC whose tumors harbored HER-2 mutations139.
Therefore, both T-DM1 and T-DXd have shown promising results in patients with HER-2 mutations and IHC 3 + NSCLC but have limited efficacy in those with IHC 2 + NSCLC. Additionally, patients with HER-2-positive tumors are more likely to have brain metastasis. In the future, combining ADCs with irreversible TKIs, such as pyrotinib, may become a trend, because TKIs can not only penetrate the blood–brain barrier but also increase the antitumor activity. In a preclinical study, researchers demonstrated that combining T-DM1 with a pan-HER irreversible inhibitor such as neratinib enhanced receptor ubiquitination and subsequent internalization of HER-2-ADC complexes, resulting in potent antitumor activity140. Furthermore, in the future, patients with IHC 2 + may present more refined subgroups, such as gastric carcinoma with IHC 2 + /fluorescence in situ hybridization (FISH) + , and these subgroups might receive a survival benefit from targeted therapy.
Neurotrophic tyrosine kinase (NTRK)
Alterations in neurotrophic tyrosine kinase (NTRK) genes (NTRK1, NTRK2, and NTRK3) are rare alterations in NSCLC, accounting for less than 1% of NSCLC cases141. Regarding the NTRK mechanism, the NTRK gene encodes the TrkA, TrkB, and TrkC transmembrane glycoproteins, which work with nerve growth factor (NGF), BDNF, neurotrophin-3 (NT-3), and NT-4 to support the development and function of the nervous system. NTRK gene fusions lead to the overexpression of Trk proteins and the activation of downstream signaling pathways, such as RAS/MAPK, PI3K/AKT, and PLC-γ pathways, resulting in cancer cell transformation, proliferation, and survival142. Although NTRK mutations, splice variants, and deletions can occur in certain types of tumor cells, these genetic alterations are generally not responsive to targeted therapy. Therefore, our article will concentrate on NTRK fusions, which are genetic changes that have been associated with a favorable response to NTRK-targeted therapy. To date, more than 80 known fusion partners have been identified, and the most frequently detected fusions are ETS Variant Transcription Factor 6 (ETV6)-NTRK3 and echinoderm microtubule associated protein like 4 (EML4)-NTRK359,143.
The first-generation TRK inhibitors entrectinib and larotrectinib were approved by the FDA due to their impressive results in phase I/II trials144. These drugs are designed to target ROS1 and ALK and have shown high response rates in clinical trials. Both entrectinib and larotrectinib yield a high response rate and are well tolerated with few adverse events in previous studies89,145,146,147,148. At the 2022 ASCO annual meeting, the updated data on larotrectinib also reported a similar result. Among 15 evaluable patients who received larotrectinib, the ORR was 73%, the mPFS was 1.8 months and the mOS was 40.7 months, and the side effects were mainly grade 1–2149. Therefore, entrectinib and larotrectinib yield high ORRs and favorable survival benefits for patients, making NTRK a major therapeutic target.
The key distinction between them is their ability to penetrate the CNS, with entrectinib being more effective at crossing the blood‒brain barrier150. This has been demonstrated in its successful treatment of patients with brain metastases. In a clinical trial, 12 patients with brain metastatic NTRK fusion-positive solid tumors received entrectinib, resulting in 6 patients who achieved a PR and 4 with SD. Nonetheless, entrectinib has been associated with several CNS-related adverse effects, such as dizziness145.
TRK inhibitors showed favorable effects. However, as with many targeted cancer therapies, the number of cases of tumor-acquired drug resistance to targeted therapy has increased in recent years. Therefore, the next generation of TRK inhibitors, such as taletrectinib (DS-6051b), selirectinib (LOXO-195), and repotrectinib (TPX-0005), are currently undergoing evaluation in ongoing phase I/II clinical trials, offering more hope for patients harboring NTRK alternations60,144.
Fibroblast growth factor receptor (FGFR)
Fibroblast growth factor receptors (FGFRs) are a family of receptor tyrosine kinases that play critical roles in numerous biological processes. The FGFR gene family consists of four members (FGFR1-4) that encode structurally similar transmembrane proteins151,152. Each FGFR contains an extracellular ligand-binding domain, a transmembrane domain, and an intracellular kinase domain. The binding of ligands, primarily fibroblast growth factors, to the extracellular domain of FGFRs leads to receptor dimerization and autophosphorylation of the intracellular domain. This causes a cascade of downstream signaling pathways, including the RAS/MAPK and PI3K/AKT pathways, and regulates cellular processes such as cell proliferation, differentiation, migration, and survival152.
Dysregulated FGFR genes include gene involved in amplification, mutation and fusion152. According to recent studies, FGFR alterations have been found to be more frequent in squamous cell histology (22%) than in adenocarcinomas (3%)152. According to a comprehensive genomic profiling study of 26,054 NSCLC specimens, FGFR fusions were found to occur in 0.2% of NSCLC patients, with squamous cell histology accounting for 0.59% and adenocarcinoma accounting for approximately 0.12%152,153. These mutations are primarily detected in patients without other driver mutations or in a subset of patients as a mechanism of acquired resistance110. Recently, the TCGA database showed that 3% of squamous-cell lung cancer (SqCLC) samples have mutations in at least one of the FGFR2 and FGFR3 genes. These mutations are mostly FGFR2 (W290C and S320C) and FGFR3 (R248C and S249C) in the extracellular domain of the gene and FGFR2 (K660E and K660N) in the kinase domain. Furthermore, the occurrence probability of FGFR gene fusion in SqCLC is approximately 2%-3.5%, with the most frequent sequence being FGFR3-TACC154.
Nonselective FGFR TKIs, such as ponatinib, dovitinib and pazopanib, have shown promising effects in NSCLC patients. However, toxicity is an important problem for nonselective FGFR inhibitors. Due to the occurrence of severe adverse events, clinical trials are frequently terminated. Therefore, researchers need to develop more effective and selective agents for cancers with FGFR aberrations (Table 6)155,156.
The next generation of FGFR inhibitors are selective FGFR TKIs. Most of the TKIs in this class, such as AZD4547 and GSK3052230, inhibit FGFR1-3. Additionally, some pan-FGFR inhibitors, such as erdafitinib and rogaratinib, can also inhibit FGFR 1–4. Currently, numerous selective FGFR inhibitors are being evaluated in clinical trials156.
AZD4547 is an oral ATP-competitive FGFR1-3 inhibitor and has a lower level of activity against FGFR 4, IGF1R, MARKs and KDR157. The SWOG S1400D trial (NCT02965378) was the first phase II trial to evaluate the efficacy of AZD4547. In 2019, the updated results enrolled 27 response-evaluable patients in the evaluable group who had various FGFR alterations were inculded. Although the safety profile was tolerable, this compound exhibited minimal activity in this predominantly FGFR-amplified cohort, with an ORR of 7%, mPFS of 2.7 months and mOS of 7.5 months158.
GSK3052230 (FP-1039) is a soluble fusion protein that acts as an FGFR ligand trap consisting of the extracellular domain of FGF receptor 1 (FGFR1) fused with the Fc region of IgG1 and subsequently inhibits tumor growth and angiogenesis159. The corresponding phase Ib study included 20 patients with metastatic or recurrent SqCLC harboring FGFR1 gene amplification who received GSK3052230 and chemotherapy. Treatment with GSK3052230 was well tolerated and had favorable antitumor activity (ORR: 47%, mPFS: 5.5 months)160.
Rogaratinib (BAY1163877) is an oral pan-FGFR inhibitor that has shown encouraging results in preclinical trials, including for lung cancers harboring FGFR alterations161. Previous data showed a 5.6% ORR162. Recently, a phase II clinical trial (NCT03762122) included 15 patients with FGFR alterations. This trial showed that rogaratinib has only limited efficacy in patients with SqCLC harboring FGFR overexpression (SD: 7; PD: 5; mPFS: 1.6 months). However, approximately 40% of patients experienced grade ≥ 3 TRAEs. Due to the high incidence of TRAEs and limited efficacy, this clinical trial has been terminated163.
In summary, the majority of clinical trials and drugs currently available are still in the research and development stage, and recent data have shown limited effects. Therefore, new drugs should be developed.
Other rare gene aberrations in lung cancer
Other rare gene aberrations have also been found in NSCLC, and some oncologic drivers have also shown excellent clinical efficacy. For example, the anaplastic lymphoma kinase (ALK) receptor gene is located on chromosome 2p23 and encodes a tyrosine kinase receptor. ALK fusions have been identified in approximately 5% of NSCLC patients, with the most common fusion partner being echinoderm microtubule-associated protein-like 4 (EML4)164. Crizotinib, the first-generation ALK inhibitor, was approved by the FDA in 2011 for the treatment of ALK-positive NSCLC165. However, second- or third-generation ALK inhibitors, such as ceritinib, alectinib, brigatinib, and lorlatinib, have been developed to overcome drug resistance to crizotinib and have shown favorable results in clinical trials166,167,168,169. Recently, fourth-generation ALK-TKIs have also shown promising results in preclinical studies. For example, NVL-655 showed antitumor activity and the ability to penetrate the blood‒brain barrier in vivo. In addition, it can also against solvent front drug-resistant mutations, a kind of genetic changes in the active site of proteins, particularly enzymes, which can render drugs ineffective by altering the binding shape or properties of site and caused drug-resistance, such as G1202R, G1202R + L1196M, and G1202R + G1269A170. On June 9th, 2022, a phase 1/2 clinical trial (ALKOVE-1; NCT05384626) is ongoing and includes patients with solid tumors harboring ALK aberrations, including NSCLC, and patients are receiving NVL-655. Recently, As of August 8th, 2023, in a cohort of 51 NSCLC patients, 20 patients achieved a PR, with an ORR of 39%. Additionally, 34 patients continued NVL-655 treatment, with an mDOR of 3.4 months. Notably, in patients with baseline brain metastases (n = 29), the ORR was 52%. Interestingly, the ORR was 54% in patients with ALK-resistant mutations (n = 28)170. TPX-0131, a next-generation ALK inhibitor, also exhibited preclinical potency against both wild-type ALK and a wide range of compound mutations, such as G1202R + L1198F, L1196M + L1198F, and G1202R + C1156F171. Recently, a relevant clinical trial (FORGE-1; NCT04849273) is ongoing. In the 2022 American Society of Clinical Oncology (ASCO) meeting, SAF-189 s, a next-generation ALK inhibitor, achieved excellent results and was well tolerated in patients with advanced, ALK-positive NSCLC, with a DCR of 100% in both the ALK inhibitor group and the ALK inhibitor-naïve group. Furthermore, it also showed excellent intracranial penetration in both groups (NCT04237805)172. Other ALK-TKIs, including entrectinib, repotrectinib (TPX-0005) and gilteritinib, are under preclinical or clinical investigation164,173,174,175.
The C-Ros 1 oncogene of the receptor tyrosine kinase (ROS1) gene, similar to the ALK-positive gene, is also called the a “diamond mutation” because it has a highly homologous structure to the ALK protein and thus has a similar oncogenic characteristics89. It was discovered in the 1980s and was first reported in lung cancer in 2007 by Rikova et al.176. ROS1 rearrangements are found in 0.9–2.6% of NSCLC cases, and patients with ROS1-positive NSCLC tend to be younger, never-smokers, and diagnosed with adenocarcinoma. ROS-1 rearrangements are considered a driver mutation in NSCLC and are mutually exclusive with other driver mutations, such as EGFR and ALK rearrangements177. Crizotinib was the first agent approved by the FDA for metastatic ROS1-positive NSCLC in March 2016. The pivotal phase I PROFILE 1001 trial (NCT00585195) showed excellent results with an ORR of 72% and a DCR of 90%. However, the emergence of drug resistance, including the G2032R point mutation, was observed. In addition, crizotinib also has poor intracranial penetration. Other agents, such as entrectinib, lorlatinib, ceritinib, cabozantinib and brigatinib, have been developed and overcome the disadvantage of weak penetration ability of the blood‒brain barrier89,178. Repotrectinib, a next-generation ROS1/TRK and ALK inhibitor, showed good tolerability and significant antitumor activity in a phase II study (TRIDENT-1; NCT03093116). The ORRs were 91% and 57% in treatment-naïve patients and patients who received previous treatment, respectively. It also exhibited excellent intracranial activity, with a 100% intracranial ORR in TKI-naïve patients and a 75% ORR in patients with 1 prior TKI179. Taletrectinib (DS-6051B) is a next-generation TKI that targets both ROS1 and NTRK. It also exhibits impressive brain-penetrant ability and activity against the ROS1 G2032R resistance mutation. In a phase II clinical study (NCT04395677), 61 eligible patients were enrolled, and the results showed 90% ORR and 95% DCR in patients with crizotinib-naïve tumors. In addition, in the crizotinib-naïve group, the ORR and DCR were 47.6% and 76.2, respectively. Moreover, it also showed excellent intracranial penetration180. Another relative phase II study (TRUST-II; NCT04919811) is ongoing181. Compared with crizotiib, ensartinib (X-396) is a TKI with tenfold greater in vitro activity against ALK than crizotinib. A recent clinical trial (NCT03608007) demonstrated the promising efficacy of ensartinib in ROS1-positive NSCLCs with an ORR of 27%. More interestingly, 75% of patients with CNS diseasesachieved disease control182.
Genetic rearrangements of neuregulin-1 (NRG1) were identified as a recently discovered oncogene driver of NSCLC that was initially reported in 2014. It has been observed in approximately 0.2–0.5% of unselected NSCLC patients60. To date, a study evaluated 21,858 tumor specimens from various solid tumors, with the most prevalent variant being CD74-NRG1 (29%), while AT1P1-NRG1 (10%) and SDC4-NRG1 (7%) were the second and third most common183. Interestingly, the rearrangement of NRG1 in solid tumors leads to aberrations in the downstream HER-2/HER-3 signaling pathway. Therefore, a previous study showed that patients harboring NRG1 fusion-positive tumors who received afatinib had disappointing results184. However, this study provided researchers with treatment ideas. Recently, GSK2849330, an agent that can inhibit the HER-3 oncologic driver, showed antitumor activity in a patient expressing the CD74-NRG1 gene fusion (NCT01966445)185. Furthermore, zenocutuzumab (MCLA-128) can inhibit HER-2/HER-3 to mediate NRG1 signaling in tumor cells. A relevant phase II basket trial (NCT02912949) is ongoing186. Seribantumab is an anti-HER3 inhibitor that has shown antitumor activity in preclinical models. The relevant clinical trial (CRESTONE; NCT04383210) enrolled patients with NRG1 fusion-positive solid tumors, including NSCLC187.
Conclusions
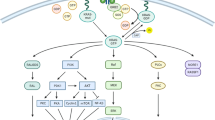
With the development of detection technology, several oncologic drivers have been identified, and corresponding agents have been developed by researchers in the last two decades. The FDA has approved multiple targeted agents for patients who have alternative oncologic drivers, such as EGFR, ALK, ROS1, BRAF, Met, Ret, FGFR and NTRK. Additionally, the development of new-generation TKIs and immunotherapeutic agents has further improved treatment outcomes, with some patients achieving long-term survival benefits. Most clinical studies have demonstrated that patients harboring some type of oncologic driver using targeted therapy can achieve better survival benefits than those receiving chemotherapy or immunotherapy. This has led to a significant shift in the management of NSCLC, with targeted therapy becoming the preferred first-line treatment option for patients with actionable mutations and guiding subsequent researchers and practitioners (Fig. 1).
Simplified overview of the molecular pathways affected by novel emerging rare targets in NSCLC and agents in clinical development. These pathways are physiologically activated by the interaction between circulating growth factors (colored circles) and transmembrane receptors (colored sticks crossing the cell membrane), leading to the downstream activation of intracellular proteins (colored ovoids) that promote cell proliferation, increased aggressiveness, or immune escape. Investigational agents that can inhibit specific pathways are also reported, along with their targeted molecules or interactions, when available. For more details, please refer to the appropriate paragraphs.
Currently, an increasing number of rare oncogenic drivers and activating mutations have been discovered and reported. Does it make sense to study corresponding TKIs and related targeted therapies? According to global cancer statistics, lung cancer is the second largest tumor in the world with a large number of patients, and lung cancer-related deaths remain the leading cause of cancer-related deaths worldwide. Lung cancer is still the leading cause of cancer-related death worldwide, and its incidence and mortality rates are 3- to fourfold greater in developed countries than in developing countries, mainly due to the tobacco epidemic in developing countries. Additionally, the incidence and mortality rates of lung cancer have shown an increasing trend not only in developed countries but also in developing countries around the world. Thus, it might be meaningful for researchers to find novel oncologic drivers and develop innovative drugs, especially in developed and developing regions.
Second, there is high funding for pan-cancer next-generation sequencing (NGS)-based gene panel testing, such as the 425-gene panel. For developing areas, not everyone has the economic conditions to complete it. Therefore, with common oncologic driver alterations, such as EGFR, and some rare oncologic driver alterations, such as RET, ALK, ROS and NTRK, we suggest that practitioners should include these in a new panel. This strategy can decrease the economic pressure on patients. Otherwise, practitioners also can use IHC, FISH, and polymerase chain reaction (PCR) to target specific gene alterations, such as MET with high GCN or HER-2 with IHC3 + , and identify corresponding patients such as patients with locally advanced and advanced lung cancer who may benefit from targeted therapies.
Third, new drugs may not lead to a relatively broad spectrum and may also generate drug resistance genes. Drug resistance can occur, such as generation of EGFR T790m or RET-related solvent front mutations. Thus, patients need to reconsider the use of a pan-cancer gene panel. Furthermore, researchers should focus on these rare mutations and develop related new agents.
Then, researchers should develop new agents that should not only cover common activating mutations, such as EGFR L858R and exon 19 deletion but also cover rare activating mutations, such as exon 20 insertion. Although some alterations in oncogenic drivers, such as HER-2 IHC + , IHC2 + , and MET amplification with low GCN and FGFR alternation, have shown limited effectiveness in clinical trials. More subgroups should be explored like IHC2 + and FISH + in gastric carcinoma and breast cancer. Moreover, researchers should develop next-generation drugs to provide significant survival benefits. In addition, researchers also can explore new regimens like TKIs plus chemotherapy and TKIs plus monoclonal antibodies or ADCs, and corresponding clinical trials should also be developed. However, clinical trials and monitoring of side effects are necessary, as some targeted agents may cause severe side effects, such as cardiac toxicity with trastuzumab. Therefore, patients should receive baseline examinations before receiving TKIs or other targeted agents. Although most of target agents are relatively safe, monitoring of side effects is necessary for each patient.
Finally, targeted therapy is now the preferred treatment option for NSCLC patients with actionable mutations. To achieve a breakthrough in molecular targeted therapy for advanced NSCLC and improve patient outcomes, researchers must continuously work toward developing new testing methods, therapies, and combination treatments. Additionally, gaining a deeper understanding of resistance mechanisms is essential for overcoming treatment resistance and improving patient response rates. These efforts could potentially lead to a paradigm shift in the treatment of locally advanced or advanced NSCLC and offer patients a more positive prognosis. In addition, researchers also cannot ignore the related side effects of targeted therapy. Therefore, each patient can achieve optimal survival benefits.
Data availability
All data generated or analysed during this study are included in this published article.
References
Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 74, 12–49. https://doi.org/10.3322/caac.21820 (2024).
Xia, C. et al. Cancer statistics in China and United States, 2022: Profiles, trends, and determinants. Chin. Med. J. (Engl.) 135, 584–590. https://doi.org/10.1097/cm9.0000000000002108 (2022).
Non-small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: A meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ 311, 899–909 (1995).
Spiro, S. G. & Silvestri, G. A. One hundred years of lung cancer. Am. J. Respir. Crit. Care Med. 172, 523–529. https://doi.org/10.1164/rccm.200504-531OE (2005).
Gridelli, C. Does chemotherapy have a role as palliative therapy for unfit or elderly patients with non-small-cell lung cancer?. Lung Cancer 38(Suppl 2), S45-50. https://doi.org/10.1016/s0169-5002(02)00357-4 (2002).
Lynch, T. J. et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 350, 2129–2139. https://doi.org/10.1056/NEJMoa040938 (2004).
Paez, J. G. et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science 304, 1497–1500. https://doi.org/10.1126/science.1099314 (2004).
Mok, T. S. et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 361, 947–957. https://doi.org/10.1056/NEJMoa0810699 (2009).
Lin, W. C. et al. Gefitinib as front-line treatment in Chinese patients with advanced non-small-cell lung cancer. Lung Cancer 54, 193–199. https://doi.org/10.1016/j.lungcan.2006.07.013 (2006).
Ettinger, D. S. et al. NCCN Guidelines® insights: Non-small cell lung cancer, version 2.2023. J. Natl. Compr. Cancer Netw. 21, 340–350. https://doi.org/10.6004/jnccn.2023.0020 (2023).
Soda, M. et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 448, 561–566. https://doi.org/10.1038/nature05945 (2007).
El-Deeb, I. M., Yoo, K. H. & Lee, S. H. ROS receptor tyrosine kinase: A new potential target for anticancer drugs. Med. Res. Rev. 31, 794–818. https://doi.org/10.1002/med.20206 (2011).
Brose, M. S. et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 62, 6997–7000 (2002).
Schneider, P. M. et al. Differential expression of the c-erbB-2 gene in human small cell and non-small cell lung cancer. Cancer Res. 49, 4968–4971 (1989).
Solomon, B. J. et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N. Engl. J. Med. 371, 2167–2177. https://doi.org/10.1056/NEJMoa1408440 (2014).
Wolf, J. et al. Capmatinib in MET exon 14-mutated or MET-amplified non-small-cell lung cancer. N. Engl. J. Med. 383, 944–957. https://doi.org/10.1056/NEJMoa2002787 (2020).
Hirsch, F. R. et al. Lung cancer: Current therapies and new targeted treatments. Lancet 389, 299–311. https://doi.org/10.1016/s0140-6736(16)30958-8 (2017).
Solomon, B. J. et al. RET solvent front mutations mediate acquired resistance to selective RET inhibition in RET-driven malignancies. J. Thorac. Oncol. 15, 541–549. https://doi.org/10.1016/j.jtho.2020.01.006 (2020).
Vikis, H. et al. EGFR-T790M is a rare lung cancer susceptibility allele with enhanced kinase activity. Cancer Res. 67, 4665–4670. https://doi.org/10.1158/0008-5472.Can-07-0217 (2007).
Rosell, R. et al. Screening for epidermal growth factor receptor mutations in lung cancer. N. Engl. J. Med. 361, 958–967. https://doi.org/10.1056/NEJMoa0904554 (2009).
D’Angelo, S. P. et al. Incidence of EGFR exon 19 deletions and L858R in tumor specimens from men and cigarette smokers with lung adenocarcinomas. J. Clin. Oncol. 29, 2066–2070. https://doi.org/10.1200/jco.2010.32.6181 (2011).
Harrison, P. T., Vyse, S. & Huang, P. H. Rare epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer. Semin. Cancer Biol. 61, 167–179. https://doi.org/10.1016/j.semcancer.2019.09.015 (2020).
Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 511, 543–550. https://doi.org/10.1038/nature13385 (2014).
Vyse, S. & Huang, P. H. Targeting EGFR exon 20 insertion mutations in non-small cell lung cancer. Signal Transduct. Target Ther. 4, 5. https://doi.org/10.1038/s41392-019-0038-9 (2019).
Fang, W. et al. EGFR exon 20 insertion mutations and response to osimertinib in non-small-cell lung cancer. BMC Cancer 19, 595. https://doi.org/10.1186/s12885-019-5820-0 (2019).
Remon, J., Hendriks, L. E. L., Cardona, A. F. & Besse, B. EGFR exon 20 insertions in advanced non-small cell lung cancer: A new history begins. Cancer Treat. Rev. 90, 102105. https://doi.org/10.1016/j.ctrv.2020.102105 (2020).
Hou, J. et al. EGFR exon 20 insertion mutations in advanced non-small-cell lung cancer: Current status and perspectives. Biomark. Res. 10, 21. https://doi.org/10.1186/s40364-022-00372-6 (2022).
Meador, C. B., Sequist, L. V. & Piotrowska, Z. Targeting EGFR exon 20 insertions in non-small cell lung cancer: Recent advances and clinical updates. Cancer Discov. 11, 2145–2157. https://doi.org/10.1158/2159-8290.Cd-21-0226 (2021).
Floc’h, N. et al. Antitumor activity of osimertinib, an irreversible mutant-selective EGFR tyrosine kinase inhibitor, in NSCLC harboring EGFR exon 20 insertions. Mol. Cancer Ther. 17, 885–896. https://doi.org/10.1158/1535-7163.Mct-17-0758 (2018).
Hirano, T. et al. In vitro modeling to determine mutation specificity of EGFR tyrosine kinase inhibitors against clinically relevant EGFR mutants in non-small-cell lung cancer. Oncotarget 6, 38789–38803. https://doi.org/10.18632/oncotarget.5887 (2015).
Kim, T. M. et al. 1529P—Phase II study of osimertinib in NSCLC patients with EGFR exon 20 insertion mutation: A multicenter trial of the Korean Cancer Study Group (LU17–19). Ann. Oncol. 30, v628. https://doi.org/10.1093/annonc/mdz260.051 (2019).
van Veggel, B. et al. Osimertinib treatment for patients with EGFR exon 20 mutation positive non-small cell lung cancer. Lung Cancer 141, 9–13. https://doi.org/10.1016/j.lungcan.2019.12.013 (2020).
Yasuda, H. et al. A phase I/II study of osimertinib in EGFR exon 20 insertion mutation-positive non-small cell lung cancer. Lung Cancer 162, 140–146. https://doi.org/10.1016/j.lungcan.2021.10.006 (2021).
Piotrowska, Z., Wang, Y., Sequist, L. V. & Ramalingam, S. S. ECOG-ACRIN 5162: A phase II study of osimertinib 160 mg in NSCLC with EGFR exon 20 insertions. J. Clin. Oncol. 38, 9513–9513 (2020).
Zwierenga, F. et al. High dose osimertinib in patients with advanced stage EGFR exon 20 mutation-positive NSCLC: Results from the phase 2 multicenter POSITION20 trial. Lung Cancer 170, 133–140. https://doi.org/10.1016/j.lungcan.2022.06.012 (2022).
Robichaux, J. P. et al. Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nat. Med. 24, 638–646. https://doi.org/10.1038/s41591-018-0007-9 (2018).
Elamin, Y. Y. et al. Poziotinib for EGFR exon 20-mutant NSCLC: Clinical efficacy, resistance mechanisms, and impact of insertion location on drug sensitivity. Cancer Cell 40, 754-767.e756. https://doi.org/10.1016/j.ccell.2022.06.006 (2022).
Sacher, A., Le, X., Cornelissen, R., Shum, E. & Garassino, M. C. 36MO Safety, tolerability and preliminary efficacy of poziotinib with twice daily strategy in EGFR/HER2 Exon 20 mutant non-small cell lung cancer. Ann. Oncol. 32, S15 (2021).
Le, X. et al. Abstract CT081: Poziotinib activity and durability of responses in previously treated EGFR exon 20 NSCLC patients—A Phase 2 study. Cancer Res. 80, CT081. https://doi.org/10.1158/1538-7445.Am2020-ct081 (2020).
Riely, G. J. et al. Activity and safety of mobocertinib (TAK-788) in previously treated non-small cell lung cancer with EGFR exon 20 insertion mutations from a phase I/II trial. Cancer Discov. 11, 1688–1699. https://doi.org/10.1158/2159-8290.Cd-20-1598 (2021).
Zhou, C. et al. Treatment outcomes and safety of mobocertinib in platinum-pretreated patients with EGFR exon 20 insertion-positive metastatic non-small cell lung cancer: A phase 1/2 open-label nonrandomized clinical trial. JAMA Oncol. 7, e214761. https://doi.org/10.1001/jamaoncol.2021.4761 (2021).
Markham, A. Mobocertinib: First approval. Drugs 81, 2069–2074. https://doi.org/10.1007/s40265-021-01632-9 (2021).
Rosa, K. Takeda to voluntarily withdraw mobocertinib for EGFR exon 20 insertion+ NSCLC. https://www.onclive.com/view/takeda-to-voluntarily-withdraw-mobocertinib-for-egfr-exon-20-insertion-nsclc (2023).
Yu, H. A. et al. Phase (Ph) 1/2a study of CLN-081 in patients (pts) with NSCLC with EGFR exon 20 insertion mutations (Ins20). J. Clin. Oncol. 40, 9007–9007. https://doi.org/10.1200/JCO.2022.40.16_suppl.9007 (2022).
Conroy, R. Investigators launch phase 3 zipalertinib combo trial in EGFR+ NSCLC. Cancer Netw. (2023).
Cho, B. C. et al. 1497PJNJ-61186372 (JNJ-372), an EGFR-cMET bispecific antibody, in advanced non-small cell lung cancer (NSCLC): An update on phase I results. Ann. Oncol. https://doi.org/10.1093/annonc/mdy292.118 (2018).
Moores, S. L. et al. A novel bispecific antibody targeting EGFR and cMet is effective against EGFR inhibitor-resistant lung tumors. Cancer Res. 76, 3942–3953 (2016).
Yun, J., Lee, S. H., Kim, S. Y., Jeong, S. Y. & Cho, B. C. Antitumor activity of amivantamab (JNJ-61186372), an EGFR-cMet bispecific antibody, in diverse models of EGFR exon 20 insertion-driven NSCLC. Cancer Discov. 10, CD-20-0116 (2020).
Park, K. et al. Amivantamab in EGFR exon 20 insertion-mutated non–small-cell lung cancer progressing on platinum chemotherapy: Initial results from the CHRYSALIS phase I study. J. Clin. Oncol. 39, 3391–3402. https://doi.org/10.1200/jco.21.00662 (2021).
Syed, Y. Y. Amivantamab: First approval. Drugs 81, 1349–1353. https://doi.org/10.1007/s40265-021-01561-7 (2021).
Vyse, S. & Huang, P. H. Amivantamab for the treatment of EGFR exon 20 insertion mutant non-small cell lung cancer. Expert Rev. Anticancer Ther. 22, 3–16. https://doi.org/10.1080/14737140.2022.2016397 (2022).
Zhou, C. et al. Amivantamab plus chemotherapy in NSCLC with EGFR exon 20 insertions. N. Engl. J. Med. 389, 2039–2051. https://doi.org/10.1056/NEJMoa2306441 (2023).
Wang, M. et al. Sunvozertinib, a selective EGFR inhibitor for previously treated non-small cell lung cancer with EGFR exon 20 insertion mutations. Cancer Discov. 12, 1676–1689. https://doi.org/10.1158/2159-8290.Cd-21-1615 (2022).
Wang, M. et al. Sunvozertinib for the treatment of NSCLC with EGFR Exon20 insertion mutations: The first pivotal study results. J. Clin. Oncol. 41, 9002–9002. https://doi.org/10.1200/JCO.2023.41.16_suppl.9002 (2023).
Xu, Y. et al. Efficacy and safety of sunvozertinib in treatment naïve NSCLC patients with EGFR exon20 insertion mutations. J. Clin. Oncol. 41, 9073–9073. https://doi.org/10.1200/JCO.2023.41.16_suppl.9073 (2023).
Dhillon, S. Sunvozertinib: First approval. Drugs 83, 1629–1634. https://doi.org/10.1007/s40265-023-01959-5 (2023).
Han, B. et al. OA03.04 A phase 1b study of furmonertinib, an oral, brain penetrant, selective EGFR inhibitor, in patients with advanced NSCLC with EGFR exon 20 insertions. J. Thorac. Oncol. 18, S49. https://doi.org/10.1016/j.jtho.2023.09.033 (2023).
Zhang, S. S. & Ou, S.-H.I. Spotlight on furmonertinib (Alflutinib, AST2818). The Swiss Army Knife (del19, L858R, T790M, exon 20 insertions, “uncommon-G719X, S768I, L861Q”) among the third-generation EGFR TKIs?. Lung Cancer Targets Ther. 13, 67–73 (2022).
Rebuzzi, S. E. et al. Novel emerging molecular targets in non-small cell lung cancer. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22052625 (2021).
Russo, A. et al. New targets in lung cancer (excluding EGFR, ALK, ROS1). Curr. Oncol. Rep. 22, 48. https://doi.org/10.1007/s11912-020-00909-8 (2020).
Ou, S. H. et al. Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J. Thorac. Oncol. 6, 942–946. https://doi.org/10.1097/JTO.0b013e31821528d3 (2011).
Pfizer’s XALKORI®(Crizotinib) Receives FDA Breakthrough Therapy Designation in Two New Indications/Pfizer. Available on- line (2018).
Chiari, R. et al. ROS1-rearranged non-small-cell lung cancer is associated with a high rate of venous thromboembolism: Analysis from a phase II, prospective, multicenter, two-arms trial (METROS). Clin. Lung Cancer 21, 15–20. https://doi.org/10.1016/j.cllc.2019.06.012 (2020).
Drilon, A. et al. Antitumor activity of crizotinib in lung cancers harboring a MET exon 14 alteration. Nat. Med. 26, 47–51. https://doi.org/10.1038/s41591-019-0716-8 (2020).
Moro-Sibilot, D. et al. Crizotinib in c-MET- or ROS1-positive NSCLC: Results of the AcSé phase II trial. Ann. Oncol. 30, 1985–1991. https://doi.org/10.1093/annonc/mdz407 (2019).
Dong, Y., Xu, J., Sun, B., Wang, J. & Wang, Z. MET-targeted therapies and clinical outcomes: A systematic literature review. Mol. Diagn. Ther. 26, 203–227. https://doi.org/10.1007/s40291-021-00568-w (2022).
Recondo, G., Che, J., Jänne, P. A. & Awad, M. M. Targeting MET dysregulation in cancer. Cancer Discov. 10, 922–934. https://doi.org/10.1158/2159-8290.Cd-19-1446 (2020).
Wu, Y. L. et al. Phase Ib/II study of capmatinib (INC280) plus gefitinib after failure of epidermal growth factor receptor (EGFR) inhibitor therapy in patients with EGFR-mutated, MET factor-dysregulated non-small-cell lung cancer. J. Clin. Oncol. 36, 3101–3109. https://doi.org/10.1200/jco.2018.77.7326 (2018).
Schuler, M. et al. Molecular correlates of response to capmatinib in advanced non-small-cell lung cancer: Clinical and biomarker results from a phase I trial. Ann. Oncol. 31, 789–797. https://doi.org/10.1016/j.annonc.2020.03.293 (2020).
Seto, T. et al. Capmatinib in Japanese patients with MET exon 14 skipping-mutated or MET-amplified advanced NSCLC: GEOMETRY mono-1 study. Cancer Sci. 112, 1556–1566. https://doi.org/10.1111/cas.14826 (2021).
Dagogo-Jack, I. et al. A phase 2 study of capmatinib in patients with MET-altered lung cancer previously treated with a MET inhibitor. J. Thorac. Oncol. 16, 850–859. https://doi.org/10.1016/j.jtho.2021.01.1605 (2021).
Engstrom, L. D. et al. Glesatinib exhibits antitumor activity in lung cancer models and patients harboring MET Exon 14 mutations and overcomes mutation-mediated resistance to type I MET inhibitors in nonclinical models. Clin. Cancer Res. 23, 6661–6672. https://doi.org/10.1158/1078-0432.Ccr-17-1192 (2017).
Markham, A. Tepotinib: First approval. Drugs 80, 829–833. https://doi.org/10.1007/s40265-020-01317-9 (2020).
Le, X. et al. Tepotinib efficacy and safety in patients with MET exon 14 skipping NSCLC: Outcomes in patient subgroups from the VISION study with relevance for clinical practice. Clin. Cancer Res. 28, 1117–1126. https://doi.org/10.1158/1078-0432.Ccr-21-2733 (2022).
Wu, Y. L. et al. Tepotinib plus gefitinib in patients with EGFR-mutant non-small-cell lung cancer with MET overexpression or MET amplification and acquired resistance to previous EGFR inhibitor (INSIGHT study): An open-label, phase 1b/2, multicentre, randomised trial. Lancet Respir. Med. 8, 1132–1143. https://doi.org/10.1016/s2213-2600(20)30154-5 (2020).
Smit, E. F. et al. INSIGHT 2: A phase II study of tepotinib plus osimertinib in MET-amplified NSCLC and first-line osimertinib resistance. Future Oncol. 18, 1039–1054. https://doi.org/10.2217/fon-2021-1406 (2022).
Markham, A. Savolitinib: First approval. Drugs 81, 1665–1670. https://doi.org/10.1007/s40265-021-01584-0 (2021).
Lu, S. et al. Once-daily savolitinib in Chinese patients with pulmonary sarcomatoid carcinomas and other non-small-cell lung cancers harbouring MET exon 14 skipping alterations: A multicentre, single-arm, open-label, phase 2 study. Lancet Respir. Med. 9, 1154–1164. https://doi.org/10.1016/s2213-2600(21)00084-9 (2021).
Hartmaier, R. J. et al. Osimertinib + savolitinib to overcome acquired MET-mediated resistance in epidermal growth factor receptor-mutated, MET-amplified non-small cell lung cancer: TATTON. Cancer Discov. 13, 98–113. https://doi.org/10.1158/2159-8290.Cd-22-0586 (2023).
Brazel, D. & Nagasaka, M. Spotlight on amivantamab (JNJ-61186372) for EGFR exon 20 insertions positive non-small cell lung cancer. Lung Cancer (Auckl) 12, 133–138. https://doi.org/10.2147/lctt.S337861 (2021).
Krebs, M. et al. Amivantamab in patients with NSCLC with MET exon 14 skipping mutation: Updated results from the CHRYSALIS study. J. Clin. Oncol. 40, 9008–9008. https://doi.org/10.1200/JCO.2022.40.16_suppl.9008 (2022).
Lu, S. et al. Abstract CT034: Phase II study of SCC244 in NSCLC patients harboring MET exon 14 skipping (METex14) mutations (GLORY study). Cancer Res. 82, CT034. https://doi.org/10.1158/1538-7445.Am2022-ct034 (2022).
Reckamp, K. L. et al. Phase II trial of cabozantinib plus erlotinib in patients with advanced epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer with progressive disease on epidermal growth factor receptor tyrosine kinase inhibitor therapy: A California Cancer Consortium Phase II trial (NCI 9303). Front. Oncol. 9, 132. https://doi.org/10.3389/fonc.2019.00132 (2019).
Yan, S. B. et al. MET-targeting antibody (emibetuzumab) and kinase inhibitor (merestinib) as single agent or in combination in a cancer model bearing MET exon 14 skipping. Investig. New Drugs 36, 536–544. https://doi.org/10.1007/s10637-017-0545-x (2018).
Park, K. et al. Phase I results of S49076 plus gefitinib in patients with EGFR TKI-resistant non-small cell lung cancer harbouring MET/AXL dysregulation. Lung Cancer 155, 127–135. https://doi.org/10.1016/j.lungcan.2021.03.012 (2021).
Fujino, T., Suda, K. & Mitsudomi, T. Emerging MET tyrosine kinase inhibitors for the treatment of non-small cell lung cancer. Expert Opin. Emerg. Drugs 25, 229–249. https://doi.org/10.1080/14728214.2020.1791821 (2020).
Goldman, J. W. et al. Phase 1/1b study of telisotuzumab vedotin (Teliso-V) + osimertinib (Osi), after failure on prior Osi, in patients with advanced, c-Met overexpressing, EGFR-mutated non-small cell lung cancer (NSCLC). J. Clin. Oncol. 40, 9013–9013. https://doi.org/10.1200/JCO.2022.40.16_suppl.9013 (2022).
Cardarella, S. et al. Clinical, pathologic, and biologic features associated with BRAF mutations in non-small cell lung cancer. Clin. Cancer Res. 19, 4532–4540. https://doi.org/10.1158/1078-0432.Ccr-13-0657 (2013).
Rodak, O., Peris-Díaz, M. D., Olbromski, M., Podhorska-Okołów, M. & Dzięgiel, P. Current landscape of non-small cell lung cancer: Epidemiology, histological classification, targeted therapies, and immunotherapy. Cancers https://doi.org/10.3390/cancers13184705 (2021).
Dankner, M., Rose, A. A. N., Rajkumar, S., Siegel, P. M. & Watson, I. R. Classifying BRAF alterations in cancer: New rational therapeutic strategies for actionable mutations. Oncogene 37, 3183–3199. https://doi.org/10.1038/s41388-018-0171-x (2018).
Hyman, D. M. et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N. Engl. J. Med. 373, 726–736. https://doi.org/10.1056/NEJMoa1502309 (2015).
Planchard, D. et al. Dabrafenib in patients with BRAF(V600E)-positive advanced non-small-cell lung cancer: A single-arm, multicentre, open-label, phase 2 trial. Lancet Oncol. 17, 642–650. https://doi.org/10.1016/s1470-2045(16)00077-2 (2016).
Planchard, D. et al. Phase 2 study of dabrafenib plus trametinib in patients with BRAF V600E-mutant metastatic NSCLC: Updated 5-year survival rates and genomic analysis. J. Thorac. Oncol. 17, 103–115. https://doi.org/10.1016/j.jtho.2021.08.011 (2022).
Swalduz, A. et al. Efficacy of dabrafenib-trametinib combination in BRAF V600E-mutated metastatic non–small cell lung cancer: Results of the IFCT-2004 BLaDE cohort. J. Clin. Oncol. 40, 9082–9082. https://doi.org/10.1200/JCO.2022.40.16_suppl.9082 (2022).
Saha, D. et al. Targeting rearranged during transfection in cancer: A perspective on small-molecule inhibitors and their clinical development. J. Med. Chem. 64, 11747–11773. https://doi.org/10.1021/acs.jmedchem.0c02167 (2021).
Yoh, K. et al. Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): An open-label, multicentre phase 2 trial. Lancet Respir. Med. 5, 42–50. https://doi.org/10.1016/s2213-2600(16)30322-8 (2017).
Gautschi, O. et al. Targeting RET in patients with RET-rearranged lung cancers: Results from the global, multicenter RET Registry. J. Clin. Oncol. 35, 1403–1410. https://doi.org/10.1200/jco.2016.70.9352 (2017).
Gainor, J. F. et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): A multi-cohort, open-label, phase 1/2 study. Lancet Oncol. 22, 959–969. https://doi.org/10.1016/s1470-2045(21)00247-3 (2021).
Griesinger, F. et al. Safety and efficacy of pralsetinib in RET fusion-positive non-small-cell lung cancer including as first-line therapy: Update from the ARROW trial. Ann. Oncol. https://doi.org/10.1016/j.annonc.2022.08.002 (2022).
Drilon, A. et al. Selpercatinib in patients with RET fusion-positive non-small-cell lung cancer: Updated safety and efficacy from the registrational LIBRETTO-001 phase I/II Trial. J. Clin. Oncol. https://doi.org/10.1200/jco.22.00393 (2022).
Subbiah, V. et al. Intracranial efficacy of selpercatinib in RET fusion-positive non-small cell lung cancers on the LIBRETTO-001 Trial. Clin. Cancer Res. 27, 4160–4167. https://doi.org/10.1158/1078-0432.Ccr-21-0800 (2021).
Drilon, A. et al. 506P - TPX-0046 is a novel and potent RET/SRC inhibitor for RET-driven cancers. Ann. Oncol. 30, v190–v191. https://doi.org/10.1093/annonc/mdz244.068 (2019).
Turning Point Therapeutics Announces Initial Clinical Data From Phase 1/2 SWORD-1 Study of RET Inhibitor TPX-0046. https://firstwordpharma.com/story/5266393 (2021).
Schoffski, P. et al. BOS172738, a highly potent and selective RET inhibitor, for the treatment of RET-altered tumors including RET-fusion+ NSCLC and RET-mutant MTC: Phase 1 study results. J. Clin. Oncol. 39, 3008–3008. https://doi.org/10.1200/JCO.2021.39.15_suppl.3008 (2021).
Suzuki, M. et al. HER2 gene mutations in non-small cell lung carcinomas: Concurrence with Her2 gene amplification and Her2 protein expression and phosphorylation. Lung Cancer 87, 14–22. https://doi.org/10.1016/j.lungcan.2014.10.014 (2015).
Hynes, N. E. & Stern, D. F. The biology of erbB-2/neu/HER-2 and its role in cancer. Biochim. Biophys. Acta 1198, 165–184. https://doi.org/10.1016/0304-419x(94)90012-4 (1994).
Swain, S. M., Shastry, M. & Hamilton, E. Targeting HER2-positive breast cancer: Advances and future directions. Nat. Rev. Drug Discov. 22, 101–126. https://doi.org/10.1038/s41573-022-00579-0 (2023).
La Salvia, A., Lopez-Gomez, V. & Garcia-Carbonero, R. HER2-targeted therapy: An emerging strategy in advanced colorectal cancer. Expert Opin. Investig. Drugs 28, 29–38. https://doi.org/10.1080/13543784.2019.1555583 (2019).
Arcila, M. E. et al. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin. Cancer Res. 18, 4910–4918. https://doi.org/10.1158/1078-0432.Ccr-12-0912 (2012).
Sankar, K., Gadgeel, S. M. & Qin, A. Molecular therapeutic targets in non-small cell lung cancer. Expert Rev. Anticancer Ther. 20, 647–661. https://doi.org/10.1080/14737140.2020.1787156 (2020).
Liu, L. et al. The role of human epidermal growth factor receptor 2 as a prognostic factor in lung cancer: A meta-analysis of published data. J. Thorac. Oncol. 5, 1922–1932. https://doi.org/10.1097/jto.0b013e3181f26266 (2010).
Riudavets, M., Sullivan, I., Abdayem, P. & Planchard, D. Targeting HER2 in non-small-cell lung cancer (NSCLC): A glimpse of hope? An updated review on therapeutic strategies in NSCLC harbouring HER2 alterations. ESMO Open 6, 100260. https://doi.org/10.1016/j.esmoop.2021.100260 (2021).
Ricciardi, G. R. et al. NSCLC and HER2: Between lights and shadows. J. Thorac. Oncol. 9, 1750–1762. https://doi.org/10.1097/jto.0000000000000379 (2014).
Yoshizawa, A. et al. HER2 status in lung adenocarcinoma: A comparison of immunohistochemistry, fluorescence in situ hybridization (FISH), dual-ISH, and gene mutations. Lung Cancer 85, 373–378. https://doi.org/10.1016/j.lungcan.2014.06.007 (2014).
Bunn, P. A. Jr. et al. Expression of Her-2/neu in human lung cancer cell lines by immunohistochemistry and fluorescence in situ hybridization and its relationship to in vitro cytotoxicity by trastuzumab and chemotherapeutic agents. Clin. Cancer Res. 7, 3239–3250 (2001).
Dziadziuszko, R. et al. Afatinib in NSCLC with HER2 mutations: Results of the prospective, open-label phase II NICHE trial of European thoracic oncology platform (ETOP). J. Thorac. Oncol. 14, 1086–1094. https://doi.org/10.1016/j.jtho.2019.02.017 (2019).
Hyman, D. M. et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 554, 189–194. https://doi.org/10.1038/nature25475 (2018).
Jebbink, M., de Langen, A. J., Boelens, M. C., Monkhorst, K. & Smit, E. F. The force of HER2—A druggable target in NSCLC?. Cancer Treat. Rev. 86, 101996. https://doi.org/10.1016/j.ctrv.2020.101996 (2020).
Kris, M. G. et al. Targeting HER2 aberrations as actionable drivers in lung cancers: Phase II trial of the pan-HER tyrosine kinase inhibitor dacomitinib in patients with HER2-mutant or amplified tumors. Ann. Oncol. 26, 1421–1427. https://doi.org/10.1093/annonc/mdv186 (2015).
Elamin, Y. Y. et al. Poziotinib for patients with HER2 exon 20 mutant non-small-cell lung cancer: Results from a phase II trial. J. Clin. Oncol. 40, 702–709. https://doi.org/10.1200/jco.21.01113 (2022).
Le, X. et al. Poziotinib in non-small-cell lung cancer harboring HER2 exon 20 insertion mutations after prior therapies: ZENITH20-2 trial. J. Clin. Oncol. 40, 710–718. https://doi.org/10.1200/jco.21.01323 (2022).
Wang, Y. et al. HER2 exon 20 insertions in non-small-cell lung cancer are sensitive to the irreversible pan-HER receptor tyrosine kinase inhibitor pyrotinib. Ann. Oncol. 30, 447–455. https://doi.org/10.1093/annonc/mdy542 (2019).
Song, Z. et al. Pyrotinib in patients with HER2-amplified advanced non-small cell lung cancer: A prospective, multicentre, single-arm trial. Clin. Cancer Res. 28, 461–467. https://doi.org/10.1158/1078-0432.Ccr-21-2936 (2022).
Estrada-Bernal, A. et al. Tarloxotinib is a hypoxia-activated pan-HER kinase inhibitor active against a broad range of HER-family oncogenes. Clin. Cancer Res. 27, 1463–1475. https://doi.org/10.1158/1078-0432.Ccr-20-3555 (2021).
Liu, S. V. et al. LBA61 first analysis of RAIN-701: Study of tarloxotinib in patients with non-small cell lung cancer (NSCLC) EGFR Exon 20 insertion, HER2-activating mutations & other solid tumours with NRG1/ERBB gene fusions. Ann. Oncol. 31, S1189. https://doi.org/10.1016/j.annonc.2020.08.2294 (2020).
Riely, G. J. et al. 1261MO updated results from a phase I/II study of mobocertinib (TAK-788) in NSCLC with EGFR exon 20 insertions (exon20ins). Ann. Oncol. 31, S815–S816. https://doi.org/10.1016/j.annonc.2020.08.1575 (2020).
Hafeez, U., Parakh, S., Gan, H. K. & Scott, A. M. Antibody-drug conjugates for cancer therapy. Molecules https://doi.org/10.3390/molecules25204764 (2020).
Hainsworth, J. D. et al. Targeted therapy for advanced solid tumors on the basis of molecular profiles: Results from mypathway, an open-label, phase IIa multiple basket study. J. Clin. Oncol. 36, 536–542. https://doi.org/10.1200/jco.2017.75.3780 (2018).
Kinoshita, I. et al. 1491PA phase II study of trastuzumab monotherapy in pretreated patients with non-small cell lung cancers (NSCLCs) harboring HER2 alterations: HOT1303-B trial. Ann. Oncol. https://doi.org/10.1093/annonc/mdy292.112 (2018).
Gatzemeier, U. et al. Randomized phase II trial of gemcitabine–cisplatin with or without trastuzumab in HER2-positive non-small-cell lung cancer. Ann. Oncol. 15, 19–27. https://doi.org/10.1093/annonc/mdh031 (2004).
Mazieres, J. et al. Combination of trastuzumab, pertuzumab, and docetaxel in patients with advanced non-small-cell lung cancer harboring HER2 mutations: Results from the IFCT-1703 R2D2 trial. J. Clin. Oncol. 40, 719–728. https://doi.org/10.1200/jco.21.01455 (2022).
van Berge Henegouwen, J. M. et al. Trastuzumab and pertuzumab combination therapy for advanced pre-treated HER2 exon 20-mutated non-small cell lung cancer. Eur. J. Cancer 171, 114–123. https://doi.org/10.1016/j.ejca.2022.05.009 (2022).
Li, B. T. et al. Ado-trastuzumab emtansine for patients with HER2-mutant lung cancers: results from a phase II basket trial. J. Clin. Oncol. 36, 2532–2537. https://doi.org/10.1200/JCO.2018.77.9777 (2018).
Iwama, E. et al. Trastuzumab emtansine for patients with non-small cell lung cancer positive for human epidermal growth factor receptor 2 exon-20 insertion mutations. Eur. J. Cancer 162, 99–106. https://doi.org/10.1016/j.ejca.2021.11.021 (2022).
Peters, S. et al. Trastuzumab emtansine (T-DM1) in patients with previously treated HER2-overexpressing metastatic non-small cell lung cancer: Efficacy, safety, and biomarkers. Clin. Cancer Res. 25, 64–72. https://doi.org/10.1158/1078-0432.Ccr-18-1590 (2019).
Li, B. T. et al. Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. N. Engl. J. Med. 386, 241–251. https://doi.org/10.1056/NEJMoa2112431 (2022).
Tsurutani, J. et al. Targeting HER2 with trastuzumab deruxtecan: A dose-expansion, phase I study in multiple advanced solid tumors. Cancer Discov. 10, 688–701. https://doi.org/10.1158/2159-8290.Cd-19-1014 (2020).
Nakagawa, K. et al. OA04.05 Trastuzumab Deruxtecan in HER2-overexpressing metastatic non-small cell lung cancer: Interim results of DESTINY-Lung01. J. Thorac. Oncol. 16, S109–S110. https://doi.org/10.1016/j.jtho.2021.01.285 (2021).
Narayan, P. et al. FDA approval summary: Fam-Trastuzumab Deruxtecan-Nxki for the treatment of unresectable or metastatic HER2-positive breast cancer. Clin. Cancer Res. 27, 4478–4485. https://doi.org/10.1158/1078-0432.Ccr-20-4557 (2021).
Li, B. T. et al. HER2-mediated internalization of cytotoxic agents in ERBB2 amplified or mutant lung cancers. Cancer Discov. 10, 674–687. https://doi.org/10.1158/2159-8290.Cd-20-0215 (2020).
Elfving, H. et al. Evaluation of NTRK immunohistochemistry as a screening method for NTRK gene fusion detection in non-small cell lung cancer. Lung Cancer 151, 53–59. https://doi.org/10.1016/j.lungcan.2020.11.023 (2021).
Okamura, K. et al. Expression of TrkB and BDNF is associated with poor prognosis in non-small cell lung cancer. Lung Cancer 78, 100–106. https://doi.org/10.1016/j.lungcan.2012.07.011 (2012).
Kummar, S. & Lassen, U. N. TRK inhibition: A new tumor-agnostic treatment strategy. Target Oncol. 13, 545–556. https://doi.org/10.1007/s11523-018-0590-1 (2018).
Farago, A. F. et al. Clinicopathologic Features of non-small-cell lung cancer harboring an NTRK gene fusion. JCO Precis. Oncol. https://doi.org/10.1200/po.18.00037 (2018).
Doebele, R. C. et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 21, 271–282. https://doi.org/10.1016/s1470-2045(19)30691-6 (2020).
Hong, D. S. et al. Larotrectinib in patients with TRK fusion-positive solid tumours: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 21, 531–540. https://doi.org/10.1016/s1470-2045(19)30856-3 (2020).
Lee, J. et al. Evaluating entrectinib as a treatment option for non-small cell lung cancer. Expert Opin. Pharmacother. 21, 1935–1942. https://doi.org/10.1080/14656566.2020.1798932 (2020).
Sartore-Bianchi, A. et al. Entrectinib for the treatment of metastatic NSCLC: Safety and efficacy. Expert Rev. Anticancer Ther. 20, 333–341. https://doi.org/10.1080/14737140.2020.1747439 (2020).
Drilon, A. et al. Efficacy and safety of larotrectinib in patients with tropomyosin receptor kinase fusion-positive lung cancers. JCO Precis. Oncol. https://doi.org/10.1200/po.21.00418 (2022).
Qin, H. & Patel, M. R. The challenge and opportunity of NTRK inhibitors in non-small cell lung cancer. Int. J. Mol. Sci. https://doi.org/10.3390/ijms23062916 (2022).
Holzmann, K. et al. Alternative splicing of fibroblast growth factor receptor IgIII loops in cancer. J. Nucleic Acids 2012, 950508. https://doi.org/10.1155/2012/950508 (2012).
Tiseo, M. et al. FGFR as potential target in the treatment of squamous non small cell lung cancer. Cancer Treat. Rev. 41, 527–539. https://doi.org/10.1016/j.ctrv.2015.04.011 (2015).
Qin, A. et al. Detection of known and novel FGFR fusions in non-small cell lung cancer by comprehensive genomic profiling. J. Thorac. Oncol. 14, 54–62. https://doi.org/10.1016/j.jtho.2018.09.014 (2019).
Dong, M., Li, T. & Chen, J. Progress on the study of targeting FGFR in squamous non-small cell lung cancer. Zhongguo Fei Ai Za Zhi 21, 116–120. https://doi.org/10.3779/j.issn.1009-3419.2018.02.05 (2018).
Ng, T. L. et al. Preselection of lung cancer cases using <em>FGFR1</em> mRNA and gene copy number for treatment with ponatinib. Clin. Lung Cancer 20, e39–e51. https://doi.org/10.1016/j.cllc.2018.09.001 (2019).
Pacini, L., Jenks, A. D., Lima, N. C. & Huang, P. H. Targeting the fibroblast growth factor receptor (FGFR) family in lung cancer. Cells https://doi.org/10.3390/cells10051154 (2021).
Gavine, P. R. et al. AZD4547: an orally bioavailable, potent, and selective inhibitor of the fibroblast growth factor receptor tyrosine kinase family. Cancer Res. 72, 2045–2056. https://doi.org/10.1158/0008-5472.Can-11-3034 (2012).
Aggarwal, C. et al. SWOG S1400D (NCT02965378), a phase II study of the fibroblast growth factor receptor inhibitor AZD4547 in previously treated patients with fibroblast growth factor pathway-activated stage IV squamous cell lung cancer (lung-MAP substudy). J. Thorac. Oncol. 14, 1847–1852. https://doi.org/10.1016/j.jtho.2019.05.041 (2019).
Harding, T. C. et al. Blockade of nonhormonal fibroblast growth factors by FP-1039 inhibits growth of multiple types of cancer. Sci. Transl. Med. 5, 178ra139. https://doi.org/10.1126/scitranslmed.3005414 (2013).
Morgensztern, D. et al. An open-label phase IB study to evaluate GSK3052230 in combination with paclitaxel and carboplatin, or docetaxel, in FGFR1-amplified non-small cell lung cancer. Lung Cancer 136, 74–79. https://doi.org/10.1016/j.lungcan.2019.08.011 (2019).
Grünewald, S. et al. Rogaratinib: A potent and selective pan-FGFR inhibitor with broad antitumor activity in FGFR-overexpressing preclinical cancer models. Int. J. Cancer 145, 1346–1357. https://doi.org/10.1002/ijc.32224 (2019).
Schuler, M. et al. Rogaratinib in patients with advanced cancers selected by <em>FGFR</em> mRNA expression: A phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 20, 1454–1466. https://doi.org/10.1016/S1470-2045(19)30412-7 (2019).
Addeo, A. et al. Fibroblast growth factor receptor (FGFR) inhibitor rogaratinib in patients with advanced pretreated squamous-cell non-small cell lung cancer over-expressing FGFR mRNA: The SAKK 19/18 phase II study. Lung Cancer 172, 154–159. https://doi.org/10.1016/j.lungcan.2022.08.016 (2022).
Peng, L. et al. Targeting ALK rearrangements in NSCLC: Current state of the art. Front. Oncol. 12, 863461. https://doi.org/10.3389/fonc.2022.863461 (2022).
Shaw, A. T. et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N. Engl. J. Med. 373, 1582. https://doi.org/10.1056/NEJMx150036 (2015).
Huber, R. M. et al. Brigatinib in crizotinib-refractory ALK+ NSCLC: 2-year follow-up on systemic and intracranial outcomes in the phase 2 ALTA trial. J. Thorac. Oncol. 15, 404–415. https://doi.org/10.1016/j.jtho.2019.11.004 (2020).
Kim, D. W. et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): Updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol. 17, 452–463. https://doi.org/10.1016/s1470-2045(15)00614-2 (2016).
Ou, S. I. et al. Continuation of lorlatinib in ALK-positive NSCLC beyond progressive disease. J. Thorac. Oncol. 17, 568–577. https://doi.org/10.1016/j.jtho.2021.12.011 (2022).
Peters, S. et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N. Engl. J. Med. 377, 829–838. https://doi.org/10.1056/NEJMoa1704795 (2017).
Pelish, H. E. et al. Abstract 1468: NUV-655 (NVL-655) is a selective, brain-penetrant ALK inhibitor with antitumor activity against the lorlatinib-resistant G1202R/L1196M compound mutation. Cancer Res. 81, 1468–1468. https://doi.org/10.1158/1538-7445.Am2021-1468 (2021).
Murray, B. W. et al. Abstract 1469: TPX-0131, a potent inhibitor of wild type ALK and a broad spectrum of both single and compound ALK resistance mutations. Cancer Res. 81, 1469–1469. https://doi.org/10.1158/1538-7445.Am2021-1469 (2021).
Yang, J.-J. et al. SAF-189s in advanced, ALK-positive, non–small cell lung cancer: Results from a first-in-human phase 1/2, multicenter study. J. Clin. Oncol. 40, 9076–9076. https://doi.org/10.1200/JCO.2022.40.16_suppl.9076 (2022).
Ardini, E. et al. Entrectinib, a Pan–TRK, ROS1, and ALK inhibitor with activity in multiple molecularly defined cancer indications. Mol. Cancer Ther. 15, 628–639. https://doi.org/10.1158/1535-7163.Mct-15-0758 (2016).
Cho, B. C. et al. Safety and preliminary clinical activity of repotrectinib in patients with advanced ROS1 fusion-positive non-small cell lung cancer (TRIDENT-1 study). J. Clin. Oncol. 37, 9011–9011. https://doi.org/10.1200/JCO.2019.37.15_suppl.9011 (2019).
Mizuta, H. et al. Gilteritinib overcomes lorlatinib resistance in ALK-rearranged cancer. Nat. Commun. 12, 1261. https://doi.org/10.1038/s41467-021-21396-w (2021).
Rikova, K. et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 131, 1190–1203. https://doi.org/10.1016/j.cell.2007.11.025 (2007).
Gendarme, S., Bylicki, O., Chouaid, C. & Guisier, F. ROS-1 fusions in non-small-cell lung cancer: Evidence to date. Curr. Oncol. 29, 641–658. https://doi.org/10.3390/curroncol29020057 (2022).
Patil, T. et al. The incidence of brain metastases in stage IV <em>ROS1</em>-rearranged non–small cell lung cancer and rate of central nervous system progression on crizotinib. J. Thorac. Oncol. 13, 1717–1726. https://doi.org/10.1016/j.jtho.2018.07.001 (2018).
Doebele, R. C. et al. TRIDENT-1: A global, multicenter, open-label Phase II study investigating the activity of repotrectinib in advanced solid tumors harboring ROS1 or NTRK1–3 rearrangements. J. Clin. Oncol. 38, TPS9637. https://doi.org/10.1200/JCO.2020.38.15_suppl.TPS9637 (2020).
Li, W. et al. The efficacy and safety of taletrectinib in patients with TKI-naïve or crizotinib-pretreated ROS1-positive non–small cell lung cancer (NSCLC). J. Clin. Oncol. 40, 8572–8572. https://doi.org/10.1200/JCO.2022.40.16_suppl.8572 (2022).
Nagasaka, M. et al. TRUST-II: A global phase II study for taletrectinib in ROS1 fusion–positive lung cancer and other solid tumors. J. Clin. Oncol. 40, TPS8601. https://doi.org/10.1200/JCO.2022.40.16_suppl.TPS8601 (2022).
Ai, X. et al. Safety but limited efficacy of ensartinib in ROS1-Positive NSCLC: A single-arm, multicenter phase 2 study. J. Thorac. Oncol. 16, 1959–1963. https://doi.org/10.1016/j.jtho.2021.06.023 (2021).
Jonna, S. et al. Detection of NRG1 gene fusions in solid tumors. Clin. Cancer Res. 25, 4966–4972. https://doi.org/10.1158/1078-0432.Ccr-19-0160 (2019).
Drilon, A. et al. Clinicopathologic features and response to therapy of NRG1 fusion-driven lung cancers: The eNRGy1 Global Multicenter Registry. J. Clin. Oncol. 39, 2791–2802. https://doi.org/10.1200/jco.20.03307 (2021).
Gan, H. K. et al. A phase I, first-in-human study of GSK2849330, an anti-HER3 monoclonal antibody, in HER3-expressing solid tumors. Oncologist 26, e1844–e1853. https://doi.org/10.1002/onco.13860 (2021).
Schram, A. M. et al. Efficacy and safety of zenocutuzumab, a HER2 x HER3 bispecific antibody, across advanced NRG1 fusion (NRG1+) cancers. J. Clin. Oncol. 40, 105–105. https://doi.org/10.1200/JCO.2022.40.16_suppl.105 (2022).
Carrizosa, D. R. et al. CRESTONE: Initial efficacy and safety of seribantumab in solid tumors harboring NRG1 fusions. J. Clin. Oncol. 40, 3006–3006. https://doi.org/10.1200/JCO.2022.40.16_suppl.3006 (2022).
Funding
This research was funded by Sichuan University’s “From 0 to 1” Innovation Research Project (2022SCUH0032); Chongqing Science and Health Joint Medical Research Project (2021MSXM318).
Author information
Authors and Affiliations
Contributions
Qitao Gou: Conceptualization, Methodology, Writing—Original Draft and Review & Editing, Funding acquisition. Qiheng Gou: Supervision, Writing—Original Draft and Review & Editing, Funding acquisition. Xiaochuan Gan: Supervision. Yuxin xie: Supervision, Conceptualization.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gou, Q., Gou, Q., Gan, X. et al. Novel therapeutic strategies for rare mutations in non-small cell lung cancer. Sci Rep 14, 10317 (2024). https://doi.org/10.1038/s41598-024-61087-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-61087-2
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.