Abstract
Coumarins are heterocycles of great interest in the development of valuable active structures in chemistry and biological domains. The ability of coumarins to inhibit biofilm formation of Gram positive bacterium (Staphylococcus aureus), Gram negative bacterium (Escherichia coli) as well as the methicillin-resistant S. aureus (MRSA) has been previously described. In the present work, new hybrid coumarin-heterocycles have been synthesized via the reaction of coumarin-6-sulfonyl chloride and 6-aminocoumarin with different small heterocycle moieties. The biological efficacy of the new compounds was evaluated towards their ability to inhibit biofilm formation and their anti-inflammatory properties. The antimicrobial activities of the newly synthesized compounds were tested against Gram positive bacterium (S. aureus ATCC 6538), Gram negative bacterium (E. coli ATCC 25922), yeast (Candida albicans ATCC 10231) and the fungus (Aspergillus niger NRRL-A326). Compounds 4d, 4e, 4f, 6a and 9 showed significant MIC and MBC values against S. aureus, E. coli, C. albicans, and methicillin-resistant S. aureus (MRSA) with especial incidence on compound 9 which surpasses all the other compounds giving MIC and MBC values of (4.88 and 9.76 µg/mL for S. aureus), (78.13 and 312.5 µg/mL for E. coli), (9.77 and 78.13 µg/mL for C. albicans), and (39.06 and 76.7 µg/mL for MRSA), respectively. With reference to the antibiofilm activity, compound 9 exhibited potent antibiofilm activity with IC50 of 60, 133.32, and 19.67 µg/mL against S. aureus, E. coli, and MRSA, (respectively) considering the reference drug (neomycin). Out of all studied compounds, the anti-inflammatory results indicated that compound 4d effectively inhibited nitric oxide production in lipopolysaccharide-(LPS-) stimulated RAW264.7 macrophage cells, giving NO% inhibition of 70% compared to Sulindac (55.2%)
Similar content being viewed by others
Introduction
Coumarins (2H-1-benzopyran-2-ones) are an elite class of compounds present in various natural products, and they have wide applications including antiviral, antimicrobial, anti-inflammatory, and other bioactivities1,2. The incorporation of another heterocyclic moiety into coumarin enriches the properties of the parent structure and the resulting compounds may exhibit promising properties. Many examples of biologically active coumarins containing heterocycles-fused were cited in the literature including antimicrobial3,4,5, antiviral6,7,8, anticancer9, antioxidant, and anti-inflammatory activities2,10,11,12. On the other hand, the carbon–sulfur bond formation plays an important role in organic synthesis13,14. Remarkably, coumarin-coupled sulfonamide15, sulfonate16, sulfonohydrazide17 is an important structural motif that is a substantial template of an emerging class of therapeutic agents.
Biofilm inhibition is recognized as a novel drug target for the broad-spectrum anti-infective strategy to combat the infections caused by drug-resistant bacterial pathogens18. Some coumarins are approved to exhibits broad-spectrum antibiofilm activity against Gram-negative bacteria19,20,21,22,23. Coumarin derivatives has been reported to inhibit the biofilm formation of Staphylococcus aureus24,25, Escherichia coli26 and Chromobacterium violaceum27.
There is an increasing body of evidence suggests that anti-inflammatory drugs can exert some antimicrobial and anti-biofilm activities against clinically relevant pathogenic bacteria like S. aureus, E. coli, and MRSA28. In this respect, in vitro studies showed that NSAIDs such as diclofenac and ibuprofen have ensured that they have anti-biofilm activity in concentrations similar to those found in human pharmacokinetic studies. The mechanisms of anti-bacterial and anti-biofilm actions of NSAIDs differ according to the microbial species28. Based on the facts stated above, and in keeping with our ongoing search for new bioactive substances, a new series of coumarin-6-heterocyclics inspired by the adaptability of the coumarin moiety were synthesized and assessed for their antimicrobial activity against a variety of bacterial strains. The most potent substances were then tested for their ability to inhibit biofilm formation as well as evaluate their anti-inflammatory effects.
Results and discussion
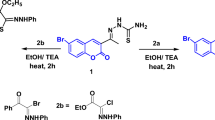
In light of our aim to synthesize new active derivatives of coumarin-based sulfonamides, sulfonohydrazide, sulfonate, sulfothioate, and formimidate, we reported a simple and appropriate coupling reaction starting with coumarin-6-sulfonyl chloride (2) and 6-aminocoumarin (3) as shown in Fig. 1. The starting coumarin-6-sulfonyl chloride (2) was synthesized via the chlorosulfonation of coumarin (1) with chlorosulfonic acid under heating (130−45 °C)29. Whereas, 6-aminocoumarin was obtained via the reduction of 6-nitrocoumarin using stannous chloride in the presence of tin granule30.
Synthetic pathway of new coumarin derivatives. Reagents and conditions: (a) HSO3Cl, 130−45 °C, 3 h; (b) i: HNO3, conc. H2SO4, 0–5 °C, 2 h, and then stirring at r.t., overnight; ii: stannous granules, TnCl2, conc. HCl, EtOH; 0–5 °C, 2 h, and then stirring at r.t., overnight; (c) EtOH, reflux, 5–30 min; (d) acetone, K2CO3, stirring, 70–80 °C; (e) dimethylformamide-dimethylacetal (DMF/DMA), dry xylene, reflux, 3 h; (f) EtOH, AcOH, reflux, 2 h.
The coupling reaction of compound (2) with various amino-heterocyclic namely, 2-aminobenzimidazole, 6-aminocoumarin, ethyl 5-amino-1-phenyl-1H-pyrazole-4-carboxylate, 3-(2-aminothiazol-4-yl)-2H-chromen-2-one31, 3-(2-aminothiazol-4-yl)-6-bromo-2H-chromen-2-one32, and 4-amino-N-(quinoxalin-2-yl)benzenesulfonamide in absolute ethanol under reflux led to the formation of the corresponding sulfonamides 4a-f (Fig. 1). On the basis of elemental analyses and NMR spectral data, the chemical structures of the newly synthesized sulfonamides 4a-f have been achieved. The 1H NMR spectra of 4a-f showed the presence of additional aromatic protons besides the aromatic proton at ≈ δ 6.50 ppm that attributed to H-2 of the coumarin moiety. Their 13C NMR spectra also demonstrated the presence of the aliphatic peaks besides the aromatic carbons in their regions. For instance, the 1H NMR (DMSO-d6) spectrum of 4d showed one singlet signal at δ 8.48 endorsing the presence of H-5 of thiazole moiety, besides a singlet signal at δ 7.51 ppm that back to NH proton. Also, the 1H NMR spectrum of 4d showed two singlet signals at δ 6.45 and 6.47 ppm attributed to the presence of (two protons) at position-2 of two coumarin moieties, in addition to the aromatic protons located on their regions (Fig. s7). Its 13C NMR spectrum (DMSO-d6) demonstrated signals at δ 169.8 ppm (C-2, thiazole moiety), 160.4 (2 C=O), 158.7, 154.0, 153.0, 144.8, 144.5, 140.3, 133.2, 129.8, 129.4, 126.0, 125.6, 118.9, 118.4, 116.9, 116.5, 116.4, 109.1 ppm (C-Ar) (Fig. s8).
The building up of new coumarin-sulfonohydrazide derivatives was the next step of our work. The reaction of 2 with the prepared benzohydrazide33 and 6-bromo-3-(2-hydrazinylthiazol-4-yl)-2H-chromen-2-one34 in absolute ethanol under reflux gave the corresponding 2-oxo-2H-chromene-6-sulfonohydrazides 5a and 5b (Fig. 1).
The 1H NMR spectra were utilized to confirm the formation of the newly coumarin derivatives 5a,b. For example, the 1H NMR (DMSO-d6) spectrum of 5a displayed two singlet signals at δ 10.72, 10.22 ppm supporting the presence of two protons of NH, besides a doublet signal at δ 6.59 (J = 8.3 Hz) authorized the presence of H-2 of coumarin (Fig. s12).
On the other hand, compound 2 demonstrated its adaptability to react with more functional groups instead of the amino group via the reaction with the hydroxyl group of 7-hydroxycoumarin and 2,6-diaminopyrimidin-4-ol, besides thiol group of N-(5-mercapto-1,3,4-thiadiazol-2-yl)acetamide to afford coumarin-sulfonates (6a,b) and coumarin-sulfothioate (7), respectively (Fig. 1). The 1H NMR (DMSO-d6) spectrum of 6a revealed one singlet signal at δ 8.74 which back to H-5 of the coumarin moiety, besides a doublet signal at δ 6.63 (J = 7.8 Hz) attributed to two CH protons of coumarin moieties at positions-2. The rest of the aromatic protons were located on their regions at δ 8.43 (d, J = 8.7 Hz, 2H), 8.17 (d, J = 9.3 Hz, 1H), 8.00 (d, J = 8.4 Hz, 1H), 7.75 (d, J = 8.4 Hz, 1H), 7.56 (d, J = 9.5 Hz, 2H), respectively (Fig. s13). Its 13C NMR (DMSO-d6) spectrum demonstrated signals at δ 159.4, 157.18 ppm supporting the presence of two C=O group, besides the remained aromatic carbon at δ 151.3, 151.2, 143.7, 137.1, 131.8, 130.2, 129.5, 128.3, 127.0, 123.3, 122.9, 118.4, 118.1 ppm (Fig. s14). On top of that, the 1H NMR (DMSO-d6) spectrum of compound 7 showed two singlet signals at δ 11.52 and 2.39 ppm endorsed the presence of (1H, NH) and (3H, CH3 of the acetyl protons), respectively, in addition to the aromatic protons which located on their regions at δ 8.53 (s, 1H), 7.82−7.66 (m, 1H), 7.58 (s, 1H), 6.54 (d, J = 8.6 Hz, 1H), 6.05 (s, 1H) (Fig. s16).
To increase the diversity of heterocyclic rings hybridized with coumarin, 6-aminocoumarin (3) was heated at reflux with N,N-dimethylformamide dimethyl acetal (DMF/DMA) to afford the corresponding enaminone (8) (Fig. 1). The acid catalytic reaction of compound 8 with 7-hydroxy coumarin in ethanol under reflux led to the formation of 2-oxo-2H-chromen-7-yl (E)-N-(2-oxo-2H-chromen-6-yl) formimidate (9) (Fig. 1). The NMR spectral data indicate the formation of compound 9. The 1H NMR (CDCl3) spectrum of 9 showed one singlet signal at δ 8.71 ppm endorsed the presence of the anil proton (CH=N), besides two doublet signals at δ 6.74−6.69 (m, 1H), 6.40−6.34 (m, 1H) attributed of two (CH) of two coumarin moieties at position-2 (Fig. s18). Its 13C NMR (CDCl3) spectrum demonstrated signals at δ 176.7, 161.4 ppm of two C=O groups, in addition to one signal at δ 152.3 ppm of CH=N. Additionally, it revealed aromatic carbons at δ 147.9, 143.4, 138.4, 136.2, 127.8, 121.8, 119.4, 119.7, 117.9, 117.5, 116.8, 111.7, 110.2 ppm (Fig. s19).
Biological evaluation
Antimicrobial activity
Using the agar well diffusion assay, the newly synthesized coumarin derivatives, were estimated for their antimicrobial activity towards S. aureus (ATCC 6538), E. coli (ATCC 25933), C. albicans (ATCC 1023) besides A. niger (NRRL-A326)35. It has been found that the newly synthesized coumarin derivatives exhibited diverse activities in relation to the test microbe (Table 1, Fig. s1). Compounds 4f and 9 had considerable antimicrobial activities against all test microbes with inhibition values of 16 and 16 mm against S. aureus, 15 and 9 mm against E. coli, 18 and 17 mm against C. albicans, and 15 and 19 mm against A. niger. It has been also found that compounds 4d, 4e, 6a, and 7 had moderate activities against S. aureus with inhibition values of 13, 10, 14, and 12 mm (respectively), whereas the other compounds exhibited low or no activities against the same test microbe. For E. coli, compounds 4b, 5b and 6b showed low activities with inhibition values of 7, 8 and 7 mm (respectively) and the other compounds showed negative results. On the other hand, compound 4d had high activity with C. albicans (18 mm) but compounds 4e and 6a exhibited moderate activities (10 and 14 mm, respectively) whereas the other compounds showed low or no activities. For A. niger, compounds 4b, 4c, 4d, 4e, 5a, 6a, and 6b had moderate activities (12, 13, 13, 12, 12, 14, and 12 mm, respectively), whereas the other compounds had low activities.
Further works including minimum inhibition concentration (MIC) and minimum bactericidal concentration (MBC) had been done for the compounds that had comparatively high antimicrobial activates, 4d, 4e, 4f, 6a and 9 (Table 2, Fig. s2)35,36.
The data from Table 2 showed that, compound 9 exhibited the lowest MIC and MBC values for all test microbes with (4.88 and 9.76 µg/mL for S. aureus), (78.13 and 312.5 µg/mL for E. coli), (9.77 and 78.13 µg/mL for C. albicans), and (39.06 and 76.7 µg/mL for MRSA). Additionally, compounds 4f, 6a, 4d, and 4e showed remarkable promising MIC values 9.77, 9.77, 19.53, and 39.06 µg/mL (respectively) and MBC values 39.06, 78.13, 78.13, and 39.06 mL (respectively) against S. aureus. They also showed higher MIC and MBC values against the remained tested microbes. With respect to neomycin, compound 9 was about 4-fold more potent against S. aureus, followed by compound 4f and 6a (about 2-fold more potent). For E. coli compound 9 was less active than neomycin (2-fold lower). In case of C. albicans, both compounds 9 and 4f were more potent than neomycin (4-fold and 2-fold, respectively, while compound 4e was equipotent with neomycin. Out of the selected five compounds MIC study against MRSA, compound 9 was equipotent with neomycin.
Considering the structure–activity relationship after antimicrobial analysis, compounds 4f, 6a, and 9 showed the antimicrobial activity of the sulfonamide -containing coumarin and the presence of di-coumarin37. Interestingly, active compound 9 may confirm that the presence of two coumarin rings has a high antimicrobial effect against most of the microorganisms studied, consistent with what was previously reported37.
Inhibition of biofilm formation
Microorganisms that can produce biofilms are known to be one of the major factors contributing to antibiotic resistance. Therefore, many experiments have been conducted to overcome these serious problems by searching for new drugs that can prevent biofilm formation38. S. aureus is one of the most frequent causes of biofilm-associated clinical infections. The increasing emergence of methicillin-resistant S. aureus (MRSA), antibiotic resistance, and biofilm-forming capacity contribute to S. aureus being the most commonly identified pathogen in both healthcare and community settings. Additionally, the ability to acquire novel antibiotic resistance mechanisms makes MRSA a major global health threat24,39,40.
Coumarin and its derivatives have attracted the attention of many microbiologists due to their antimicrobial effectiveness41,42. There are also further studies on the effectiveness of coumarin as an inhibitor of biofilm formation20,43. A recent study has proven that 3-hyrdroxy-coumarin, a marine bacterium-derived compound, showed antibiofilm formation44. So, the inhibition of biofilm formation was performed for the five most active compounds 4d, 4e, 4f, 6a, and 9 using neomycin as a reference control compound. Table 3 and Fig. s3 explained the ability of the most active compounds with potent antibiofilm formation expressed as IC50 values. It was found that, compound 9 exhibited the best antibiofilm activities against S. aureus, E. coli, and MRSA with IC50 values of 60, 133.32, and 19.67 µg/mL, respectively in comparison to neomycin (IC50 = 19.67, 79.289, and 39.34 µg/mL, respectively). For the other compounds, it has been reported that compounds 4d and 6a had considerably acceptable results with S. aureus (IC50 of 185.51 and 355.52 µg/mL, respectively). The other compounds had appreciable IC50 values against the same test microbe. For E. coli, compounds 4d and 4e showed noticeable IC50 values 321.25 and 345.40 µg/mL (respectively). All the other compounds (4d, 4e, 4f and 6a) when tested as antibiofilm formation by MRSA, compound 4e and 6a showed promising results (IC50: 85.02 and 40.73 µg/mL, respectively) in respect to neomycin. Out of the five selected compounds for inhibition of biofilm formation, compound 9 was the potent one and showing antibiofilm activity against MRSA (about twofold more potent) and about fivefold and threefold lower against S. aureus, E. coli, respectively.
Effect of compounds on nitric oxide levels in LPS-stimulated RAW 264.7 macrophages
Coumarins represent an important family of oxygen-containing heterocycles, widely distributed in nature45,46. Coumarin and its derivatives exhibited a broad range of biological and pharmacological activities47. A previous study indicated that imperatorin (a coumarin derivative) has an anti-inflammatory effect in lipopolysaccharide-stimulated mouse macrophages (RAW264.7) in an in-vitro model of edema, as it inhibits the protein expression of nitric oxide synthase (NOS) and a cyclooxygenase-2 (COX-2)48. The effect of the active compounds (4d, 4f, 6a, and 9) on levels of Nitric Oxide (Fig. 2A) in LPS-stimulated RAW 264.7 cells was investigated according to the method of Elshahid et al.49. All the cells were treated with the studied compounds along with LPS or LPS alone for 24 h. To determine the level of NO production, the released of nitrite into the culture medium was measured using Griess reagent. As a result, LPS alone markedly induced NO production compared with that generated by the control. However, pretreatment with the studied compounds affected NO levels that significantly produced in LPS-stimulated RAW 264.7 cells as shown in Fig. 2A. Moreover, compounds (4d) induce marked inhibition on NO production by (70%) as compared to LPS (Table 4).
(A) Effects of the studied compounds on the production of nitric oxide (NO) in LPS-stimulated RAW264.7 macrophages. Cells were treated with the studied compounds at concentration 100 µg/mL plus LPS (1 μg/mL) or LPS alone for 24 h. Sulindac (NSAID) was used as a positive control. (B) Cytotoxic effect of the studied compounds and Sulindac on Raw-264.7 macrophages at concentrations (100 μg/mL). Values are expressed as the means ± SD (n = 3) p < 0.0001 (versus LPS alone, 2A).
In a parallel experiment, to examine the cytotoxicity of the studied compounds on RAW 264.7 cells, the cells were treated with each compound for 24 h in the presence or absence of LPS, and the cytotoxic potential was measured by the MTT assay50.
The results showed that compound (4d) was the least cytotoxic compound (≈ 20% cytotoxicity) indicating high cell viability. Meanwhile, compound (4f) and Sulindac (positive control) showed higher cytotoxic effect as indicated by the MTT reduction assay (Fig. 2B). These results clearly indicate that the anti-inflammatory activity of 4d in LPS-stimulated RAW 264.7 macrophages was not due to direct cell death. Accumulating evidence indicates that NO is a critical mediators of inflammation51,52. NO plays a pivotal role in many body functions; however, its overproduction, particularly in macrophages, can lead to cytotoxicity, inflammation, and autoimmune disorders51,52. Our data are in agreement with several in-vitro studies performed with LPS-stimulated RAW264.7 cells, which showed that coumarin and its derivatives have shown a therapeutic effect against edema, eliminating proteins and fluid from injured tissue by activating mechanisms such as phagocytosis, enzyme release, and proteolysis48,53,54.
Experimental part
Chemistry
All reagents and solvents were of commercial grade. Coumarin (Sigma-Aldrich Chemie GmeH, Taufkirchen, Germany). Melting points of the synthesized coumarins were measured on the digital melting point apparatus (Electro thermal 9100, Electro thermal Engineering Ltd., serial No. 8694, Rochford, United Kingdom) and are uncorrected. A Bruker Avance spectrometer (Bruker, Germany) was used to measure the 1H and 13C NMR spectra of new coumarins at 500 and 125 MHz, respectively. Elemental analyses were carried out on a Perkin–Elmer 2400 analyzer (USA) and were found within ± 0.4% of the theoretical values. TMS was used as the internal standard and hydrogen coupling patterns are described as (s) singlet, (d) doublet, (t) triplet, (q) quartet and (m) multiple. Chemical shifts were defined as parts per million (ppm) relative to the solvent peak.
General Procedure for the preparation of coumarin sulfonamide derivatives 4a-f. An equal proportion of coumarin-6-sulfonyl chloride and applicable amino-compounds (10 mmol) in absolute ethanol (10 mL) was refluxed under stirring for 5–30 min. The precipitate formed on hot was collected by filtration and recrystallized from the proper solvent.
N-(1H-benzo[d]imidazol-2-yl)-2-oxo-2H-chromene-6-sulfonamide (4a). Recrystallized from ethanol\DMF as colorless crystals; MP. 295−7 °C; yield: 0.19 g, 55%; 1H NMR (500 MHz, DMSO-d6) δ 12.48 (s, 1H), 8.45 (s, 1H), 8.08 (t, J = 7.5 Hz, 1H), 7.95 (s, 1H), 7.77 (d, J = 8.3 Hz, 1H), 7.45−7.06 (m, 4H), 6.43 (t, J = 8.3 Hz, 1H), 6.14 (s, 1H). Analysis Calc. for C16H11N3O4S (341.34): C, 56.30; H, 3.25; N, 12.31; S, 9.39; Found: C, 56.41; H, 3.33; N, 12.44; S, 9.44.
2-Oxo-N-(2-oxo-2H-chromen-6-yl)-2H-chromene-6-sulfonamide (4b). Recrystallized from ethanol-DMF (5:1) as faint brown crystals; MP. 203−5 °C; yield: 0.30 g, 80%; 1H NMR (500 MHz, DMSO- d6) δ 10.56 (s, 1H), 8.12 (ddd, J = 12.1, 15.3, 7.3 Hz, 1H), 8.06−7.91 (m, 1H), 7.90−7.83 (m, 1H), 7.76 (dd, J = 11.0, 8.2 Hz, 1H), 7.59 (d, J = 10.9 Hz, 1H), 7.50 (dt, J = 10.2, 11.7 Hz, 1H), 7.41 (t, J = 9.7 Hz, 1H), 7.35−7.18 (m, 1H), 6.56 (td, J = 9.8, 6.1 Hz, 1H), 6.46 (ddd, J = 10.7, 9.4, 5.9 Hz, 1H). Analysis Calc. for C18H11NO6S (369.35): C, 58.54; H, 3.00; N, 3.79; S, 8.68; Found: C, 58.45; H, 2.98; N, 3.85; S, 8.73.
Ethyl 5-((2-oxo-2H-chromene)-6-sulfonamido)-1-phenyl-1H-pyrazole-4-carboxylate (4c). Recrystallized from ethanol-DMF (5:1) as colorless crystals; MP. 220−2 °C; yield: 0.29 g, 65%; 1H NMR (500 MHz, DMSO- d6) δ 9.92 (dd, J = 11.7, 9.9 Hz, 1H), 9.61 (s, 1H), 8.28−8.16 (m, 1H), 8.09 (d, J = 12.1 Hz, 1H), 7.95 (dd, J = 2.4, 2.8 Hz, 1H), 7.85 (s, 1H), 7.82−7.71 (m, 1H), 7.68−7.28 (m, 4H), 6.55 (t, J = 8.0 Hz, 1H), 3.34 (q, J = 7.8.0 Hz, 2H), 1.01 (t, J = 6.7 Hz, 3H). Analysis Calc. for C21H17N3O6S (439.44): C, 57.40; H, 3.90; N, 9.56; S, 7.30; Found: C, 57.32; H, 3.98; N, 9.66; S, 7.22.
2-Oxo-N-(2-(2-oxo-2H-chromen-3-yl)thiazol-4-yl)-2H-chromene-6-sulfonamide (4d). Recrystallized from ethanol-DMF (5:1) as green crystals; MP. 260−2 °C; yield: 0.41 g, 90%; 1H NMR (500 MHz, DMSO- d6) δ 8.48 (s, 1H), 8.09 (d, J = 9.5 Hz, 1H), 7.95 (s, 1H), 7.78 (d, J = 9.0 Hz, 1H), 7.73 (d, J = 7.6 Hz, 1H), 7.62 (t, J = 7.7 Hz, 1H), 7.51 (s, 1H), 7.42 (d, J = 8.3 Hz, 1H), 7.37 (t, J = 7.5 Hz, 1H), 7.32 (d, J = 8.5 Hz, 1H), 6.46 (2 s, 2H); 13C NMR (126 MHz, DMSO- d6) δ 169.8, 160.4, 158.7, 154.0, 153.0, 144.8, 144.5, 140.3, 133.2, 129.8, 129.4, 126.0, 125.6, 118.9, 118.4, 116.9, 116.5, 116.4, 109.1. Analysis Calc. for C21H12N2O6S2 (452.46): C, 55.75; H, 2.67; N, 6.19; S, 14.17; Found: C, 55.69; H, 2.77; N, 6.25; S, 14.22.
N-(2-(6-Bromo-2-oxo-2H-chromen-3-yl)thiazol-4-yl)-2-oxo-2H-chromene-6-sulfonamide (4e). Recrystallized from ethanol-DMF (5:1) as crystals; MP. 250−2 °C; yield: 0.43 g, 80%; 1H NMR (500 MHz, DMSO- d6) δ 8.39 (s, 1H), 8.12 (d, J = 9.6 Hz, 1H), 8.04 (d, J = 2.0 Hz, 2H), 7.95 (d, J = 1.6 Hz, 1H), 7.77 (dd, J = 8.5, 1.6 Hz, 1H), 7.70 (dd, J = 8.7, 2.1 Hz, 1H), 7.50 (s, 1H), 7.37 (t, J = 10.7 Hz, 1H), 7.31 (d, J = 8.5 Hz, 1H), 6.51 (d, J = 9.5 Hz, 1H). Analysis Calc. for C21H11BrN2O6S2 (531.35): C, 47.47; H, 2.09; Br, 15.04; N, 5.27; S, 12.07; Found: C, 47.51; H, 2.21; Br, 14.99; N, 5.33; S, 11.98.
2-Oxo-N-(4-(N-(quinoxalin-2-yl)sulfamoyl)phenyl)-2H-chromene-6-sulfonamide (4f) Recrystallized from ethanol-DMF (5:1) as colorless crystals; MP. 203−5 °C; yield: 0.28 g, 55%; 1H NMR (500 MHz, DMSO- d6) δ 12.84 (s, 2H), 7.92 (d, J = 8 Hz, 7H), 7.63 (s, 2H), 7.27 (s, 2H), 6.78 (d, J = 9.6 Hz, 3H). 13C NMR (126 MHz, DMSO- d6) δ 169.5, 167.1, 141.9, 135.5, 135.3, 131.9, 127.9, 127.0, 125.1, 124.1, 109.0; Analysis Calc. for C23H16N4O6S2 (508.05): C, 54.32; H, 3.17; N, 11.02; S, 12.61; Found: C, 54.35; H, 3.22; N, 12.43; S, 12.51.
General Procedure for the preparation of coumarin sulfonohydrazide derivatives 5a and 5b. These compounds were prepared as described for 4 from 2 (10 mmol) and benzohydrazide or 6-bromo-3-(2-hydrazinylthiazol-4-yl)-2H-chromen-2-one (10 mmol).
N'-benzoyl-2-oxo-2H-chromene-6-sulfonohydrazide (5a). Recrystallized from ethanol-DMF (5:1) as colorless crystals; MP. 235−7 °C; yield: 0.17 g, 50%; 1H NMR (500 MHz, DMSO- d6) δ 10.72 (s, 1H), 10.22 (s, 1H), 8.29−8.13 (m, 2H), 7.91 (dd, J = 9.4, 11.1 Hz, 2H), 7.65 (s, 2H), 7.49 (d, J = 8.8 Hz, 2H), 7.39 (s, 1H), 6.59 (d, J = 8.3 Hz, 1H). Analysis Calc. for C16H12N2O5S (344.34): C, 55.81; H, 3.51; N, 8.14; S, 9.31; Found: C, 55.78; H, 3.65; N, 8.22; S, 9.46.
2-Oxo-N'-(2-(2-oxo-2H-chromen-3-yl)thiazol-4-yl)-2H-chromene-6-sulfonohydrazide (5b). Recrystallized from ethanol-DMF (5:1) as brown crystals; MP. 250−2 °C; yield: 0.42 g, 90%; 1H NMR (500 MHz, DMSO- d6) δ 9.68 (s, 1H), 9.59 (s, 1H), 8.31 (d, J = 8.5 Hz, 2H), 8.19−8.05 (m, 2H), 7.98−7.88 (m, 2H), 7.73 (t, J = 8.8 Hz, 2H), 7.54−7.20 (m, 2H), 6.47 (d, J = 9.5 Hz, 1H). Analysis Calc. for C21H12BrN3O6S2 (546.37): C, 46.16; H, 2.21; Br, 14.62; N, 7.69; S, 11.74. Found: C, 46.10; H, 2.30; Br, 14.72; N, 7.80; S, 11.82.
Synthesis of 2-oxo-2H-chromen-7-yl 2-oxo-2H-chromene-6-sulfonate (6a). This compound was prepared as described for 4a from 2 (10 mmol) and 7-hydroxy coumarin (10 mmol). The product (6a) recrystallized from ethanol-DMF (5:1) as yellow crystals; MP. 259−61 °C; yield: 0.22 g, 60%; 1H NMR (500 MHz, DMSO- d6) δ 8.74 (s, 1H), 8.43 (d, J = 8.7 Hz, 2H), 8.17 (d, J = 9.3 Hz, 1H), 8.00 (d, J = 8.4 Hz, 1H), 7.75 (d, J = 8.4 Hz, 1H), 7.56 (d, J = 9.5 Hz, 2H), 6.63 (d, J = 7.8 Hz, 2H); 13C NMR (126 MHz, DMSO- d6) δ 159.4, 157.18, . Analysis Calc. for C18H10O7S (370.33): C, 58.38; H, 2.72; S, 8.66; Found: C, 58.42; H, 2.65; S, 8.74.
2,6-Diaminopyrimidin-4-yl 2-oxo-2H-chromene -6-sulfonate (6b). To a solution of 2,6-diaminopyrimidin-4-ol (10 mmol) in acetone containing (10 mmol) of K2CO3 was added compound 2 (10 mmol). The reaction mixture was stirring under heat (70–80 °C) for 4 h. The crude product was obtained upon filtration. Recrystallized from ethanol\DMF (5:1) as yellow crystals; MP. 255−7 °C; yield: 0.18 g, 55%; 1H NMR (500 MHz, DMSO- d6) δ 8.17 (d, J = 9.5 Hz, 1H), 7.78 (d, J = 8.1 Hz, 1H), 7.43 (s, 2H), 7.31 (t, J = 7.7 Hz, 1H), 6.48 (d, J = 9.5 Hz, 1H), 4.94 (s, 1H), 2.46 (s, 2H). Analysis Calc. for C13H10N4O5S (334.31): C, 46.71; H, 3.02; N, 16.76; S, 9.59; Found: C, 46.76; H, 3.12; N, 16.89; S, 9.60.
Synthesis of S-(5-acetamido-1,3,4-thiadiazol-2-yl) 2-oxo-2H-chromene-6-sulfonothioate (7). This compound was prepared as described for 4a from 2 (10 mmol) and N-(5-mercapto-1,3,4-thiadiazol-2-yl)acetamide (10 mmol). The product recrystallized from ethanol-DMF (5:1) as crystals; MP. 260−2 °C; yield: 0.27 g, 70%; 1H NMR (500 MHz, DMSO-d6) δ 11.52 (s, 1H), 8.53 (s, 1H), 7.82−7.66 (m, 1H), 7.58 (s, 1H), 6.54 (d, J = 8.6 Hz, 1H), 6.05 (s, 1H), 2.39 (s, 3H). Analysis Calc. for C13H9N3O5S3 (383.41): C, 40.72; H, 2.37; N, 10.96; S, 25.09; Found: C, 40.69; H, 2.40; N, 11.01; S, 24.98.
Synthesis of (E)-N,N-Dimethyl-N'-(2-oxo-2H-chromen-6-yl)formimidamide (8). N,N-Dimethylformamide dimethyl acetal (10 mmol) was added to 6-aminocoumarin (10 mmol) in xylene (2 mL). The reaction mixture was heated at reflux for (3 h) then left to cool. The brown crystals formed after cooling were collected by scratch. Recrystallized from acetone as honey crystals; MP. 180 °C; yield: 0.13 g, 60%; 1H NMR (500 MHz, CDCl3) δ 7.72−7.50 (m, 2H), 7.29−7.08 (m, 2H), 7.03 (s, 1H), 6.43−6.30 (m, 1H), 3.08 (d, J = 8.8 Hz, 6H). Analysis Calc. for C12H12N2O2 (216.24): C, 66.65; H, 5.59; N, 12.96; Found: C, 66.56; H, 5.64; N, 13.00.
Synthesis of 2-oxo-2H-chromen-7-yl (E)-N-(2-oxo-2H-chromen-6-yl) formimidate (9). Compound 8 (10 mmol) and 7-hydroxy coumarin (10 mmol) in absolute ethanol (10 mL) containing (0.5 mL) of glacial acetic acid was heated under reflux for 2 h. The crude product was collected and recrystallized from acetone as brown crystals; MP. 108−10 °C; yield: 0.2 g, 60%; 1H NMR (500 MHz, CDCl3) δ 8.71 (s, 1H), 8.17 (d, J = 9.3 Hz, 1H), 7.60−7.54 (m, 1H), 7.44 (tdd, J = 9.2, 5.8, 3.0 Hz, 1H), 7.36−7.30 (m, 1H), 7.27−7.24 (m, 1H), 7.20−7.16 (m, 1H), 7.13 (d, J = 8.8 Hz, 1H), 6.90−6.83 (m, 1H), 6.74−6.69 (m, 1H), 6.40−6.34 (m, 1H); 13C NMR (126 MHz, CDCl3) δ 176.7, 161.4, 152.3, 147.9, 143.4, 138.4, 136.2, 127.8, 121.8, 119.4, 119.7, 117.9, 117.5, 116.8, 111.7, 110.2; Analysis Calc. for C19H11NO5 (333.30): C, 68.47; H, 3.33; N, 4.20; Found: C, 68.32; H, 3.21; N, 4.44.
Biological assays
Antimicrobial assay
The antimicrobial activity of the synthesized compounds were assessed against Staphylococcus aureus ATCC 6538-P as Gram positive bacterium, Escherichia coli ATCC 25933 as Gram negative bacterium, Candida albicans ATCC 10231 as yeast as well as the filamentous fungal test microbe Aspergillus niger NRRL-A326 by the agar well diffusion method35. Bacterial and yeast test microbes were inoculated on nutrient agar medium plates seeded with 0.1 mL of 105–106 cells/mL whereas the fungal test strain was cultivated on plates having potato dextrose agar medium that seeded by 0.1 mL (106 cells/mL) of the fungal inoculum. 5 mg of each sample was dissolved in 2 mL of DMSO. 100 µl from each sample were distributed in holes developed in each inoculated plate. Then plates were kept at 4 °C for more than 2 h to allow extreme dispersion. The plates were then kept at 37 °C overnight for bacteria and yeast and kept at 30 °C for 2 days for the fungus in vertical location to permit maximum microbial growth. Neomycin was used as reference drug for Gram-positive and Gram-negative bacteria as well as yeast. Cyclohexamide was used as reference drug for fungi (A. niger). The clear zone diameters expressed in millimeter (mm) were used to differentiate the antimicrobial activity of tested compounds. The experiment was carried out twice and their mean were considered.
Evaluation of minimum inhibitory concentration (MIC) and Minimum bactericidal Methicillin Resistant S. aureus concentration (MBC)
MIC was performed using S. aureus ATCC 6538, Gram-positive bacterium, and E. coli ATCC 25922, Gram-negative bacterium, Candida albicans ATCC 10231 as yeast, and Methicillin Resistant S. aureus (MRSA) as tested microbes that are grown on a Mueller Hinton medium. Test microbes were cultivated in 100 mL bottles with each test at 35 °C for 24 h. Cells were obtained by centrifugation (4000 rpm) under a sterile condition at 4 °C for 15 min. The cells were washed using sterile saline until the supernatant was clear. Cells with an optical density of 0.5 to 1 (at 550 nm) giving an actual number of colony-forming units of 5 × 106 cfu/mL were obtained. Resazurin solution was prepared by dissolving 270 mg tablet in 40 mL of sterile distilled water. Then, 96-well sterile microplates were prepared. Then, 50μL of test material in DMSO was pipetted into the first row of the plate. To all other wells, 50μL of broth medium was added. Two-fold serial dilutions were performed. Then, 10μL of resazurin indicator solution was added, 10μL of bacterial suspension was added to each well. The plates were prepared in duplicate and placed in an incubator set at 37 °C for 18–24 h. Any colour changes from purple to pink or colourless were recorded as positive. The lowest concentration at which colour change occurred was taken as the MIC value. MBC has been performed by streaking of the two concentrations higher than MIC and the plates exhibiting no growth were considered as MBC35,36. Neomycine has been used as positive control55.
Inhibition of biofilm formation (crystal violet method)
Bacterial strains were incubated in test tubes with TSB (5 mL) containing 2% w/v glucose at 37 °C for 24 h. After that, the bacterial suspensions were diluted to achieve turbidity equivalent to a 0.5 McFarland standard. The diluted suspension (2.5μL) was added to each well of a single cell culture polystyrene sterile, flat-bottom 96-well plate filled with TSB (200μL) with 2% w/v glucose. Sub-MIC concentration values of compounds 4d, 4e, 4f, 6a, and 9 were directly added to the wells to reach concentrations ranging from 100 to 0.1 μM to assess BIC50 values that are, the concentration at which the percentage of inhibition of biofilm formation is equal to 50%. Plates were incubated at 37 °C for 24 h. After biofilm growth, the content of each well was removed, wells were washed twice with sterile NaCl 0.9% and stained with 200μL of 0.1% w/v crystal violet solution for 15 min at 37 °C. The excess solution was removed, and the plate was washed twice, using tap water. A volume of 200 μL of ethanol was added to each stained well to solubilize the dye35,38. Neomycine has been used as positive control55. Optical density (O.D.) was read at 600 nm using a microplate reader (GloMax®-Multi Detection System, Milan, Italy). The experiments were run at least in triplicates, and three independent experiments were performed. The percentage of inhibition was calculated through the formula:
Anti‑inflammatory assay
Cell culture (seeding and treatment)
The RAW 264.7 macrophage cell line were supplied from ATCC (American type culture collection).The cells were sub-cultured in Roswell Park Memorial Institute's RPMI 1640 medium49.
Procedure
The following procedures were all completed in a biosafety level II Laminar flow cabinet in a clean environment. RAW 264.7 cells were suspended at concentration of 1 × 105 cells per well (in 96 well plates). The cells were then incubated with the test compounds, LPS (lipopolysaccharide, negative control) and Sulindac (positive control drug) according to the method49. After 24 h, the supernatant was gently transferred to new 96-well plates for measuring nitric oxide (NO) while cells were used for cell viability testing using the MTT method. The percentage change in viability was calculated according to the below formula
Nitric oxide assay
The generation of nitric oxide (NO) was measured in the supernatants of cultivated RAW 264.7 cells. With slight modification, the Nitric Oxide (NO) measurement was carried out as described by Eid et al.50 using the Griess reagent. In detail, 50 μl of cell culture media were added to 50 μl of Griess reagent then incubated at room temperature for 15 min before being measured at 540 nm50. A sodium nitrite standard curve was used to calculate the amount of nitrite, as shown in equation:
Statistical analysis
All statistical analysis and IC50 values were calculated using the concentration–response curve fit to the non-linear regression model and One-way ANOVA with Dunnet’s posttest was performed using GraphPad Prism® v7.0 (GraphPad Software Inc., San Diego, CA, USA).
Conclusion
Coumarins are considered as brilliant groups of compounds existed in nature with diverse chemical skeleton and biological activities. This study focused on the synthesis of new coumarin-conjugated sulfonamides, sulfonohydrazide, sulfonate, sulfothioate, and formimidate. The new compounds were assessed as antimicrobial, antibiofilm, and anti-inflammatory activities. 2-oxo-2H-chromen-7-yl (E)-N-(2-oxo-2H-chromen-6-yl) formimidate (9) exhibited brilliant antimicrobial activity, as well as antibiofilm activity. On the other side, 2-oxo-N-(2-(2-oxo-2H-chromen-3-yl)thiazol-4-yl)-2H-chromene-6-sulfonamide (4f) effectively inhibited nitric oxide production in lipopolysaccharide- (LPS-) stimulated RAW264.7 macrophage cells and could be considered as anti-inflammatory agent. Noteworthy, it is not a requirement, that a compound that has a strong antimicrobial effect may play an anti-inflammatory role. This may be due to the role of the active groups in the two compounds.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Wu, Y., Xu, J., Liu, Y., Zeng, Y. & Wu, G. A review on anti-tumor mechanisms of coumarins. Front. Oncol. 10, 592853 (2020).
Kirsch, G., Abdelwahab, A. B. & Chaimbault, P. Natural and synthetic coumarins with effects on inflammation. Molecules 21, 1322 (2016).
Ranjan Sahoo, C. et al. Coumarin derivatives as promising antibacterial agent(s). Arab. J. Chem. 14, 102922 (2021).
Yang, G. et al. The synthesis of coumarin thiazoles containing a trifluoromethyl group and their antifungal activities. Arab. J. Chem. 14, 102880 (2021).
Singh, L. K., Priyanka, Singh, V. & Katiyar, D. Design, synthesis and biological evaluation of some new coumarin derivatives as potential antimicrobial agents. Med. Chem. 11, 128–134 (2015).
Hassan, M. Z., Osman, H., Ali, M. A. & Ahsan, M. J. Therapeutic potential of coumarins as antiviral agents. Eur. J. Med. Chem. 123, 236–255 (2016).
Mishra, S., Pandey, A. & Manvati, S. Coumarin: An emerging antiviral agent. Heliyon 6, e03217 (2020).
Abdelmohsen, U. R. et al. Natural coumarins as potential anti-SARS-CoV-2 agents supported by docking analysis. RSC Adv. 11, 16970–16979 (2021).
Ngoc Toan, V., Dinh Thanh, N. & Minh Tri, N. 1,3,4-Thiadiazoline−coumarin hybrid compounds containing D-glucose/D-galactose moieties: Synthesis and evaluation of their antiproliferative activity. Arab. J. Chem. 14, 103053 (2021).
Husain, A., Al Balushi, K., Akhtar, M. & Khan, S. Coumarin linked heterocyclic hybrids: A promising approach to develop multi target drugs for Alzheimer’s disease. J. Mol. Struct. 1241, 130618 (2021).
Kontogiorgis, C. A., Savvoglou, K. & Hadjipavlou-Litina, D. J. Antiinflammatory and antioxidant evaluation of novel coumarin derivatives. J. Enzyme Inhib. Med. Chem. 21, 21–29 (2006).
Chandak, N. et al. Dual evaluation of some novel 2-amino-substituted coumarinylthiazoles as anti-inflammatory-antimicrobial agents and their docking studies with COX-1/COX-2 active sites. J. Enzyme Inhib. Med. Chem. 29, 476–484 (2013).
Qiao, Z. & Jiang, X. Recent developments in sulfur-carbon bond formation reaction involving thiosulfates. Org. Biomol. Chem. 15, 1942–1946 (2017).
El-Sawy, E. R., Abdelwahab, A. B. & Kirsch, G. Insight on mercapto-coumarins: Synthesis and reactivity. Molecules 27, 2150 (2022).
Irfan, A. et al. Coumarin sulfonamide derivatives: An emerging class of therapeutic agents. Heterocycl. Commun. 26, 46–59 (2020).
Novaroli, L. et al. Human recombinant monoamine oxidase B as reliable and efficient enzyme source for inhibitor screening. Bioorg. Med. Chem. 13, 6212–6217 (2005).
Chethan Prathap, K. N. & Lokanath, N. K. Synthesis, characterization, crystal structure and quantum chemical investigations of three novel coumarin-benzenesulfonohydrazide derivatives. J. Mol. Struct. 1158, 26–38 (2018).
Donlan, R. M. Biofilm formation: A clinically relevant microbiological process. Clin. Infect. Dis. 33, 1387–1392 (2001).
Qais, F. A. et al. Coumarin exhibits broad-spectrum antibiofilm and antiquorum sensing activity against gram-negative bacteria: In vitro and in silico investigation. ACS Omega 6, 18823–18835 (2021).
Zhang, Y. et al. Coumarin reduces virulence and biofilm formation in Pseudomonas aeruginosa by affecting quorum sensing, type III secretion and C-di-GMP levels. Front. Microbiol. 9, 1952 (2018).
Thakur, S., Ray, S., Jhunjhunwala, S. & Nandi, D. Insights into coumarin-mediated inhibition of biofilm formation in Salmonella Typhimurium: Biofouling. Biofouling 36, 479–491 (2020).
He, Z. et al. Anti-biofilm activities of coumarin as quorum sensing inhibitor for Porphyromonas gingivalis. J. Oral Microbiol. 14, 2055523 (2022).
Zou, J. et al. An in vitro coumarin-antibiotic combination treatment of Pseudomonas aeruginosa biofilms. Nat. Prod. Commun. 16, 1934578X2098774 (2021).
Qu, D. et al. A new coumarin compound DCH combats methicillin-resistant Staphylococcus aureus biofilm by targeting arginine repressor. Sci. Adv. 6, eaay9597 (2020).
Das, T. et al. Modulation of S. aureus and P. aeruginosa biofilm: An in vitro study with new coumarin derivatives. World J. Microbiol. Biotechnol. 34, 170 (2018).
Lee, J.-H. et al. Coumarins reduce biofilm formation and the virulence of Escherichia coli O157:H7. Phytomedicine 21, 1037–1042 (2014).
D’Almeida, R. E. et al. Comparison of seven structurally related coumarins on the inhibition of Quorum sensing of Pseudomonas aeruginosa and Chromobacterium violaceum. Bioorg. Chem. 73, 37–42 (2017).
Paes Leme, R. C. & da Silva, R. B. Antimicrobial activity of non-steroidal anti-inflammatory drugs on biofilm: Current evidence and potential for drug repurposing. Front. Microbiol. 12, 707629 (2021).
Sabt, A. et al. Novel coumarin-6-sulfonamides as apoptotic anti-proliferative agents: synthesis, in vitro biological evaluation, and QSAR studies. J. Enzyme Inhib. Med. Chem. 33, 1095–1107 (2018).
El-Sawy, E. et al. Synthesis and molecular docking of novel non-cytotoxic anti-angiogenic sulfonyl coumarin derivatives against hepatocellular carcinoma cells in vitro. J. Appl. Pharm. Sci. 7, 049–066 (2017).
Godugu, K. et al. Solid state thiazole-based fluorophores: Promising materials for white organic light emitting devices. Dyes Pigments 187, 109077 (2021).
Pardo-Jiménez, V., Navarrete-Encina, P. & Díaz-Araya, G. Synthesis and biological evaluation of novel thiazolyl-coumarin derivatives as potent histone deacetylase inhibitors with antifibrotic activity. Molecules 24, 739 (2019).
Hashemzadeh, T. et al. Luminescent iridium(III)–boronic acid complexes for carbohydrate sensing. Dalton Trans. 49, 11361–11374 (2020).
Abdel-Aziem, A., Baaiu, B., Elbazzar, A. & Elabbar, F. A facile synthesis of some novel thiazoles, arylazothiazoles, and pyrazole linked to thiazolyl coumarin as antibacterial agents. Synth. Commun. 50, 2522–2530 (2020).
Abo-Salem, H. M., Abd El Salam, H. A., Abdel-Aziem, A., Abdel-Aziz, M. S. & El-Sawy, E. R. Synthesis, molecular docking, and biofilm formation inhibitory activity of bis(indolyl)pyridines analogues of the marine alkaloid nortopsentin. Molecules 26, 4112 (2021).
Sarker, S. D., Nahar, L. & Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 42, 321–324 (2007).
Muratović, S. et al. Synthesis of biscoumarin derivatives as antimicrobial agents. Asian J. Pharm. Clin. Res. 6, 132–134 (2013).
Ceri, H., Olson, M., Morck, D. & Storey, D. In Biofilms, Infection, and Antimicrobial Therapy. in Minimal Biofilm Eradication Concentration (MBEC) Assay: Susceptibility (CRC Press, 2005).
Allen, H. B. et al. The presence and impact of biofilm-producing staphylococci in atopic dermatitis. JAMA Dermatol. 150, 260–265 (2014).
Oyama, T. et al. Biofilm-forming methicillin-resistant Staphylococcus aureus survive in Kupffer cells and exhibit high virulence in mice. Toxins 8, 198 (2016).
Cheke, R. S. et al. Molecular insights into coumarin analogues as antimicrobial agents: Recent developments in drug discovery. Antibiotics 11, 566 (2022).
Smyth, T., Ramachandran, V. N. & Smyth, W. F. A study of the antimicrobial activity of selected naturally occurring and synthetic coumarins. Int. J. Antimicrob. Agents 33, 421–426 (2009).
Tajani, A. S. et al. Anti-quorum sensing and antibiofilm activity of coumarin derivatives against Pseudomonas aeruginosa PAO1: Insights from in vitro and in silico studies. Iran J. Basic Med. Sci. 26, 445–452 (2023).
Sushmitha, T. J. et al. 3-Hydroxy coumarin demonstrates anti-biofilm and anti-hyphal efficacy against Candida albicans via inhibition of cell-adhesion, morphogenesis, and virulent genes regulation. Sci. Rep. 13, 11687 (2023).
El-Sawy, E. R., Abdelwahab, A. B. & Kirsch, G. Synthetic routes to coumarin(benzopyrone)-fused five-membered aromatic heterocycles built on the α-pyrone moiety. Part 1: Five-membered aromatic rings with one heteroatom. Molecules 26, 483 (2021).
Matos, M. J. et al. Coumarins—An Important Class of Phytochemicals. in Phytochemicals - Isolation, Characterisation and Role in Human Health (IntechOpen, 2015). https://doi.org/10.5772/59982.
Annunziata, F., Pinna, C., Dallavalle, S., Tamborini, L. & Pinto, A. An Overview of coumarin as a versatile and readily accessible scaffold with broad-ranging biological activities. Int. J. Mol. Sci. 21, 4618 (2020).
Huang, G.-J. et al. Inducible nitric oxide synthase and cyclooxygenase-2 participate in anti-inflammatory activity of imperatorin from Glehnia littoralis. J. Agric. Food Chem. 60, 1673–1681 (2012).
Elshahid, Z. et al. Antimicrobial, cytotoxic, and α-glucosidase inhibitory potentials using the one strain many compounds technique for red sea soft corals associated fungi’ secondary metabolites and chemical composition correlations. J. Biol. Active Prod. Nat. 11, 467–489 (2021).
Eid, M. et al. Plasmonic superparamagnetic SPION@Ag@chitosan core-shell: Uptake and nitric oxide inhibition by colorectal cell lines. J. Inorg. Organomet. Polym. Mater. 32, 1–10 (2022).
Yoon, W.-J., Kim, S.-S., Oh, T.-H., Lee, N. H. & Hyun, C.-G. Cryptomeria japonica essential oil inhibits the growth of drug-resistant skin pathogens and LPS-induced nitric oxide and pro-inflammatory cytokine production. Pol. J. Microbiol. 58, 61–68 (2009).
Jeong, J.-W. et al. Anti-inflammatory effects of cordycepin via suppression of inflammatory mediators in BV2 microglial cells. Int. Immunopharmacol. 10, 1580–1586 (2010).
Chen, L. Z. et al. New arylpyrazoline-coumarins: Synthesis and anti-inflammatory activity. Eur. J. Med. Chem. 138, 170–181 (2017).
Zamani Taghizadeh Rabe, S., Iranshahi, M. & Mahmoudi, M. In vitro anti-inflammatory and immunomodulatory properties of umbelliprenin and methyl galbanate. J. Immunotoxicol. 13, 209–216 (2016).
Abd El-Lateef, H. M. et al. Design and synthesis of 2-(4-bromophenyl)quinoline-4-carbohydrazide derivatives via molecular hybridization as novel microbial DNA-gyrase inhibitors. ACS Omega 8, 17948–17965 (2023).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
A.A., conceptualization of research topics and formulation of specific aims; G.E.A., and A.A., equally performed the synthesis; M.S.A., evaluation of the antimicrobial and biofilm activities and analyzed the data; Z.A.E.-S., evaluation of the anti-inflammatory activity, performed the statistical analysis and wrote the data; E. R. E. conceived the experiments and wrote/edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ahmed, G.E., Elshahid, Z.A., El-Sawy, E.R. et al. Synthesis, biofilm formation inhibitory, and inflammation inhibitory activities of new coumarin derivatives. Sci Rep 14, 9106 (2024). https://doi.org/10.1038/s41598-024-59072-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-59072-w
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.