Abstract
Antibacterial properties of 3′,4′-difluoroquercetin (di-F-Q), a fluorine-substituted stable quercetin derivative, were investigated. Even though di-F-Q itself did not show interesting antibacterial activity, treatment of the Staphylococcus aureus strains with di-F-Q resulted in a dose-dependent reduction in biofilm formation with IC50 values of 1.8 ~ 5.3 mg/L. Also, the antibacterial activity of ceftazidime (CAZ) against carbapenem-resistant Pseudomonas aeruginosa (CRPA) showed eightfold decrease upon combination with di-F-Q. Assessment of the antimicrobial activity of CAZ in combination with di-F-Q against 50 clinical isolates of P. aeruginosa confirmed 15.7% increase in the percentages of susceptible P. aeruginosa isolates upon addition of di-F-Q to CAZ. Further mechanistic studies revealed that di-F-Q affected the antibiotics efflux system in CRPA but not the β-lactamase activity. Thus, di-F-Q was almost equally effective as carbonyl cyanide m-chlorophenyl hydrazine in inhibiting antibiotic efflux by P. aeruginosa. In vivo evaluation of the therapeutic efficacy of CAZ-(di-F-Q) combination against P. aeruginosa showed 20% of the mice treated with CAZ-(di-F-Q) survived after 7 days in IMP carbapenemase-producing multidrug-resistant P. aeruginosa infection group while no mice treated with CAZ alone survived after 2 days. Taken together, di-F-Q demonstrated unique strain-specific antimicrobial properties including anti-biofilm and antibiotic-potentiating activity against S. aureus and P. aeruginosa, respectively.
Similar content being viewed by others
Introduction
Quercetin (Fig. 1) is one of the most famous flavonoids, and it is characterized by broad spectrum biological activity including antioxidant, anti-inflammatory, antiviral, and anticancer effects1. Quercetin has also been reported to have a potential as an antibacterial agent1, and its anti-biofilm activity against clinical isolates of gram-positive bacteria, albeit low2, is noteworthy. However, several unfavorable physicochemical properties of quercetin such as low chemical stability limits its pharmaceutical use. Previously, in order to tackle this problem, we prepared 3′,4′-difluoroquercetin (di-F-Q, Fig. 1) through bioisosteric replacement of the catecholic hydroxyl groups of quercetin with fluorine atoms, which showed significantly improved chemical stability as well as anticancer activity compared with quercetin3. In this context, antibacterial properties of di-F-Q appears worth investigating and, in this study, we attempted to evaluate antibacterial, anti-biofilm, and antibiotic-potentiating activity of di-F-Q.
Structures of quercetin and di-F-Q3.
Materials and methods
Materials
Dimethyl sulfoxide (DMSO), crystal violet and phosphate buffered saline (PBS) were purchased from Merck (St. Louis, MO, USA). Mueller–Hinton broth (MHB) and tryptic soy broth (TSB) were obtained from BD Biosciences (San Jose, CA, USA) and Fisher Scientific (Pittsburgh, PA, USA), respectively.
Microorganisms
The bacterial strains used in this study are listed in Table 1.
Assessment of antibacterial activity of di-F-Q
Minimum inhibitory concentration (MIC) of di-F-Q was determined for all isolates in three replicates using broth microdilution with cation-adjusted MHB according to the Clinical and Laboratory Standards Institute (CLSI) guidelines14. DMSO stock solution of di-F-Q was serially diluted to the desired concentrations in MHB. Then, 10 μl of the bacterial suspension [5 × 105 colony forming units (CFU)/ml] was combined with 200 μl of di-F-Q in 96-well microtiter plates (1% DMSO, final). After incubation of the plate at 37 °C for 24 h, bacterial growth was visibly evaluated by monitoring the turbidity of the resulting suspension, and the MICs were determined as the lowest di-F-Q concentration that inhibited the visible growth of the bacteria.
Anti-biofilm activity
The inhibitory effect of di-F-Q on biofilm formation of various bacterial strains was determined by 96-well plate-based crystal violet assay as described previously15. Bacterial strains were cultured with 0.5 × TSB and diluted to 5 × 105 CFU/ml in fresh 0.5 × TSB with 1% glucose. In a 96-well plate, 10 μl of di-F-Q was mixed with 190-μl of the bacterial suspension to final concentrations of 0, 1, 5, 10, 25, 50, and 100 mg/l. The plate was incubated for 24 h and gentle aspiration of media was followed by fixing the biofilm by addition of 100% ethanol. Ethanol was then removed, and the plate was air-dried. The wells were treated with 0.1% crystal violet for 10 min. The plates were washed with deionized water (dH2O), and the crystal violet-stained biofilms were solubilized with 33% acetic acid for 1 h. Absorbance of the resulting solution at 600 nm was monitored.
Antibiotic resistance potential
Antibacterial activity (MICs) of various antibiotics including ampicillin (AMP), ceftazidime (CAZ), cefepime (FEP), meropenem (MER), and vancomycin (VAN) were evaluated in the absence and presence of di-F-Q by using the checkerboard synergy test16,17. A total of 100 μl of cation-adjusted MHB was distributed in each well of the 96-well microtiter plate. Di-F-Q was then serially diluted along the ordinate of the plate (0.25 ~ 128 μg/ml), while the second antibiotic was diluted along the abscissa (0.06 ~ 512 μg/ml). From each bacterial isolate, an inoculum standardized with 0.5 McFarland turbidity standard was prepared in cation-adjusted MHB. Each microtiter well was inoculated with 200 μl of a bacterial inoculum (5 × 105 CFU/ml), and the plates were incubated at 35 °C for 20 h under aerobic conditions. The MIC values of each antibiotics in combination with di-F-Q was determined as described above. At least three independent experiments were performed for each strain.
Inhibition of β-lactamase
Inhibitory activity of di-F-Q against the β-lactamase activity was explored by using a β-lactamase Inhibition Screening Assay Kit (K804-100) (Biovision, San Francisco, USA) which includes nitrocefin, a β-lactamase substrate generating a colorimetric species, as a marker for β-lactamase activity. Assay was performed in accordance with the manufacturer’s instructions, and changes in absorbance A490 for nitrocefin was monitored. Clavulanic acid was used as a positive control.
Inhibition of efflux pumps
The efflux activity of CRPA was assessed by determining the accumulation of ethidium bromide (EtBr) as described previously18. Overnight culture of CRPA strain was inoculated in MHB. After incubation at 37 °C for 4 h, the bacterial cells were centrifuged (3,000 rpm, 15 min) and the pellet was resuspended in PBS. After adjusting the OD600 to 0.1, the bacterial suspension (186 µl) was added to each well of the flat bottomed, black 96-well plate. EtBr (10 µl) was also added to each well to a final concentration of 2.5 µM. Finally, 4 µl of di-F-Q or a positive control (carbonyl cyanide m-chlorophenyl hydrazine, CCCP) was added to the plate and the bacteria were incubated at 37 °C. The fluorescence emission from EtBr was taken (λex 535 nm/λem 600 nm) for 30 min with a 1-min interval using Cytation 5 imaging multi-mode reader (BioTek Instruments, Inc., Winooski, VT, USA).
In vivo antibacterial activity of CAZ in combination with di-F-Q
Animal experiments were carried out in accordance with established practices as described in the National Institutes of Health Guide for Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the Asan Institute for Life Sciences, Seoul, Korea. Six-week-old female outbred immunocompetent CD-1 mice were randomly distributed into 2 groups (10 mice per group), which were infected with 1.0 × 107 CFU/mouse of P. aeruginosa ATCC 27853 (CSPA) or IMP carbapenemase-producing MRPA admixed with 5% porcine mucin. The mice of each group were further allocated in two subgroups (n = 5 for each subgroup) and, after 2 h, the mice in each subgroup were treated with CAZ (10 mg/kg/day) or CAZ-(di-F-Q) (10 mg/kg/day–40 mg/kg/day) for 7 days.
Statistical analysis
Data are representative of at least three independent experiments and expressed as means ± SD. Tests used for nonparametric data included one-way analysis of variance (ANOVA) with Tukey’s post hoc test (GraphPad, Prism 5).
Results and discussion
Antimicrobial activity of di-F-Q
The antibacterial activity of di-F-Q against various gram-positive and gram-negative bacterial strains was investigated (Table 1). Gram-positive bacteria included six S. aureus strains [MSSA, MRSA with different sequence types (ST 5, ST72, and ST239), hVISA, and VISA] and two Enterococcus strains (VSE and VRE) (Table 1). Eight gram-negative bacterial strains [P. aeruginosa (CSPA and CRPA), A. baumannii (CSAB and CRAB), K. pneumoniae (wild type and CRE KPC type), and E. coli (wild type and CRE NDM-1)] were also tested for their susceptibility to di-F-Q by using the broth microdilution checkerboard (CB) method (Table 1). The gram-positive strains were weakly sensitive to di-F-Q (MIC, 8–32 mg/L), but gram-negative strains were highly resistant (MIC, > 128 mg/L) (Table 2). This difference may be explained by the structure and composition of bacterial cell wall. Gram-positive bacteria consist of a relatively simple envelope, and its cytoplasmic membrane is surrounded by peptidoglycan layer. Previously, sub-MICs of quercetin and its derivatives were shown to disrupt or alter cytoplasmic membrane to exhibit antibacterial activity against S. aureus19,20. Based on the structural similarity, di-F-Q is presumed to have the similar mode of action as quercetin against gram-positive bacteria. On the other hand, gram-negative bacterial cell envelope is more complex and relatively impermeable structure due to their distinctive outer membrane containing lipopolysaccharide21. As a result, gram-negative bacteria are protected from many antibiotics, especially large and hydrophobic ones, to which gram-positive bacteria are susceptible. The lack of antibacterial activity of di-F-Q against the gram-negative bacteria might be attributed to its hydrophobic nature (cLogP = 3.78) and resulting inability to cross the bacterial cell wall. In this context, it is worth to note that Wang et al. reported that higher concentration of quercetin (50 × MIC) was required to damage the membrane of E. coli compared with S. aureus (10 × MIC)20.
Anti-biofilm activity of di-F-Q
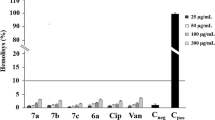
The anti-biofilm activity of di-F-Q was then investigated because, by using this protective mechanism22,23, bacteria are known to become significantly more resistant to antibiotics24. The bacterial strains tested above were monitored for their biofilm-formation activity in the presence of increasing concentrations of di-F-Q, and a noticeable change was observed only in the S. aureus strains which are well characterized for pronounced ability to form biofilms25 (Fig. 2). Treatment of the six S. aureus strains with di-F-Q resulted in a noticeable dose-dependent reduction in biofilm formation with IC50 values of 1.8 ~ 5.3 mg/l (Table 3). As mentioned above, quercetin and its derivatives more easily disrupt the membrane of gram-positive bacteria compared with that of gram-negative bacteria. In addition, previous studies showed that quercetin and its derivatives inhibit hemolysis and biofilm formation in S. aureus by reducing alpha-toxin secretion26,27,28,29, which might explain the S. aureus-specific anti-biofilm effect of di-F-Q. On the other hand, quercetin is also known to inhibit the aqr quorum-sensing system which modules biofilm formation in S. aureus28. However, in current investigation, anti-biofilm effect of di-F-Q was observed in both agr-functional MRSA strains (AMC-SA 5013, AMC-SA 5016) and agr-dysfunction MRSA strain (AMC-SA 3416). In addition, although quercetin exerted strong anti-biofilm effects on P. aeruginosa stain PAO1 by repressing expression levels of quorum-sensing associated genes30, di-F-Q did not exert anti-biofilm effect on our P. aeruginosa strains (data not shown). Taken together, it seems that inhibition of quorum-sensing system may not be essential for anti-biofilm effect of di-F-Q.
Antibiotics-potentiating activity of di-F-Q
The potent anti-biofilm activity of di-F-Q prompted further investigation on its antibiotic properties particularly in combination with other antibiotics. Thus, antibiotic-potentiating activity of di-F-Q was evaluated and, in the presence of sub-MICs (MIC/4) of di-F-Q, antibacterial activity of ampicillin (AMP, aminopenicillin), ceftazidime (CAZ, 3rd generation cephalosporin), cefepime (FEP, 4th generation cephalosporin), meropenem (MEM, carbapenem) and vancomycin (VAN, glycopeptide) were determined against various antibiotic-resistant gram-positive and gram-negative bacteria (Table 4). In general, the antibacterial activity of the antibiotics was not significantly affected by combination with di-F-Q. To our surprise, however, MICs of CAZ against the gram-negative carbapenem-resistant strains such as CRPA and CRAB showed significant decreases (eight- and four-fold, respectively) upon combination with di-F-Q (MIC/4, Table 4).
The remarkable effect of di-F-Q on the selective restoration of the antibacterial activity of CAZ against carbapenem-resistant gram-negative strains such as CRPA and CRAB is of critical interest because carbapenems are used as last resorts for the treatment of infections caused by multidrug-resistant gram-negative bacteria31. Thus, the antimicrobial activity of CAZ alone and in combination with di-F-Q was further assessed against 50 clinical isolates of P. aeruginosa using broth microdilution method. The MIC distributions of CAZ against P. aeruginosa isolates were obtained in the absence and presence of di-F-Q, which showed significant shifts to lower values upon combination with di-F-Q (Fig. 3). The ceftazidime-potentiating activity of di-F-Q and thereby sensitization of the P. aeruginosa isolates to CAZ was found to be concentration-dependent, and the shifts in MICs were more pronounced with increasing concentrations of di-F-Q (Fig. 3).
Based on the Clinical and Laboratory Standards Institute (CLSI) susceptibility interpretive criteria (breakpoints) for CAZ against P. aeruginosa14, the CAZ-(di-F-Q) susceptibility testing results against 50 P. aeruginosa isolates were categorized into percentages of susceptible (%S, MIC ≤ 8), intermediate (%I, MIC = 16) and resistant (%R, MIC ≥ 32) in Table 4. Also included in Table 4 are the MIC50 and the MIC90 values, the lowest concentration of CAZ at which 50% and 90% of the isolates were inhibited, respectively. CAZ-(di-F-Q) activity (MIC50/90, 2/8 mg/l; 94.1% susceptible at 8 mg/l) against all 50 P. aeruginosa isolates (with the di-F-Q concentration fixed at 32 mg/l) was noticeably enhanced in comparison to the CAZ single treatment (MIC50/90, 4/ > 32 mg/l; 78.4% susceptible at 8 mg/l), which amounts to 15.7% increase in the percentages of susceptible P. aeruginosa isolates upon addition of di-F-Q to CAZ (Table 5).
The significantly increased sensitization of P. aeruginosa isolates to CAZ by di-F-Q is reminiscent of CAZ-avibactam (AVI) combination (MIC50/90, 2/8 mg/l; 96.3% susceptible at 8 mg/l) which showed 7.4 ~ 16.3% increases in the percentages of the susceptible P. aeruginosa isolates compared with those of CAZ single treatment32,33. As the CAZ-potentiation activity of avibactam is attributed to its β-lactamase inhibitory activity33, we evaluated the effect of di-F-Q on the enzymatic activity of β-lactamase. However, the β-lactamase activity was not affected by treatment with di-F-Q (Fig. 4).
On the other hand, overexpression of efflux pumps is generally considered as the major mechanism for intrinsic and acquired antibiotic resistance in P. aeruginosa34; a diverse array of antibiotics including β-lactams, quinolones, tetracyclines, macrolides, linomycins chloramphenicols and novobiocins are known to suffer from efflux pump-mediated resistance by P. aeruginosa35. Thus, efflux pump inhibitory activity of di-F-Q was investigated by using ethidium bromide (EtBr) as the fluorescent substrate of efflux pumps in P. aeruginosa (Fig. 5)36. In CRPA, di-F-Q increased EtBr fluorescence in a dose-dependent manner indicating inhibition of efflux pumps and thereby intracellular retention of EtBr; significant efflux pump inhibitory activity of di-F-Q was observed at concentrations 32 mg/l or above, which is in line with the eightfold reduction of the MIC of CAZ against CRPA by the same amount of di-F-Q (Table 3). Carbonyl cyanide m-chlorophenyl hydrazine (CCCP) is known to inhibit the efflux pump in P. aeruginosa36, and we observed that treatment of CRPA with CCCP (16 mg/l) increased fluorescence from EtBr (Fig. 5). These results collectively suggest that di-F-Q (32 mg/l) and CCCP (16 mg/l) are almost equally effective in inhibiting antibiotic efflux by P. aeruginosa. The MexAB-OprM multidrug efflux pump system is known to pump out mostly lipophilic and amphiphilic drugs, which include EtBr.
Therapeutic efficacy of CAZ-(di-F-Q) combination against P. aeruginosa was then evaluated in mice infected with CSPA or multidrug resistant P. aeruginosa (MRPA) (Fig. 6). Thus, six-week-old female outbred immunocompetent CD-1 mice were infected with 1.0 × 107 CFU/mouse of P. aeruginosa ATCC 27853 (CSPA) (Fig. 6a) or MRPA (Fig. 6b) admixed with 5% porcine mucin and, after 2 h, treated with CAZ (10 mg/kg/day) or CAZ-(di-F-Q) (10–40 mg/kg/day) for 7 days (n = 5 for each treatment group). The CSPA- or MRPA-infected mice without antibiotic treatment all died after 1 day (infected control, Fig. 6). Also, in both CSPA and MRPA infection groups, no mice treated with CAZ alone survived after 2 days (CAZ, Fig. 6). However, 20% of the mice treated with CAZ-(di-F-Q) survived after 7 days in CSPA as well as MRPA infection group [CAZ + (di-F-Q), Fig. 6]. Interestingly, between 3 to 5 days after infection, CAZ-(di-F-Q) was more effective in protecting MRPA-infected mice (60 ~ 40% survival rate, Fig. 6b) than the CSPA-infected ones (20% survival rate, Fig. 6a).
In summary, we evaluated antibacterial properties of di-F-Q, a fluorinated quercetin analogue. Di-F-Q was not effective in killing bacteria but showed potent anti-biofilm activity against S. aureus with IC50 values of 1.8 ~ 5.3 mg/l. Also, di-F-Q potentiated the antibacterial activity of the 3rd generation cephalosporin CAZ against the gram-negative carbapenem-resistant strains such as CRPA and CRAB (eight- and four-fold decreases in MICs, respectively). Assessment of the antimicrobial activity of CAZ in combination with di-F-Q against 50 clinical isolates of P. aeruginosa confirmed a remarkable enhancement in antibacterial activity (MIC50/90, 2/8 mg/l; 94.1% susceptible at 8 mg/l) in comparison to the CAZ single treatment (MIC50/90, 4/ > 32 mg/l; 78.4% susceptible at 8 mg/l), which amounts to 15.7% increase in the percentages of susceptible P. aeruginosa isolates upon addition of di-F-Q to CAZ. Further mechanistic studies revealed that di-F-Q affected the antibiotics efflux system in CRPA but not the β-lactamase activity. Thus, di-F-Q was almost equally effective as CCCP in inhibiting antibiotic efflux by P. aeruginosa. In vivo evaluation of the therapeutic efficacy of CAZ-(di-F-Q) combination against P. aeruginosa showed 20% of the mice treated with CAZ-(di-F-Q) survived after 7 days in MRPA infection group while no mice treated with CAZ alone survived after 2 days. Taken together, di-F-Q demonstrated the peculiar strain-specific anti-biofilm and antibiotic-potentiating activity against S. aureus and P. aeruginosa, respectively.
References
Wang, W. et al. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 56, 21–38. https://doi.org/10.1016/j.tifs.2016.07.004 (2016).
Costa Júnior, S. D. et al. Antibacterial and antibiofilm activities of quercetin against clinical isolates of Staphyloccocus aureus and Staphylococcus saprophyticus with resistance profile. Int. J. Environ. Agric. Biotechnol. 3, 1948–1958. https://doi.org/10.22161/ijeab/3.5.50 (2018).
Cho, S. Y., Kim, M. K., Mok, H., Choo, H. & Chong, Y. Separation of quercetin’s biological activity from its oxidative property through bioisosteric replacement of the catecholic hydroxyl groups with fluorine atoms. J. Agric. Food Chem. 60, 6499–6506. https://doi.org/10.1021/jf3018645 (2012).
Soni, I., Chakrapani, H. & Chopra, S. Draft genome sequence of methicillin-sensitive Staphylococcus aureus ATCC 29213. Genome Announc. 3, e01095-e1115. https://doi.org/10.1128/genomeA.01095-15 (2015).
Joo, E.-J. et al. Emergence of community-genotype methicillin-resistant Staphylococcus aureus in Korean hospitals: Clinical characteristics of nosocomial infections by community-genotype strain. Infect. Chemother. 49, 109–116. https://doi.org/10.3947/ic.2017.49.2.109 (2017).
Price, C. S., Kon, S. E. & Metzger, S. Rapid antibiotic susceptibility phenotypic characterization of Staphylococcus aureus using automated microscopy of small numbers of cells. J. Microbiol. Methods 98, 50–58. https://doi.org/10.1016/j.mimet.2013.12.021 (2014).
Kim, E. B., Kopit, L. M., Harris, L. J. & Marco, M. L. Draft genome sequence of the quality control strain Enterococcus faecalis ATCC 29212. J. Bacteriol. 194, 6006–6007. https://doi.org/10.1128/JB.01423-12 (2012).
Lee, Y. J. et al. Sesquiterpenoids from Tussilago farfara flower bud extract for the eco-friendly synthesis of silver and gold nanoparticles possessing antibacterial and anticancer activities. Nanomaterials 9, 819. https://doi.org/10.3390/nano9060819 (2019).
Fang, X. et al. Draft genome sequence of Pseudomonas aeruginosa strain ATCC 27853. J. Bacteriol. 194, 3755. https://doi.org/10.1128/JB.00690-12 (2012).
Kim, M. K., Jung, M., Park, K.-H. & Chong, Y. Quercetin-pivaloxymethyl conjugate potentiates antibiotics against Pseudomonas aeruginosa and Acinetobacter baumannii. Bull. Korean Chem. Soc. 39, 879–881. https://doi.org/10.1002/bkcs.11493 (2018).
Davenport, K. W. et al. Draft genome assembly of Acinetobacter baumannii ATCC 19606. Genome Announc. 2, e00832-e914. https://doi.org/10.1128/genomeA.00832-14 (2014).
Arivett, B. A. et al. Draft genome sequences of Klebsiella pneumoniae clinical type strain ATCC 13883 and three multidrug-resistant clinical isolates. Genome Announc. 3, e01385-e1414. https://doi.org/10.1128/genomeA.01385-14 (2015).
Minogue, T. D. et al. Complete genome assembly of Escherichia coli ATCC 25922, a serotype O6 reference strain. Genome Announc. 2, e00969-e1014. https://doi.org/10.1128/genomeA.00969-14 (2014).
Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial susceptibility testing; 25th informational supplement. CLSI M100-S25. Clinical and Laboratory Standards Institute, Wayne, PA.
Brahma, U. et al. Antimicrobial and anti-biofilm activity of hexadentated macrocyclic complex of copper (II) derived from thiosemicarbazide against Staphylococcus aureus. Sci. Rep. 8, 8050. https://doi.org/10.1038/s41598-018-26483-5 (2018).
Sopirala, M. M. et al. Synergy testing by Etest, microdilution checkerboard, and time-kill methods for pan-drug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 54, 4678–4683. https://doi.org/10.1128/AAC.00497-10 (2010).
Kim, S. et al. Pilot screening to determine antimicrobial synergies in a multidrug-resistant bacterial strain library. Microb. Drug Resist. 22, 372–378. https://doi.org/10.1089/mdr.2015.0251 (2016).
Smith, H. E. & Blair, J. M. A. Redundancy in the periplasmic adaptor proteins AcrA and AcrE provides resilience and an ability to export substrates of multidrug efflux. J. Antimicrob. Chem. 69, 982–987. https://doi.org/10.1093/jac/dkt481 (2014).
Amin, M. U., Khurram, M., Khattak, B. & Khan, J. Antibiotic additive and synergistic action of rutin, morin and quercetin against methicillin resistant Staphylococcus aureus. BMC Complement Altern. Med. 15, 59. https://doi.org/10.1186/s12906-015-0580-0 (2015).
Wang, S. et al. Bacteriostatic effect of quercetin as an antibiotic alternative in vivo and its antibacterial mechanism in vitro. J. Food Prot. 81, 68–78. https://doi.org/10.4315/0362-028X.JFP-17-214 (2018).
Silhavy, T. J., Kahne, D. & Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2, a000414. https://doi.org/10.1101/cshperspect.a000414 (2010).
Kiedrowski, M. R. & Horswill, A. R. New approaches for treating staphylococcal biofilm infections. Ann. N. Y. Acad. Sci. 1241, 104–121. https://doi.org/10.1111/j.1749-6632.2011.06281.x (2011).
de la Fuente-Nunez, C., Reffuveille, F., Fernandez, L. & Hancock, R. E. Bacterial biofilm development as a multicellular adaptation: Antibiotic resistance and new therapeutic strategies. Curr. Opin. Microbiol. 16, 580–589. https://doi.org/10.1016/j.mib.2013.06.013 (2013).
Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2, 114–122. https://doi.org/10.1038/nrd1008 (2003).
Archer, N. K. et al. Staphylococcus aureus biofilms: Properties, regulation, and roles in human disease. Virulence 2, 445–459. https://doi.org/10.4161/viru.2.5.17724 (2011).
Kim, M. K., Lee, T.-G., Jung, M., Park, K.-H. & Chong, Y. In vitro synergism and anti-biofilm activity of quercetin–pivaloxymethyl conjugate against Staphylococcus aureus and Enterococcus Species. Chem. Pharm. Bull. 66, 1019–1022. https://doi.org/10.1248/cpb.c18-00380 (2018).
Cho, H. S., Lee, J.-H., Cho, M. H. & Lee, J. Red wines and flavonoids diminish Staphylococcus aureus virulence with anti-biofilm and anti-hemolytic activities. Biofouling 31, 1–11. https://doi.org/10.1080/08927014.2014.991319 (2015).
Lee, J. H. et al. Anti-biofilm activities of quercetin and tannic acid against Staphylococcus aureus. Biofouling 29, 491–499. https://doi.org/10.1080/08927014.2013.788692 (2013).
López, G. C. & Sánchez, C. A. C. Quercetin attenuates Staphylococcus aureus virulence by reducing alpha-toxin secretion. Rev. Argent. Microbiol. 50, 131–135. https://doi.org/10.1016/j.ram.2017.07.002 (2017).
Ouyang, J. et al. Quercetin inhibits Pseudomonas aeruginosa biofilm formation via the vfr-mediated lasIR system. Microb. Pathog. 149, 104291 (2020).
Willyard, C. The drug-resistant bacteria that pose the greatest health threats. Nature 543, 15. https://doi.org/10.1038/nature.2017.21550 (2017).
Hidalgo, J. A., Vinluan, C. M. & Antony, N. Ceftazidime/avibactam: A novel cephalosporin/nonbeta-lactam beta-lactamase inhibitor for the treatment of complicated urinary tract infections and complicated intra-abdominal infections. Drug Des. Dev. Ther. 10, 2379–2386. https://doi.org/10.2147/DDDT.S110946 (2016).
Huband, M. D. et al. In vitro activity of ceftazidime-avibactam against contemporary Pseudomonas aeruginosa isolates from U.S. medical centers by census region, 2014. Antimicrob. Agents Chemother. 60, 2537–2541. https://doi.org/10.1128/AAC.03056-15 (2016).
Aeschlimann, J. R. The role of multidrug efflux pumps in the antibiotic resistance of Pseudomonas aeruginosa and other gram-negative bacteria. Insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 23, 916–924. https://doi.org/10.1592/phco.23.7.916.32722 (2003).
Masuda, N. et al. Substrate specificities of MexAB-OprM, MexCD-OprJ, and MexXY-oprM efflux pumps in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 44, 3322–3327. https://doi.org/10.1128/aac.44.12.3322-3327.2000 (2000).
Lomovskaya, O. et al. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: Novel agents for combination therapy. Antimicrob. Agents Chemother. 45, 105–116. https://doi.org/10.1128/AAC.45.1.105-116.2001 (2001).
Acknowledgements
This study was supported by a grant from the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2019R1C1C1003195).
Author information
Authors and Affiliations
Contributions
M.K.K., K.P., and Y.C. designed the research; W.K., M.J. and M.K.K. conducted the whole experiment, including data acquisition and analysis; Y.P.C. and Y.S.K. performed animal model study; K.P. and Y.C. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kho, W., Kim, M.K., Jung, M. et al. Strain-specific anti-biofilm and antibiotic-potentiating activity of 3′,4′-difluoroquercetin. Sci Rep 10, 14162 (2020). https://doi.org/10.1038/s41598-020-71025-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-71025-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.