Abstract
Microbial infections are currently a widespread disease in hospitals and community health centres and are a major cause of death worldwide. In pursuit of searching new antimicrobial agents, coumarin linked to thiazoles, pyridines and pyrazoles have been developed and evaluated for their antimicrobial properties against two Gram + bacteria, two Gram − bacteria as well as two fungi. Some of the prepared coumarins displayed high to moderate activity against the tested microorganisms with respect to the reference drugs. However, compound 3 exhibited antimicrobial effect equal to the reference drug Ciprofloxacin for Gram − baceria Enterobacter cloacae. Compound 12 was found to be the most potent compound against Bacillus pumilis with MIC of 7.69 (µmol/ml). Compounds 3, 4 and 12 showed remarkable activity against Streptococcus faecalis with MIC of 14.34, 3.67 and 15.36 (µmol/ml), respectively. Regarding Escherichia coli, most compounds recorded high to moderate MIC values (4.73–45.46 µmol/ml). Moreover, in case of E. cloacae compound 9 was the most potent compound with MIC value of 22.76 (µmol/ml).
Similar content being viewed by others
Introduction
One of the main factor in lowering the worldwide burden of infectious illnesses is antimicrobial agents1. When bacteria, viruses, fungi, and parasites, among other microbes, are able to adapt and flourish in the presence of drugs, this phenomenon is known as antimicrobial resistance (AMR)2. Due to the unreasonable usage of antibiotics, the appearance of multidrug-resistant (MDR) pathogens increased resulting in greater mortality and morbidity. Methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococci (VRE), and MDR Gram-negative bacteria in particular cause numerous therapeutic medications to lose their effectiveness or completely stop working. Additionally, the highly invasive fungal infections have posed an unprecedented challenge to the health sector3,4,5. Depending on how they work, antimicrobial agents can be categorised into several classes. Inhibitors of protein synthesis, inhibitors of nucleic acid synthesis, inhibitors of metabolic processes, and agents that depolarize cell membranes are the primary categories6,7. Antibiotics can no longer be used to treat bacterial infections, indicating an uncertain future for healthcare. In order to combat medication resistance on clinically important infections, it is necessary to find novel compounds with antibacterial activity that may function through mechanisms of action that are different from those of well-known classes of antimicrobial drugs8,9,10,11.
Coumarins are heterocycles that are widely distributed in plants. Apricots, cherries, cinnamon, strawberries, and other foods are some excellent sources of coumarins12. Natural and synthetic coumarins demonstrated a broad range of therapeutic applications13, including antimicrobial14,15,16, anti-HIV17,18, antioxidant19, anticoagulant20,21, anti-infammatory22,23, anticonvulsant24,25, anticancer26,27,28 and antiviral29. Also, they attract the major interest of chemists due to their wide variety of uses such as laser dyes30, cosmetics31, fluorescence probes32, photosensitizers33, food and perfumes34. Additionally, they have proven to be novel lipid-lowering agents with mild triglyceride-lowering capability35.
Thiazole ring is present in a lot of commercial medications as an active component36 due to its marvelous biological activity such as antiviral37, antimicrobial38,39, anti-inflammatory40, anti-HIV41, antitumor42,43 and antioxidant. Moreover, thiazolyl–coumarin are a significant group of heterocycles having a wide range of biological functions such as antibacterial, anticancer, antiviral, antioxidant44,45,46,47,48. It is well known that coumarin derivatives with pyridine heterocycles have anticoagulant, antibacterial, antifungal properties and antiproliferative activity against cancer cell49,50,51,52. In this regard and in accordance with the data offered, we have been concentrating on the synthesis of molecular hybrids based on bioactive heterocycles and coumarins as well as the evaluation of their antimicrobial activity.
Results and discussion
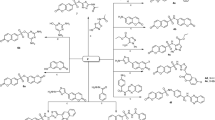
A new series of thiazoles attached to 6-bromocoumarin moiety were synthesized by Hantzsch thiazole synthesis. Thus, interaction of 2-(1-(6-bromo-2-oxo-2H-chromen-3-yl)ethylidene)hydrazine-1-carbothioamide (1)53 with hydrazonoyl halides 2a, b in ethanol and triethylamine under reflux gave the derivatives 3 and 4, respectively, in good yield (Fig. 1). The structures of the new derivatives were elucidated by elemental analysis and spectroscopic data.
The infrared spectra of compounds 3 and 4 revealed the presence of stretching frequencies at 3429 and 3428 cm−1 attributable to the imino group, in addition to the presence of a strong absorption bands at 1735 and 1736 cm−1 due to carbonyl group as well as the other absorption bands. Moreover 1H NMR spectrum in DMSO-d6 for compound 3 revealed singlet signals at chemical shifts 2.37 and 8.30 ppm for methyl and imino protons, respectively, beside other signals due to aromatic protons. Its 13C NMR (100 MHz) recorded signals at δ 17.90 (CH3), 116.82–159.43 (Ar–C) and 183.16 ppm (C=O). While in 1H NMR spectrum of compound 4 (DMSO-d6), triplet and quartet signals were appeared at chemical shifts 1.31 and 4.37 ppm, besides other three singlet signals at chemical shifts 2.34, 7.23 and 8.59 ppm assignable to methyl and 2NH protons, respectively. The 13C NMR (100 MHz) of 4 displayed signals at δ 14.31, 17.89 (2CH3), 63.40 (CH2), 118.76, 118.94–159.11 (Ar–C), 166.28 and 195.45 ppm (2C=O).
Next, condensation of enaminone 554 with active methylene compounds such as ethyl acetoacetate, acetylacetone, trifloroacetylacetone and ethyl cyanoacetate in AcOH and AcONH4 resulted in the formation of new pyridines 6–9, respectively as shown in Fig. 2. The structure of the new products was assigned based on the spectral data and elemental analysis. The IR spectra of pyridines 6–9 displayed two absorption bands at the region of 1675–1715 cm−1 and 1740–1710 cm−1 accounted for two carbonyl groups. 1H NMR spectrum for compound 6 as an example, in DMSO-d6 showed triplet and quartet signals at δ 1.34 and 4.27 ppm for (CH3CH2O-) and singlet signal at 2.90 ppm for methyl protons. In the 13C NMR spectrum of compound 6 two CH3 and one CH2 appeared at 14.35, 25.06 and 61.62 ppm, respectively. Signals at 158.76 and 166.21 ppm are attributed to two carbonyl groups. Whereas compound 7 recorded two singlet signals at δ 1.90 and 2.43 ppm for two methyl groups, beside four doublet signals at δ 6.80, 7.30, 7.70 and 8.40 ppm for six aromatic protons. In the 13C NMR spectrum of compound 7 two methyl groups appeared at 25.03 and 29.97 ppm, respectively. Signals at 168.38 and 170.41 are attributed to two carbonyl groups. Furthermore, compound 8 showed one singlet signal at δ 2.71 ppm assigned to COCH3 protons, all other signals were for CH aromatic protons. Compound 9 displayed a singlet signal at δ 7.95 ppm for NH proton as well as other signals for CH aromatic protons.
The most likely pathway for the formation of pyridine derivatives 6–9 is outlined in Fig. 3. The reaction proceeded via initial Michael addition of the active methylene compound to the activated double bond of enaminone 5 to give I. Secondly, nucleophilic addition of NH3 molecule (generated from dissociation of ammonium acetate) to carbonyl carbon and subsequent cyclization via elimination of dimethyl amine and two water molecules lead to final product.
However, new pyrazoles 10a–c were synthesized from the reaction of enaminone 5 and hydrazonyl halides 2b–d in boiling benzene containing trimethylamine under reflux as outlined in Fig. 4. By using spectroscopic data and elemental analysis, all structures were clarified. 1H NMR spectrum of compound 10a revealed new triplet and quartet signals for the ethoxy group at 1.16 and 4.20 ppm, while, pyrazole derivative 10b recorded a singlet signal for the methyl protons at 2.57 ppm, beside the aromatic protons. The 13C NMR spectrum of compound 10b showed one methyl groups at 25.8 ppm while three signals for carbonyl groups appeared at 146.10, 154.21 and 158.41 ppm, respectively. Also, compound 10c recorded new triplet and quartet signals for the ethoxy group at δ 1.35 and 4.44 ppm in 1H NMR spectrum, its 13C NMR spectrum showed signals at 27.83 (CH3), 116.99–158.36 (Ar–C), 184.74, 194.51 (3C=O).
The mechanism55 for the formation of compounds 10a–c is illustrated in Fig. 4. The reaction proceeded via 1,3-dipolar cycloaddition reaction between the imine 2′ (produced from the reaction of compound 2 with TEA) and the activated double bond in compound 5 giving the non-isolable cyclo adduct I. The intermediate I loses dimethylamine molecule to give the final pyrazole derivatives 10a–c.
On the next side, enaminone 5 was reacted with 5-Amino-1-phenyl-1H-pyrazole-4-carboxylic acid ethyl ester (11)56 in ethanol and drops of acetic acid to give 5-[3-(6-bromo-2-oxo-2H-chromen-3-yl)-3-oxo-propenylamino]-1-phenyl-1H-pyrazole-4-carboxylic acid ethyl ester (12) through elimination of dimethylamine molecule (Fig. 5). The IR spectrum of compound 12 revealed strong absorption peaks at ν 3265, 1703 and 1682 cm−1 for NH and two carbonyl groups respectively. Its 1H NMR spectrum recorded triplet and quartet signals at δ 1.2 and 4.19 ppm due to ethoxy group, beside a singlet signal due to NH proton at δ 8.59 ppm. In the 13C NMR spectrum of compound 12 one CH3 and one CH2 appeared at 14.93 and 45.02 ppm, respectively. Signals at 154.06, 158.76 and 164.03 are attributed to three carbonyl groups.
Finally, reaction of enaminone 5 with 6-aminothiouracil 13 in glacial acetic acid furnished 5-(6-bromo-2-oxo-2H-chromen-3-yl)-2-thioxo-2,3-dihydro-1H-pyrido[2,3-d]pyrimidin-4-one (14) in a good yield.
Biological studies
Antimicrobial activity
The primary antimicrobial screening for the new compounds was assessed against six microbes using the agar well diffusion method. The chosen pathogenic microbes were Bacillus pumilis (MTCC-2296) and Streptococcus faecalis (MTCC-0459) as Gram + bacteria, Escherichia coli (ATCC-25955) and Enterobacter cloacae (ATCC-23355) as Gram – bacteria, while Saccharomyces cerevisiae (ATCC-9763) and Candida albicans (ATCC-10231) were used as fungi. Penicillin G was used as standard antibacterial (Gram +) drug while Ciprofloxacin was used as standard antibacterial (Gram −) drug and Ketoconazole as antifungal drug. The broth dilution method was performed to measure the minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC) and the minimum fungicidal concentrations (MFC).
Preliminary antimicrobial testing results (Table 1) demonstrated that thiazole derivative 3 having 5-phenyl azo group was high active against the two gram + bacteria with IZs of 20 and 20 mm and has the same IZ (23 mm) as Ciprofloxacin in the case of E. cloacae. While, compound 4 with phenyl hydrazo group attached to 5-position of thiazole nucleus revealed promising antibacterial effect against E. coli and C. albicans with IZs of 22 and 19 mm and was moderate active against B. pumilis (IZ = 18 mm). Regarding pyridine derivatives 6, 8 and 9, it was observed that compound 8 with CF3 group attached to 2-position of pyridine ring showed high activity towards one bacteria and one fungi comparing with compounds 6 and 9 with CH3 and C=O groups which was high active against one of tested bacteria. Potent antimicrobial effects were observed for compound 12 with ester group attached to 4-position of pyrazole ring towards all the tested pathogenic microbes (IZs = 19–21 mm) except with C. albicans whereas, pyrimidine thione 14 was high active only towards one bacteria and one fungi.
MIC and MBC
The results for the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) (Table 2) indicated that compound 12 has the highest MIC of 7.69 (µmol/ml) for B. pumilis while other compounds revealed moderate MIC. Compounds 3, 4 and 12 exhibited high MIC of 14.34, 3.67 and 15.36 (µmol/ml), respectively for S. faecalis. For E. coli, all compounds showed high MIC except compounds 4 and 9 which have moderate MIC of 29.41 and 45.46 (µmol/ml), respectively. Moreover, in case of E. cloacae, all compounds revealed moderate MIC except compound 9 which showed high MIC of 22.76 (µmol/ml). In case of the minimum bactericidal concentrations (MBC), all compounds exhibited moderate MBC for the two gram + bacteria. While, for gram – bacteria all compounds showed moderate MBC except compound 8 which has MBC of 37.84 (µmol/ml) for E. coli.
MIC and MFC
The results for the minimum inhibitory concentrations (MIC) and the minimum fungicidal concentrations (MFC) were illustrated in Table 3. All compounds revealed moderate MIC values for both S. cerevisiae and C. albicans except compound 12 which showed the highest MIC of 15.36 (µmol/ml) for S. cerevisiae. On the other hand, all compounds exhibited moderate MFC for the two fungi except compound 3 which has the lowest MFC of 459.2 (µmol/ml).
Experimental protocols
General informations
All reagents and solvents were of commercial grade. Melting points of all the prepared coumarins were detected on an electro thermal apparatus and may be uncorrected. IR spectra were recorded using the Nicolet is 10 FTIR instrument in the wavenumber range of 4000–400 cm−1. Some of 1H and 13C NMR data were measured with a Bruker Avance spectrometer (Bruker, Germany) at 400 and 100 MHz and others were measured with a Jeol spectrometer (Japan) at 500 and 125 MHz, respectively, using TMS as the internal standard. TMS was used as the internal standard and hydrogen coupling patterns were described as singlet (s), doublet (d), triplet (t), quartet (q) and multiplet (m). Chemical shifts were defined as parts per million (ppm) relative to the solvent peak. Antimicrobial activity was performed at Department of microbiology, Faculty of Pharmacy (Boys), Al-Azhar University, Cairo 11754, Egypt.
General method for the synthesis of compounds 3 and 4
A mixture of thiosemicarbazone 1 (0.01 mol) and hydrazonoyl halides 2a, b (0.01 mol) in ethanol containing trimethylamine was refluxed for 2 h. The colored precipitated solid was filtered on hot and recrystallized from DMF afforded the desired compounds 3 and 4.
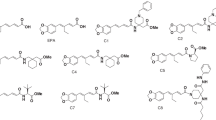
6-Bromo-3-{1-[(4-phenyl-5-phenylazo-thiazol-2-yl)-hydrazono]-ethyl}-chromen-2-one (3)
Orange powder, yield: 80%; m.p: 235–236 °C; IR (KBr) cm−1: 3429 (N–H), 3068, 2924 (CH), 1735 (C=O), 1643 (C=N), 1599 (C=C); 1H NMR: (400 MHz, DMSO-d6, δ, ppm): 2.37 (s, 3H), 7.41 (t, 2H, J = 8.8 Hz), 7.58–7.64 (m, 5H), 7.73 (t, 1H, J = 8 Hz), 7.79, 7.81 (dd, 1H, J = 1.6 Hz, J = 2.4 Hz), 8.02 (d, 2H, J = 9.2 Hz), 8.23–8.26 (m, 3H), 8.30 (s, NH); 13C NMR (125 MHz, DMSO-d6 δ, ppm): 17.90 (CH3), 116.82, 118.75, 121.26, 123.05, 123.39, 127.37, 128.03, 129.27, 129.71, 130.78, 131.95, 134.61, 134.80, 135.35, 139.21, 140.94, 150.35, 151.79, 153.09, 158.93, 159.43 (Ar–C), 183.16 (C=O). Anal. calcd. for C26H18BrN5O2S (544.42): C, 57.36; H, 3.33; Br, 14.68; N, 12.86; S, 5.89. Found: C, 57.43; H, 3.26; Br, 14.75; N, 12.80; S, 5.97.
(E)-2-(1-(6-bromo-2-oxo-2H-chromen-3-yl)ethylidene)hydrazine-1-carbimidic (Z)-2-ethoxy-2-oxo-N-phenylacetohydrazonic thioanhydride (4)
Yellow powder, yield: 77%; m.p: 209–210 °C; IR (KBr) cm−1: Broad 3428 (2N–H), 3050, 2921 (CH), 1736 (2C=O), 1599 (C=N), 1546 (C=C); 1H NMR: (400 MHz, DMSO-d6, δ, ppm): 1.31 (t, 3H, J = 7.6 Hz), 2.34 (s, 3H), 4.37 (q, 2H, J = 7.2 Hz), 7.23 (s, NH), 7.40–7.45 (m, 3H), 7.56 (t, 2H, J = 8.4 Hz), 7.79, 7.83 (dd, 2H, J = 8.8 Hz, J = 8.8 Hz), 7.95 (d, 1H, J = 8.9 Hz), 8.19 (s, 1H), 8.26 (s, NH), 8.59 (s, NH); 13C NMR (125 MHz, DMSO-d6 δ, ppm): 14.31, 17.89 (2CH3), 63.40 (CH2), 118.76, 118.94, 122.54, 122.85, 127.95, 129.08, 129.68, 131.93, 135.33, 139.12, 140.90, 141.17, 153.08, 153.48, 158.47, 159.11, 166.28 (Ar–C), 195.45 (2C=O). Anal. calcd. for C22H20BrN5O4S (530.39) C, 49.82; H, 3.80; Br, 15.07; N, 13.20; S, 6.05 Found: C, 49.78; H, 2.87; Br, 15.17; N, 13.11; S, 60.08.
General method for the synthesis of compounds 6–9
A mixture of enaminone 5 (0.01 mol) and each of ethyl acetoacetate, acetylacetone, trifloroacetylacetone or ethyl cyanoacetate (0.01 mol) in acetic acid containing ammonium acetate was heated under reflux for 2 h. The colored solid obtained on hot was filtered, washed with ethanol, dried and recrystallized from DMF/EtOH mixture yielding the title compounds 6–9.
6-(6-Bromo-2-oxo-2H-chromen-3-yl)-2-methyl-nicotinic acid ethyl ester (6)
Beige crystals, yield: 78%; m.p: 220–222 °C; IR (KBr) cm−1: 3059, 2986 (CH), 1740, 1715 (2C=O), 1612 (C=N), 1563 (C=C); 1H NMR: (400 MHz, DMSO-d6, δ, ppm): 1.34 (t, 3H, J = 7.2 Hz), 2.90 (s, 3H), 4.29 (q, 2H, J = 6.8 Hz), 7.28 (d, 1H, J = 8.8 Hz), 7.66, 7.68 (dd, 1H, J = 2 Hz, J = 2 Hz), 7.93 (s, 1H), 7.97 (d, 1H, J = 2 Hz), 8.19–8.24 (m, 1H), 8.85 (s, 1H); 13C NMR (100 MHz, DMSO-d6 δ, ppm): 14.53, 25.06 (2CH3), 61.62 (CH2), 116.83, 118.68, 121.38, 125.73, 132.13, 135.67, 139.44, 142.73, 152.74, 153.02 (Ar–C), 158.76, 166.21 (2C = O). Anal. calcd. for C18H14NBrNO4 (388.21) C, 55.69; H, 3.63; Br, 20.58; N, 3.61. Found: C, 55.60; H, 3.75; Br, 20.66; N, 3.69.
3-(5-Acetyl-6-methyl-pyridin-2-yl)-6-bromo-chromen-2-one (7)
Beige powder, yield: 82%; m.p: 230–233 °C; IR (KBr) cm−1: 3070, 2987 (CH) 1728, 1681 (2C=O), 1613 (C=N), 1562 (C=C); 1H NMR: (400 MHz, DMSO-d6, δ, ppm): 2.62 (s, 3H), 2.71 (s, 3H), 6.8 (d, 1H, J = 8.4 Hz), 7.39–7.53 (m, 1H), 7.79 (t, 1H, J = 9.2 Hz), 8.22–8.51 (m, 2H), 8.88 (s, 1H); 13C NMR (100 MHz, DMSO-d6 δ, ppm): 25.03, 29.97 (2CH3), 116.83, 118.68, 119.93, 121.32, 125.34, 127.02, 132.09, 135.35, 138.76, 142.49, 152.96, 157.39, 159.38 (Ar–C), 168.38, 170.41 (2C=O). Anal. calcd. for C17H12BrNO3 (358.19) C, 57.00; H, 3.38; Br, 22.31; N, 3.91. Found: C, 57.08; H, 3.29; Br, 22.41; N, 3.99.
3-(5-Acetyl-6-trifluoromethyl-pyridin-2-yl)-6-bromo-chromen-2-one (8)
Beige Powder, yield: 80%; m.p: 300 °C; IR (KBr) cm−1: 3066, 2922 (CH), 1731, 1682 (2C=O), 1645 (C=N), 1562 (C=C); 1H NMR: (300 MHz, DMSO-d6, δ, ppm): 2.43 (s, 3H), 6.86 (d, 1H, J = 6 Hz), 7.38 (d, 2H, J = 9 Hz), 7.79 (d, 1H, J = 8.7 Hz), 8.40 (d, 2H, J = 6 Hz). Anal. calcd. for C17H9BrF3NO3 (412.16) C, 49.54; H, 2.20; Br, 19.39; F, 13.83; N, 3.40. Found: C, 49.63; H, 2.29; Br, 19.49; F, 13.75; N, 3.47.
6-(6-Bromo-2-oxo-2H-chromen-3-yl)-2-oxo-1,2-dihydro-pyridine-3-carbonitrile (9)
Yellow powder, yield: 75%; m.p: above 300 °C; 1H NMR: (400 MHz, DMSO-d6, δ, ppm): 6.9 (d, 1H, J = 4.4 Hz), 7.39–7.49 (m, 1H), 7.81, 7.83 (dd, 2H, J = 2 Hz, J = 2.4 Hz), 7.95 (s, NH), 8.49 (s, 2H). Anal. calcd. for C15H7BrN2O3 (343.13) C, 52.50; H, 2.06; Br, 23.29; N, 8.16. Found: C, 52.58; H, 2.13; Br, 23.20; N, 8.23.
General method for the synthesis of pyrazoles 10a–c
To a mixture of the appropriate hydrazonoyl halide 2b–d (0.01 mol) and the enaminone 5 (0.01 mol) in dry benzene (10 ml), was added triethylamine (0.2 ml) and the mixture was refluxed for 2 h. The mixture was filtered on hot and let to cool, the precipitate obtained after cooling was washed with EtOH, dried and recrystallized from DMF/EtOH mixture.
4-(6-Bromo-2-oxo-2H-chromene-3-carbonyl)-1-phenyl-1H-pyrazole-3-carboxylic acid ethyl ester (10a)
Orange powder, yield: 80%; m.p: 150–152 °C IR (KBr) cm−1: 3137, 3060 (CH), 1745, 1720, 1645 (3C=O), 1603 (C=N), 1556 (C=C); 1H NMR: (400 MHz, DMSO-d6, δ, ppm): 1.16 (t, 3H, J = 6.8 Hz), 4.20 (q, 2H, J = 6.8 Hz), 7.36–8.09 (m, 7H), 8.17 (s, 1H), 8.24 (s, 1H), 8.56 (s, 1H). Anal. calcd. for C22H15BrN2O5 (467.27) C, 56.55; H, 3.24; Br, 17.10; N, 6.00. Found: C, 56.44; H, 3.32; Br, 17.19; N, 6.11.
3-(3-Acetyl-1-phenyl-1H-pyrazole-4-carbonyl)-6-bromo-chromen-2-one (10b)
Yellow crystal, yield: 82%; m.p: 232–233 °C IR (KBr) cm−1: 3248, 3151 (CH), 1739, 1681, 1634 (3C=O), 1603 (C=N), 1554 (C=C); 1H NMR: (500 MHz, DMSO-d6, δ, ppm): 2.54 (s, 3H), 7.44–7.57 (m, 5H), 7.87–8.16 (m, 3H), 8.48 (s, 1H), 9.20 (s, 1H); 13C NMR (125 MHz, DMSO-d6 δ, ppm): 27.83 (CH3), 116.99, 119.09, 120.03, 120.80, 123.96, 128.18, 128.76, 130.35, 132.71, 134.30, 136.74, 138.86, 144.45, 149.95, 153.74, 158.36 (Ar–C), 184.74, 194.51 (3C=O). Anal. calcd. for C21H13BrN2O4 (437.24) C, 57.69; H, 3.00; Br, 18.27; N, 6.41. Found: C, 57.77; H, 2.91; Br, 18.35; N, 6.49.
4-(6-Bromo-2-oxo-2H-chromene-3-carbonyl)-1-(4-nitro-phenyl)-1H-pyrazole-3-carboxylic acid ethyl ester (10c)
Beige powder, yield: 75%; m.p: 220–222 °C 1H NMR: (500 MHz, DMSO-d6, δ, ppm):1.35 (t, 3H, J = 6.65 Hz), 4.44 (q, 2H, J = 6.7 Hz), 7.33 (s, 1H), 7.48 (d, 1H, J = 8.55 Hz), 7.64–7.72 (m, 5H), 8.55 (s, 1H), 9.15 (s, 1H); 13C NMR (125 MHz, DMSO-d6 δ, ppm): 14.61 (CH3), 62.42 (CH2), 117.00, 117.28, 119.68, 124.28, 127.02, 128.09, 128.50, 129.37, 133.26, 134.99, 137.52, 138.63, 150.80, 151.80 (Ar–C), 161.98 (3C=O). Anal. calcd. for C22H14BrN3O7 (512.27) C, 51.58; H, 2.75; Br, 15.60; N, 8.20. Found: C, 51.49; H, 2.80; Br, 15.66; N, 8.12.
Synthesis of 5-[3-(6-bromo-2-oxo-2H-chromen-3-yl)-3-oxo-propenylamino]-1-phenyl-1H-pyrazole-4-carboxylic acid ethyl ester (12)
A mixture of enaminone 5 (0.01 mol) and 5-Amino-1-phenyl-1H-pyrazole-4-carboxylic acid ethyl ester (11) (0.01 mol) in 10 ml ethanol and few drops of acetic acid was heated in reflux for 2 h. The solid that is separated on hot was filtered, dried and recrystallized from DMF. Brown powder, yield: 79%; m.p: 120–121 °C; IR (KBr) cm−1: 3396 (N–H), 3074, 2989, 2944 (CH), 1730, 1682 (3C=O), 1622 (C=N), 1556 (C=C); 1H NMR: (400 MHz, DMSO-d6, δ, ppm): 1.26 (t, 3H, J = 6.8 Hz), 4.19 (q, 2H, J = 6.8 Hz), 6.33 (s, 2H), 7.38–8.21 (m, 10H), 8.59 (s, NH); 13C NMR (100 MHz, DMSO-d6 δ, ppm): 14.93 (CH3), 59.45 (CH2), 95.22, 116.54, 116.82, 118.59, 118.81, 120.47, 121.15, 124.05, 127.97, 129.91, 132.97, 137.01, 138.33, 140.62, 146.10, 150.19, 153.32 (Ar–C), 154.06, 158.76, 164.03 (3C=O). Anal. calcd. for C24H18BrN3O5 (508.32): C, 56.71; H, 3.57; Br, 15.72; N, 8.27. Found: C, 56.80; H, 3.66; Br, 15.82; N, 8.36.
Synthesis of 5-(6-bromo-2-oxo-2H-chromen-3-yl)-2-thioxo-2,3-dihydro-1H-pyrido[2,3-d]pyrimidin-4-one (14)
A mixture of enaminone 5 (0.01 mol) and 6-aminothiouracil 14 (0.01 mol) in glacial acetic acid was heated in reflux for 2 h. The solid that is separated on hot was filtered, dried and recrystallized from DMF. Orange powder; yield: 77%; m.p: above 300 °C; IR (KBr) cm−1: 3425, 3320 (2N-H), 3072, 2902 (CH), 1733, 1675 (2C=O), 1610 (C=N), 1557 (C=C), 1172 (C=S); 1H NMR: (500 MHz, DMSO-d6, δ, ppm): 7.41 (d, 1H, J = 8.55 Hz), 7. 80 (s, 1H), 8.03–8.09 (m 2H), 8.34, 8.95 (2 s, 2H), 12.56, 13.11 ppm (s, 2NH); 13C NMR (125 MHz, DMSO-d6 δ, ppm): 112.38, 117.05, 118.93, 120.56, 121.10, 125.16, 131.92, 136.18, 137.73, 143.60, 151.67, 153.23, 156.00, 159.01 (Ar–C), 162.83, 176.6 (2C=O). Anal. calcd. for C16H8BrN3O3S (402.22) C, 47.78; H, 2.00; Br, 19.87; N, 10.45; S, 7.97. Found: C, 47.72; H, 2.06; Br, 19.81; N, 10.49; S, 7.94.
Biological studies
Antimicrobial activity
Evaluation of the antimicrobial activity of the prepared compounds was performed against four bacteria and two fungal species using the agar plate diffusion method57. Penicillin G and ciprofloxacin were used as reference drugs for gram positive and gram negative bacteria, while ketoconazole was used as standard antifungal drug.
MIC Measurement
The microdilution method (broth dilution)58 was performed to measure the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC). Two fold serial dilutions of the test compounds (up to 10) and one quality control (QC) antibiotic in a microdilution plate. (Start with 1000 µg, 500 µg, 250 µg, 125 µg, 62.5 µg, 31.3 µg, 7.81 µg, 3.91 µg and 1.95 µg/ml). Create the inoculum by taking a few colonies from an agar plate with a sterile swab, prepare overnight broth then from broth preparing a McFarland standard (half McFarland ), and diluting the McFarland standard into media. (With Optical Density 0.1 at wavelength 580 nm). Dispense the inoculum into the microdilution plate with the serial diluted test compounds and incubate the microdilution plate overnight. Read the microdilution plate to determine the MIC value. Plate a portion of each well on an appropriate agar media, incubate the agar, and check for colonies to determine the MBC and MFC. The MIC was defined as the lowest concentration of the compound at which no visible growth occurred after 48 h of inoculation. The MBC showing the lowest concentration at which no visible growth occurred after 96 h of inoculation.
Conclusions
This study describes the activity of new coumarin-based thiazoles, pyridines and pyrazoles as antimicrobial agents. The newly synthesized compounds were screened for in vitro antimicrobial activity against two gram + bacteria, two gram – bacteria as well as two fungal strains. Compound 12 was found to be the most potent compound against B. pumilis with MIC of 7.69 (µmol/ml). Compounds 3, 4 and 12 showed remarkable activity against S. faecalis with MIC of 14.34, 3.67 and 15.36 (µmol/ml), respectively. Regarding E. coli, most compounds recorded high to moderate MIC values (4.73–45.46 µmol/ml). Moreover, in case of E. cloacae compound 9 was the most potent compound with MIC value of 22.76 (µmol/ml).
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information file.
References
Manandhar, S., Luitel, S. & Dahal, R. K. In vitro antimicrobial activity of some medicinal plants against human pathogenic bacteria. J. Trop. Med. 2019(1895340), 2019. https://doi.org/10.1155/2019/1895340 (2019).
Dadgostar, P. Antimicrobial resistance: Implications and costs. Infect. Drug Resist. 12, 3903–3910 (2019).
Wang, J., Ansari, M. F., Lin, J. M. & Zhou, C. H. Design and synthesis of sulfanilamide aminophosphonates as novel antibacterial agents towards Escherichia coli. Chin. J. Chem. 39, 2251–2263. https://doi.org/10.1002/cjoc.202100165 (2021).
Xie, Y. P., Ansari, M. F., Zhang, S. L. & Zhou, C. H. Novel carbazole-oxadiazoles as potential Staphylococcus aureus germicides. Pestic. Biochem. Physiol. 175, 104849. https://doi.org/10.1016/j.pestbp.2021.104849 (2021).
Yang, X. C. et al. Coumarin thiazoles as unique structural skeleton of potential antimicrobial agents. Bioorg. Chem. 124, 105855. https://doi.org/10.1016/j.bioorg.2022.105855 (2022).
Reygaert, W. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 4, 482–501 (2018).
Manukumar, H. M. et al. Novel T-C@AgNPs mediated biocidal mechanism against biofilm associated methicillin-resistant Staphylococcus aureus (Bap-MRSA) 090, cytotoxicity and its molecular docking studies. MedChemComm 8, 2181–2194 (2017).
Kawase, M. et al. Antimicrobial activity of new coumarin derivatives. Arzneimittel-Forschung/Drug Res. 51, 67–71 (2001).
Zha, G. F. et al. Benzimidazole analogues as efficient arsenals in war against methicillin-resistance staphylococcus aureus (MRSA) and its SAR studies. Bioorg. Chem. 115, 105175 (2021).
Ravindar, L. et al. Aryl fluorosulfate analogues as potent antimicrobial agents: SAR, cytotoxicity and docking studies. Bioorg. Chem. 81, 107–118 (2018).
Verma, S. K. et al. A key review on oxadiazole analogs as potential methicillin-resistant Staphylococcus aureus (MRSA) activity: Structure-activity relationship studies. Eur. J. Med. Chem. 219, 113442 (2021).
Sarker, S. D. & Nahar, L. Progress in the chemistry of naturally occurring coumarins. Prog. Chem. Org. Nat. Prod. 106, 241–304 (2017).
Adimule, V. M., Nandi, S. S., Kerur, S. S., Khadapure, S. A. & Chinnam, S. Recent Advances in the One-Pot Synthesis of Coumarin Derivatives from Different Starting Materials Using Nanoparticles: A Review Topics in Catalysis (Springer, 2022). https://doi.org/10.1007/s11244-022-01571-z.
Sahoo, C. R. et al. Coumarin derivatives as promising antibacterial agent(s). Arab. J. Chem. 14, 102922 (2021).
Gouda, M. A. et al. Recent progress on coumarin scaffold-based anti-microbial agents (Part III). J. Heterocycl. Chem. 57, 3784–3817 (2020).
Hu, X.-L., Xu, Z., Liu, M.-L., Feng, L.-S. & Zhang, G.-D. Recent developments of coumarin hybrids as anti-fungal agents. Curr. Top. Med. Chem. 17, 3219–3231 (2017).
Xu, Z., Chen, Q., Zhang, Y. & Liang, C. Coumarin-based derivatives with potential anti-HIV activity. Fitoterapia 150, 104863 (2021).
Liu, Y. P. et al. Prenylated coumarins from the fruits of manilkara zapota with potential anti-inflammatory effects and anti-HIV activities. J. Agric. Food Chem. 67, 11942–11947 (2019).
Antonijević, M. R. et al. Green one-pot synthesis of coumarin-hydroxybenzohydrazide hybrids and their antioxidant potency. Antioxidants 10, 1106 (2021).
Kraimi, A., Belboukhari, N., Sekkoum, K. & Aboul-Enein, H. Y. Chiral anticoagulants drugs based on coumarin. Aditum J. Clin. Biomed. Res. 2, 1 (2021).
MMS-HM Journal. The antioxidant and anticoagulant effects of coumarin and quercetin from cinnamon methanolic extract, and the assessment of cinnamon powder effect on plasma. Eprints. Lums. Ac. Ir 4, 103–113 (2019).
SandraLiliana, P. D. et al. Isolation, chemical characterization, and anti-inflammatory activity of coumarins, flavonoids, and terpenes from Tagetes lucida. Nat. Prod. Res. 36, 4751–4756 (2022).
Fattah, T. A. et al. Functionalized furo[3,2-c]coumarins as anti-proliferative, anti-lipolytic, and anti-inflammatory compounds: Synthesis and molecular docking studies. J. Mol. Struct. 1179, 390–400 (2019).
Keri, R. S., Budagumpi, S. & BalappaSomappa, S. Synthetic and natural coumarins as potent anticonvulsant agents: A review with structure–activity relationship. J. Clin. Pharm. Ther. 47, 915–931 (2022).
Mohammadi-Khanaposhtani, M. et al. Design, synthesis, in vivo, and in silico evaluation of new coumarin-1,2,4-oxadiazole hybrids as anticonvulsant agents. Bioorg. Chem. 89, 102989 (2019).
Rawat, A. & Vijaya Bhaskar Reddy, A. Recent advances on anticancer activity of coumarin derivatives. Eur. J. Med. Chem. Rep. 5, 100038 (2022).
Menezes, J. C. J. M. D. S. & Diederich, M. Translational role of natural coumarins and their derivatives as anticancer agents. Future Med. Chem. 11, 1057–1082 (2019).
Trommenschlager, A. et al. Gold(I)–coumarin–caffeine-based complexes as new potential anti-inflammatory and anticancer trackable agents. ChemMedChem 13, 2408–2414 (2018).
Mishra, S., Pandey, A. & Manvati, S. Coumarin: An emerging antiviral agent. Heliyon 6, e03217 (2020).
Jathar, L. V., Kulkarni, M., Himatsinghani, D., Ajwani, N. & Achalawat, D. G. Comparative study of coumarin-120 (C-120) and stilbine-3 (STB-3) laser dyes doped in sol-gel glasses. New J. Glas. Ceram. 11, 57–82 (2021).
Hroboňová, K. & Špačková, A. Application of selective polymeric sorbents for simple coumarins extraction from deodorant samples. Acta Chim. Slovaca 13, 56–62 (2020).
Sun, X. Y., Liu, T., Sun, J. & Wang, X. J. Synthesis and application of coumarin fluorescence probes. RSC Adv. 10, 10826–10847 (2020).
Abdallah, M., Hijazi, A., Dumur, F. & Lalevée, J. Coumarins as powerful photosensitizers for the cationic polymerization of epoxy-silicones under near-UV and visible light and applications for 3D printing technology. Molecules 25, 2063 (2020).
Lončar, M. et al. Coumarins in food and methods of their determination. Foods 9, 645 (2020).
Mohareb, R. M. & Abdo, N. Y. M. Synthesis and cytotoxic evaluation of pyran, dihydropyridine and thiophene derivatives of 3-acetylcoumarin. Chem. Pharm. Bull. 63, 678–687. https://doi.org/10.1248/cpb.c15-00115 (2015).
Mamidala, S. et al. Microwave irradiated one pot, three component synthesis of a new series of hybrid coumarin based thiazoles: Antibacterial evaluation and molecular docking studies. J. Mol. Struct. 1225, 129114 (2021).
Singh, I. P., Gupta, S. & Kumar, S. Thiazole compounds as antiviral agents: An update. Med. Chem. 16, 4–23 (2020).
Reddy, G. M. et al. Synthesis, antimicrobial activity and advances in structure-activity relationships (SARs) of novel tri-substituted thiazole derivatives. Eur. J. Med. Chem. 123, 508–513 (2016).
Eryılmaz, S. et al. Derivatives of pyridine and thiazole hybrid: Synthesis, DFT, biological evaluation via antimicrobial and DNA cleavage activity. Bioorg. Chem. 95, 103476 (2019).
Ramagiri, R. K., Vedula, R. R. & Thupurani, M. K. A facile one-step multi-component approach toward the synthesis of 3-(2-amino-4-thiazolyl)coumarins by using trimethylsilyl isothiocyanate and their antioxidant and anti-inflammatory activity. Phosphor. Sulfur. Silicon Relat. Elem. 190, 1393–1397 (2015).
Zia, M., Akhtar, T., Hameed, S. & Al-Masoudi, N. A. New aryl-1,3-thiazole-4-carbohydrazides, their 1,3,4-oxadiazole-2-thione, 1,2,4-triazole, isatin-3-ylidene and carboxamide derivatives. Synthesis and anti-HIV activity. Z. Nat. B 67, 747–758 (2012).
Prashanth, T. et al. Synthesis of coumarin analogs appended with quinoline and thiazole moiety and their apoptogenic role against murine ascitic carcinoma. Biomed. Pharmacother. 112, 108707 (2019).
Anuradha, et al. Design, computational studies, synthesis and biological evaluation of thiazole-based molecules as anticancer agents. Eur. J. Pharm. Sci. 134, 20–30 (2019).
Pardo-Jiménez, V., Navarrete-Encina, P. & Díaz-Araya, G. Synthesis and biological evaluation of novel thiazolyl-coumarin derivatives as potent histone deacetylase inhibitors with antifibrotic activity. Molecules 24, 739 (2019).
Abdel-Aziem, A., Baaiu, B. S., Elbazzar, A. W. & Elabbar, F. A facile synthesis of some novel thiazoles, arylazothiazoles, and pyrazole linked to thiazolyl coumarin as antibacterial agents. Synth. Commun. 50, 2522–2530 (2020).
Bensalah, D. et al. Synthesis and antioxidant properties of some new thiazolyl coumarin derivatives. Green Chem. Lett. Rev. 13, 155–163 (2020).
Ngoc Toan, V. & Dinh Thanh, N. Novel thiazoline-coumarin hybrid compounds containing sugar moieties: Synthesis, biological evaluation and molecular docking study as antiproliferative agents. New J. Chem. 45, 10636–10653 (2021).
Osman, H. et al. New thiazolyl-coumarin hybrids: Design, synthesis, characterization, X-ray crystal structure, antibacterial and antiviral evaluation. J. Mol. Struct. 1166, 147–154 (2018).
Babaei, E. et al. Novel coumarin-pyridine hybrids as potent multi-target directed ligands aiming at symptoms of Alzheimer’s disease. Front. Chem. 10, 1–13 (2022).
Dorababu, A. Coumarin-heterocycle framework: A privileged approach in promising anticancer drug design. Eur. J. Med. Chem. Rep. 2, 100006 (2021).
Kenchappa, R. et al. Synthesis of some 2, 6-bis (1-coumarin-2-yl)-4-(4-substituted phenyl) pyridine derivatives as potent biological agents. Arab. J. Chem. 10, S1336–S1344 (2017).
Fayed, E. A., Sabour, R., Harras, M. F. & Mehany, A. B. M. Design, synthesis, biological evaluation and molecular modeling of new coumarin derivatives as potent anticancer agents. Med. Chem. Res. 28, 1284–1297 (2019).
Arshad, A. et al. Synthesis and antimicrobial properties of some new thiazolyl coumarin derivatives. Eur. J. Med. Chem. 46, 3788–3794 (2011).
Gomha, S. M., Zaki, Y. H., Abdelhamid, A. O. & Bunce, R. A. Utility of 3-acetyl-6-bromo-2H-chromen-2-one for the synthesis of new heterocycles as potential antiproliferative agents. Molecules 20, 21826–21839 (2015).
Gomha, S. M. & Abdel-Aziz, H. A. Enaminones as building blocks in heterocyclic preparations: Synthesis of novel pyrazoles, pyrazolo-[3,4-d]pyridazines, pyrazolo[1,5-a]pyrimidines, pyrido[2,3-d]pyrimidines linked to imidazo[2,1-b]thiazole system. Heterocycles 85, 2291–2303 (2012).
Li, J. F., Zhu, Y. Q., Wang, X. & Yang, H. Z. Synthesis and herbicidal activities of a series of di(aminopyrazoly) ketone derivatives. J. Heterocycl. Chem. 44, 749–755 (2007).
Bauer, A. W., Kirby, W. M., Sherris, J. C. & Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 45, 493–496 (1966).
Barchiesi, F. et al. EUCAST Discussion Document E.Dis 7.1 June 2002. Method for the determination of minimum inhibitory concentration (MIC) by broth dilution of fermentative yeasts. Clin. Microbiol. Infect. 1, 1–8 (2003).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
S.A.E. and A.A., conceptualization of research topics and formulation of specific aims; M.T.S., performed the synthesis; A.A. analyzed the data and wrote/edited the manuscript. All authors have agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sayed, M.T., Elsharabasy, S.A. & Abdel-Aziem, A. Synthesis and antimicrobial activity of new series of thiazoles, pyridines and pyrazoles based on coumarin moiety. Sci Rep 13, 9912 (2023). https://doi.org/10.1038/s41598-023-36705-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-36705-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.