Abstract
Morchella esculenta is an edible fungus with a uniquely delicious flavor and remarkable benefits for health. Herein, the molecular mechanism underlying the anti-inflammatory effects of Morchella esculenta was elucidated using molecular docking and network pharmacology. NPASS, Super-pred, SEA, Swiss Target Prediction, GeneCards, DisGeNET, Omim database, and STRING platform were used to select anti-inflammatory targets and construct target protein interaction networks using the active ingredients of Morchella esculenta. The OmicShare cloud platform was used to analyze GO functions and KEGG pathways related to the target, and the AutoDock Vina software was used to perform molecular docking and molecular dynamics (MD) simulation on the main target. Based on Cytoscape’s “Network Analysis”, the degree was used to identify potential key targets, and different inflammatory transcriptome data sets were used to evaluate core targets showing clinical significance. The active ingredient of Morchella esculenta identified from the NPASS database was EOYA, which had 43 anti-inflammatory targets, including NR1I2, PTGS1, PTGS2, CYP4F2, CYP3A4, TLR4, MAPK1, PLA2G4A, and PTPN11, and was mainly implicated in arachidonic acid metabolism, vascular endothelial growth factor signal pathway, and sphingomyelin signal transduction pathway, indicating that the anti-inflammatory effects of EOYA were mainly related to these biological processes. The degree was used to select 9 potential effective targets, namely NR1I2, PTGS1, PTGS2, CYP4F2, CYP3A4, TLR4, MAPK1, PLA2G4A, and PTPN11, among which NR1I2, PTGS1, PTGS2, PLA2G4A, MAPK1, CYP3A4, and TLR4 showed clinical significance. Molecular docking results showed that (E)-Octadec-11-En-9-Ynoic Acid (EOYA) could spontaneously bind to the 9 core targets, and the binding fractions of NR1I2, PTGS1, PTGS2, CYP4F2, and CYP3A4 were the highest. The MD simulation results showed that EYOA did indeed bind well NR1I2 to PTGS2, and the complex has high stability. Morchella esculenta can regulate the activity of prostaglandin endoperoxide synthetase, and affect the biosynthesis of prostaglandins, thereby impacting the metabolic pathway of arachidonic acid.
Similar content being viewed by others
Introduction
Inflammation, mediated by inflammatory cytokines and immune cells, is a defense response to injury, infection, allergy, and necrosis1. It is characterized by fever, redness, pain, and swelling2. Inflammatory responses are essential for defense against external stimuli and critical to the healing process3. However, chronic inflammation is associated with several diseases, including cancer, arthritis, cardiovascular disease, atherosclerosis, nervous system disorders, and autoimmune diseases4. Identifying promising non-toxic anti-inflammatory drugs is a target area of biomedical research. Aspirin, diclofenac, and other commonly used drugs for treating inflammation have serious side effects. Thus, anti-inflammatory agents based on natural products have become popular in recent years5.
Morchella esculenta is a delicious edible fungus with a unique flavor and remarkable health benefits6, and it contains various nutrients and exerts anti-oxidation, anti-inflammatory, and anti-tumor pharmacological activities7. Morchella esculenta produces several primary and secondary metabolites, including proteins, polysaccharides, sterols, polyphenols, alkaloids, fatty acids, flavonoids, and other small molecules6. A heteropolysaccharide isolated from the fruiting body of Morchella esculenta has an anti-inflammatory role as it blocks the activation of the NF-κB signaling pathway8. Simultaneously, total flavonoids of Morchella-fermented liquid show anti-inflammatory effects in RAW264.7 macrophages stimulated by lipopolysaccharide9. The hot water–ethanol extract of Morchella mycelia has an obvious inhibitory effect on acute and chronic inflammation10. EOYA is a natural octadecyl-conjugated enyne acid, wherein the special enyne-conjugated structure endows it with many biological activities, such as antibacterial11, antifungal12, larvicidal13 and anti-inflammatory14,15 properties.
In recent years, basic research on the active substances of traditional Chinese medicines has gained traction. To better clarify the pharmacological mechanisms underlying traditional Chinese medicine in the treatment of diseases, computational methods like molecular docking, network pharmacology, and dynamic simulation have emerged as effective tools16,17. At present, most of the researches on network pharmacology are Chinese herbal medicines and prescriptions18,19,20, but there are few studies on edible and medicinal fungi.The pharmacological activity of cordycepin, the active component of Cordyceps militaris, was studied by means of network pharmacology and molecular docking, and the core targets for improving Alzheimer's disease were screened21. However, there is no related research on Morchella esculenta.Herein, we employed network pharmacology to assess the anti-inflammatory targets of the active ingredient of Morchella esculenta, EOYA, and conducted protein interaction analysis, GO function analysis, and KEGG pathway analysis of these targets. We also used the GEO database to evaluate the clinical significance of the core targets and performed molecular docking and molecular dynamics (MD) simulation verification for the core targets to comprehensively elucidate the molecular mechanism underlying the anti-inflammatory effects of the active ingredient of Morchella esculenta.
Target selection of active components from Morchella esculenta
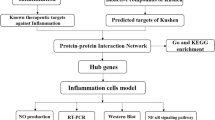
In the NPASS database (http://bidd.group/NPASS/), “Morchella esculenta” was entered as the organism to search for the active ingredients of morel. In the Superpred (http://prediction.charite.de/), Swiss Target Prediction (http://swisstargetprediction.ch/), and SEA (http://sea.bkslab.org/) databases, the “SMILES” value of the active ingredient of Morchella esculenta was entered; the structure diagram of the substance was drawn, and the target of the active ingredient was assessed. The target of the active ingredient of Morchella esculenta was obtained by combining and removing the targets obtained from the above three databases.
Selection of anti-inflammatory targets and construction of target network using the active components of Morchella esculenta
The keyword “anti-inflammatory” was entered in DisGeNET (www.disgenet.org/), GeneCards (www.genecards.org/), and Omim databases (https://www.omim.org/) to search for related targets, which were scored according to the photographic significance. Subsequently, the anti-inflammation targets of the three databases were combined to obtain significant anti-inflammation targets. The disease targets and the active ingredient targets of Morchella esculenta were input into Venny2.1.0 (https://bioinfogp.cnb.csic.es/tools/venny/), and the intersection of the two yielded the anti-inflammation targets of the active ingredient of Morchella esculenta, which were together imported into the Cytoscape 3.9.1 software to build the active ingredient_anti-inflammation target network.
Construction of an anti-inflammatory target interaction network (PPI) using active components of Morchella esculenta
The anti-inflammatory targets of the active ingredient of Morchella esculenta obtained as described above were imported into STRING (www.string-db.org/) to construct the protein–protein interaction networks (PPI). The organism was set as “Homo Sapiens”; the minimum interaction threshold value was set at “medium confidence = 0.4”, and default values were retained for other parameters. The PPI network was imported into the Cytoscape 3.9.1 software, and the “cytoHubba” function was used to calculate the degree, betweenness, closeness, and other topological parameters for each network node. The greater the betweenness, closeness, and degree of the node, the more important the position of the node in the network. The targets with a degree, betweenness, and closeness above the corresponding average values were selected as the main anti-inflammation target of the active ingredient of Morchella esculenta.
GO function and KEGG pathway enrichment analysis for anti-inflammatory targets of Morchella esculenta
The anti-inflammatory targets of the active ingredient of Morchella esculenta were analyzed by GO and KEEG analyses using the DAVID web tool (https://david.ncifcrf.gov/), and the obtained data were further analyzed by OmicShare (http://omicshare.com/) cloud platform for assessing dynamic GO enrichment. The UNIPROT website (www.uniprot.org/) was used to convert the anti-inflammatory targets of the active ingredient of Morchella esculenta into corresponding Gene Symbols, and the OmicShare cloud platform was used to conduct KEGG pathway (www.kegg.jp/kegg/kegg1.html) enrichment analysis for the anti-inflammatory targets of the active ingredient of Morchella esculenta.
Clinical characteristics and tissue enrichment of key targets
The transcriptome data for inflammation were retrieved through the GEO database (https://www.ncbi.nlm.nih.gov/genome) to obtain the clinical significance of core targets. The differences in core target gene expression before and after inflammation were analyzed. Graphpad prism8 was used to draw a box chart. The Human eFP Browser (http://bar.utoronto.ca/efp_human/cgi-bin/efpWeb.cgi)22 was used to study the distribution of expression of the key targets, and the whole-body expression distribution map was obtained.
Molecular docking verification of active components in Morchella esculenta and the major anti-inflammatory targets
The 2D structure of the active ingredient of Morchella esculenta was obtained from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/), and the structure of ligand was sketched, hydrogens added, energy minimized and saved as mol2 files using the Chem3D software for storage. The target protein complex was searched in the PDB database (http://www.rcsb.org/), We manually curated the PDB to choose structures that described continuous, well resolved (resolution less than 2.5 Å), single-chain proteins and ligand binding. and the Discovery studio software was used to remove phosphate radicals, water molecules, and inactive ligands from the target protein. The target protein was imported into AutoDock23 Tools software for hydrogenation and charging. Subsequently, the target protein and active components of Morchella esculenta were introduced into the molecular docking website, CB-DOCK2 (CB-Dock2: An accurate protein–ligand blind docking tool (labshare.cn)) for molecular docking, and the docking combination with the lowest binding free energy was selected. The receptor-ligand binding image was visualized using the Pymol (2.5.0) software (www.pymol.org/)24.
Molecular dynamics (MD) simulation
Molecular dynamics (MD) simulation of ligand-receptor docked complex EYOA-NR1I2 and EYOA-PTGS2 with the lowest Binding Free Energy were carried out using GROMACS25 (version 2021.2). Protein topology file was generated using the AMBER99SB-ILDN force field, whereas ligand topology file was generated by ACPYPE script using the AMBER fore field. MD simulation carried out in a triclinic box filled with TIP3 water molecules and periodic bounding conditions were applied. System was neutralized with NaCl counter ions. Before MD simulation, the complex was minimized for 1000 steps and equilibrated by running NVT and NPT for 100 ps. Then MD simulation was performed for 100 ns for each system under periodic boundary conditions at 310 K temperature and 1.0 bar pressure.
Results
Identification of the anti-inflammatory target of active ingredients in Morchella esculenta
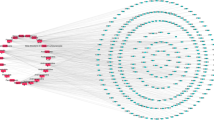
The active ingredient of “Morchella esculenta” according to the NPASS database was (E)-Octadec-11-En-9-Yinoic Acid (EOYA) (Fig. 1), numbered NPC179764, with a chemical formula of C18H30O2, and ‘SMILE’ value, CCCCCC/C=C/C#CCCCCCCCC(=O)O. The targets obtained by searching the “EOYA Smile Value” in the Super-pred, SEA, Swiss Target Prediction databases were merged and de-weighed, and 107 targets of EOYA were obtained. A total of 1654 anti-inflammation-related targets were obtained from GeneCards, DisGeNET, and Omim databases. The EOYA and anti-inflammatory targets were imported into the Venny 2.1.0, and 43 EOYA–anti-inflammatory targets were obtained after de-duplication (Fig. 2).
Establishing the anti-inflammatory-target PPI Network using active components in Morchella esculenta
EOYA and its 43 anti-inflammation targets were imported into the Cytoscape 3.9.1 software to obtain the EOYA anti-inflammation target network (Fig. 3). The PPI network of anti-inflammatory targets is shown in Fig. 3. Each target participating in the interaction is represented by a circle. The larger the circle area, the greater its degree. The thicker the circle’s border, the greater betweenness. The darker the circle, the greater its closeness. There witsere 43 nodes and 104 interactions in the PPI network, with an average degree, betweenness, and closeness of 4.84, 59.63, and 17.70, respectively. The simultaneous measures of degree, betweenness, and closeness above the average yielded 9 targets (Table 1), indicating their key position in the PPI network and role as the main targets of EOYA responsible for its anti-inflammatory effect. Nine main targets, PTGS2, TLR4, MAPK1, PLA2G4A, CYP3A4, CYP4F2, PTGS1, NR1I2, and PTPN11 were analyzed. MAPK1, PTPN11, and TLR4 were involved in inflammation; PTGS1, PLA2G4A, CYP3A4, CYP4F2, PTGS1, and NR1I2 were involved in arachidonic acid-related pathways, and CYP3A4, PTGS2, NR1I2, and PTGS1 were involved in the synthesis of prostaglandins.
GO function and enrichment analysis for the anti-inflammatory target of Morchella esculenta
The DAVID database was used to obtain enrichment data for 43 targets, and the dynamic GO enrichment analysis based on the OmicShare cloud platform was used to select 322 GO entries with P < 0.05, including 22 molecular functions, 24 cellular components, and 276 biological processes. As shown in Fig. 4, biological processes mainly included biosynthesis, cell reaction, cell metabolism, and signal transduction; molecular functions included binding, enzyme activity, and regulation, and the cellular components included organelles, vesicles, and extracellular matrix. The target functions were enriched in prostaglandin biosynthesis, prostaglandin endoperoxide synthetase activity, and cell response to angioprogressive hormone, indicating that the anti-inflammatory effects of EOYA were mainly related to these biological processes.
KEGG pathway enrichment analysis for the anti-inflammatory targets of the active component in Morchella esculenta
Using P < 0.001, 20 KEGG pathways related to the anti-inflammatory targets of EOYA, were selected. Figure 5 shows the enrichment bubble diagram for each pathway. The enrichment fraction of the target was the degree of EOYA enrichment for the target in a pathway and all the involved proteins. The greater the value, the higher the enrichment degree. The color represents the P-value. The redder the color, the smaller the P-value, and the more significant the enrichment. The size of the point indicates the number of enrichment targets in the pathway. The larger the point, the more the number of enrichment targets. The pathways with large target values mainly included arachidonic acid metabolism, vascular endothelial growth factor signal pathway, and sphingomyelin signal transduction pathway. Among them, the arachidonic acid metabolism26 pathway (Fig. 6) showed the greatest number of enriched targets, the highest target value, and the lowest P-value.
Expression of anti-inflammatory core genes targeted by the active components of Morchella esculenta
The data set of inflammation was screened according to the significant difference in the expression of core targets. Using the transcription data of the dermatitis GSE153007 dataset, neuroinflammation GSE135511 dataset, rhinitis GSE19187 dataset, pneumonia GSE35716 dataset, hepatitis GSE83148 dataset, and gastritis GSE60427 dataset, the corresponding expressions of the nine core genes were analyzed. As shown in Figs. 7, 8, 9, 10, 11, 12, in the transcriptome data of contact dermatitis, the expression of CYP4F2 reduced significantly. The expressions of PTGS1 and CYP3A4 were markedly increased in patients with rhinitis accompanied by asthma, while the expression of NR1I2 decreased synonym. The mRNA expressions of PLA2G4A and PTGS2 in neuritis causing multiple cerebral infarctions increased significantly as compared to those in normal subjects. In cases of bacterial pneumonia, the mRNA expression of MAPK1 and CYP3A4 increased significantly, while those of PTGS1 and PTGS2 decreased synonym. In HBV-induced hepatitis, the expressions of MAPK1, PTGS1, PTGS2, PLA2G4A, and TLR4 increased significantly. On comparing the transcriptome of patients with gastric inflammation and normal individuals, mRNA expression of TLR4 was found to be synonym increased in the former group.
The expressions of NR1I2, PTGS1, PTGS2, CYP4F2, and CYP3A4 were assessed to analyze the levels of core genes in the skeletal, immune, and digestive systems. The core gene expressions were different throughout the body. As shown in Fig. 13A–E, the expressions of NR1I2, PTGS1, CYP4F2, and CYP3A4 was ubiquitous in the skeletal, immune, and digestive systems of normal individuals. The expression of NR1I2 was the highest in the liver, while that of PTGS1 was the highest in smooth muscles. CYP4F2 and CYP3A4 were mainly expressed in the liver, mandible, and hindbrain. PTGS2 showed the highest expression in the omental adipose tissues of the skeletal, immune, and digestive systems, mainly in the human bone marrow, lymph node, cardiac stomach, and cecum.
Molecular docking between the active ingredient of Morchella esculenta, EOYA, and its main anti-inflammatory targets
As shown in Table 2, the affinity between the nine target proteins and EOYA was less than − 5 kcal/mol, among which NR1I2, PTGS2, PTGS1, CYP3A4, and CYP4F2 (Figs. 14, 15) showed the highest affinity with − 7.0, − 7.0, − 6.8, − 6.8, 6.7 kcal/mol, respectively. These targets may be the key anti-inflammation targets of the active ingredient of Morchella esculenta, EOYA.
MD simulation
The top two best categorized docked complexes (EYOA-NR1I2 and EYOA-PTGS2) were additionally considered for molecular dynamic simulations to validate the network pharmacology and molecular docking results (Fig. 16). RMSD analysis of the protein give insights into its structural conformation during the simulations, providing an indication of the stability of the protein and whether the simulation has equilibrated. The results showed RMSD was less than 2.5 Å for two complexes until the end of the simulations, indicating that the complexes were overall stable, and it demonstrated that ETOA forms a stable complex with NR1I2 and PTGS2.RMSF was calculated to survey the fluctuation of every amino acid of the ligand–protein complex.In an RMSF plot, the peak indicates which region of the protein fluctuates most during the simulation, while lower RMSF values represent smaller conformational change.The RMSF values of the amino acid residues in loop regions were found to highly fluctuate in EYOA-NR1I2 model (142–434). The high value of RMSF fluctuation denotes strong variation of protein structure over all MD simulation. This is mainly due to strand breaks of amino acid from this portion of the protein. The EYOA-PTGS2 occurred some minor conformational changes that reflect the stability of the complex. For a more detailed characterization of hydrogen bond (H-bond) between protein and ligand atoms throughout MD trajectory, We counted the H-bonds for all systems during the course of 100 ns of simulation time. The result show that in the process of dynamics simulation, the hydrogen bonds of the complex always existed, and the number of hydrogen bonds were greater than or equal to 1 in EYOA-NR1I2 and EYOA-PTGS2 complex. These results indicate that small molecules form stable complex with proteins. Furthermore, no significant change in SASA for two proteins was observed, indicating that no significant part of the proteins was exposed to water, and the structure remained compact throughout the simulation time.We found that SASA analysis revealed a steady decline in SASA from 0 to 100 ns in EYOA-PTGS2 complex, indicating favourable binding and progressive protein tightening, and made the complex structure stable gradualy throughout the simulation.SASA also presents small decline from 0 to 20 ns upon ligand binding in EYOA-NR1I2, It might be that small molecules, after constantlycolliding with the active site in the protein pocket, found the rightconformation so as to reach the balance. The final results showed that EYOA did indeed bind well NR1I2 to PTGS2, and the complex has high stability.
MD simulation results for key anti-inflammatory targets of EOYA, the active ingredient of Morchella esculenta (A): RMSD, (B): Number of hydrogen bonds, (C): Radius of gyration, (D): Solvent Accessible Surface, (E): The RMS fluctuation of EOYA-NR1I2, (F): The RMS fluctuation of EOYA-PTGS2). In all systerm, the color indicates-NR1l2 protein (blue), PTGS2 protein (red).
Discussion
Morchella esculenta is a fungus mushroom used both as medicine and food. It has the functions of invigorating the yang, resolving phlegm, tonifying the kidney, regulating the qi, invigorating the brain, pleen, and stomach, and aiding digestion. The role of Morchella esculenta in the arachidonic acid metabolic pathway has been studied. For instance, a specific LOX activity was determined from the crude extract of Morchella esculenta, and the affinity of LOX activity to the substrate, arachidonic acid, was strong (83%)27. The extracellular vesicles of Morchella esculenta contain some specific lipids, miRNAs, and proteins, which can inhibit LPS-induced inflammation by attenuating the production of ROS and reducing the phosphorylation levels in the p38 MAPK signaling pathway28. The active extract of Morchella esculenta can inhibit the expression of LPS-induced COX, and significantly inhibit the activity of NF-κB, indicating its preventive effect on inflammation29. To further analyze the anti-inflammatory mechanisms of action of Morchella esculenta, we then applied a network pharmacological method. However, the previous TCM network pharmacological research predicted drug targets through some databases only. This may be not fully consistent with the actual target of Morchella esculenta. To overcome these shortcomings, we use the 6 differents inflammation GEO database to provided more compelling evidence. Our results showed that we obtained one compounds EYOA derived from Morchella esculenta though the NPASS database, we searched multiple compound target databases and disease target databases and identified 43 anti-inflammatory targets of EOYA. According to the network pharmacology analysis, EOYA mainly played an anti-inflammatory role through arachidonic acid metabolism, vascular endothelial growth factor signaling pathway, and sheath phosphorus signal transduction pathway.
9 potential targets were chosen as core targets based on the ‘Degree’, ‘Betweenness’ and ‘Closeness’ values in the PPI network, NR1I2, PTGS1, PTGS2, CYP4F2, CYP3A4, TLR4, MAPK1, PLA2G4A, and PTPN11. we expect to screen protein targets related to inflammation using 6 GEO databases related to inflammatory, Clinically relevant gene association networks obtained from these inflammation database. we obtained 5 tagerts significant differences with inflammation, including as NR1I2, PTGS1, PTGS2, CYP4F2 and CYP3A4. By using Autodock software EYOA is docked with 5 protein targets. The results show that all 5 proteins can bind well to EYOA. and the best characterized of these are NR1I2 and PTGS2. EYOA can form the strong interactions with the NR1I2 active site HIS 327 and GLN285, the PTGS2 active site ARG121. The studies of molecular dynamics simulations were carried out to understand more deeply the modes of interaction of the selected EYOA with the target proteins.The simulation result showed EYOA can form strong hydrogen bond interactions with the NR1I2 active site HIS 327 and GLN285, the PTGS2 active site ARG121 and TRY356, which was the main interaction force of EYOA bound with the active site of NR1I2 and PTGS2. The RMSD, RMSF, RG, hydrogen bond, and SASA define the complexes and show that they are more stable (Fig. 17). The analysis results of GEO dataset validating inflammatory core targets show that different types of inflammation have different significantly different targets, which may be related to the location of inflammation. But it also shows the application potential of EYOA in different inflammation.
Chronic inflammation is caused by various stimuli from natural immune cells through different pathogen-related modes. Its reaction process is continuous and complex, and these are mainly transmitted through the NF-κB signaling pathway30. The continuously increasing NF-κB activity can accelerate the aging of certain immune cells, leading to an increase in the levels of related inflammatory factors31. Simultaneously, the increase in the levels of reactive oxygen species (ROS) from mitochondria and cytoplasm and defective antioxidant protection result in continually altered redox metabolism32, and this redox imbalance changes the signaling response to growth, nutrition factors, cytokines, and chemokines, eventually leading to cell aging and chronic inflammation33. To date, hormone drugs are commonly used to treat inflammation. Dexamethasone is a glucocorticoid used to treat inflammatory diseases; it has been used for more than 50 years but its various side effects have been confirmed, including hypertension, adrenal insufficiency, and hyperglycemia34. Therefore, it is necessary to identify drugs with significant anti-inflammatory effects and fewer side effects. For centuries, traditional Chinese medicine has been used to treat various diseases35.
At present, the metabolites of fungi have become the main source of anti-inflammatory lead compounds because of their novel structure, low toxicity and significant inhibitory effect36. Many new compounds have been obtained from plant endophytic fungi, which can inhibit the excessive production of endotoxin-induced NO and pro-inflammatory cytokines IL-6, tumor necrosis factor-α and monocyte chemoattractant protein-1 at both gene and protein levels37. The compounds isolated from algae-producing fungi could inhibit the excessive production of NO and pro-inflammatory cytokines in lipopolysaccharide-treated RAW264.7 macrophages in a dose-dependent manner without any cytotoxicity38. New polyketones were isolated from marine fungus Eutyellahoraria. Nitric oxide was induced by lipopolysaccharide in RAW264.7 macrophages and showed strong anti-inflammatory activity. When the concentration was 50.0 μg/mL, the inhibition rate was 54.9%39. Edible and medicinal fungi are natural products with effective anti-inflammatory and antiviral properties. During the SARS-CoV-2 pandemic, people often ingested, reducing the impact of SARS-CoV-240. Agaricus blazei Murrill has the properties of anti-allergy, anti-infection, asthma and anti-tumor. In mouse model, it also has anti-inflammatory effect in patients with inflammatory bowel disease41.
Inflammation, accompanied by increased vascular expansion permeability and leukocyte exudation, is an acute reaction involving and mediated by several chemical factors. The importance of arachidonic acid metabolism lies in the fact that it can be metabolized by three different enzymes, namely cyclooxygenases (COXs, also known as PGG/H synthetases), lipoxygenases (LOXs), and cytochrome P450 (CYP) enzymes (ω-hydroxylase and cyclooxygenase)42 (Fig. 16), which have attracted extensive attention in the inflammatory processes and diseases43 Prostaglandins, the products of arachidonic acid metabolism, are inflammatory mediators. Inflammation stimulates arachidonic acid metabolism and releases metabolites, such as prostaglandins and leukotrienes, causing fever, pain, and vasodilation in patients. In clinical practice, the most common manifestations of inflammatory reactions are redness, swelling, heat and pain, increased permeability, and leukocyte exudation.
PTGS1 is an essential enzyme responsible for prostaglandin synthesis44. PTGS1 can regulate peripheral vascular resistance, maintain renal blood flow, protect the gastric mucosa, and regulate platelet aggregation45. The expression of PTGS1 is related to several inflammatory pathways46. PTGS1 is distributed in microglia and plays a key role in regulating inflammation in the central nervous system47. Ursolic acid can treat autoimmune thyroiditis by inhibiting the mechanism and inflammatory pathways of the MALAT1/miR206/PTGS1 axis48. PTGS2 (or COX-2) is induced by hormones, growth factors, and inflammatory stimuli, and is considered an important source of prostaglandin formation in proliferative (such as cancer) and inflammatory diseases. CYP4F2 and CYP3A4 are enzymes of the cytochrome P450 family. The most prominent role of the CYP pathway is the metabolism of fat-soluble exogenous substances. The expression and activity of CYP are controlled by hormones, growth factors, and transcription factors49. The NR1I2-nuclear receptor subfamily, whose members are transcription factor coding proteins characterized by the presence of a DNA binding domain and a ligand binding domain. Its encoded protein is a transcriptional regulator of the cytochrome P450 gene CYP3A4.
The comparative and enrichment analyses of the effective components of Morchella esculenta showed that MAPK1, PTPN11, and TLR4 were involved in inflammation; PTGS2, PLA2G4A, CYP3A4, CYP4F2, PTGS1, and NR1I2 were involved in arachidonic acid-related pathways. CYP3A4, PTGS2, NR1I2, and PTGS1 were involved in the synthesis of prostaglandins. NR1I2, CYP3A4, and CYP4F2 participated in the cytochrome P450 pathway (Fig. 18). This showed that Morchella esculenta could participate in multiple metabolic pathways involving arachidonic acid, directly or indirectly regulate the activity of prostaglandin endoperoxide synthetase, and inhibit the release of prostaglandins, thereby reducing inflammation.
Three metabolic pathways involving arachidonic acid42.
Conclusion
In summary, Morchella esculenta has a multi-target pharmacological effect in the treatment of inflammation through the arachidonic acid metabolism pathway, which is expected to shed new insights into the treatment of inflammation. Herein, the network pharmacological strategy was used to study the multi-pathway and multi-target mechanisms of the active ingredient of Morchella esculenta, EOYA, in the prevention and treatment of inflammation. Morchella esculenta may be effective for the treatment of inflammation and provide a new direction for addressing the current shortcomings in treating inflammation.
Data availability
The data used to support the findings of this study are available from the corresponding authors upon reasonable request.
Abbreviations
- CYP3A4:
-
Cytochrome p450 family 3 subfamily a polypeptide 4
- CYP4F2:
-
Phylloquinone omega-hydroxylase CYP4F2
- EOYA:
-
(E)-Octadec-11-En-9-Ynoic Acid
- NR1I2:
-
Nuclear receptor subfamily 1 group I member 2
- PLA2G4A:
-
Cytosolic phospholipase A2
- PTGS1:
-
Prostaglandin G/H synthase 1
- PTGS2:
-
Prostaglandin G/H synthase 2
- PTPN11:
-
Tyrosine-protein phosphatase non-receptor type 11
- TLR4:
-
Toll-like receptor 4
References
Xu, Q. et al. New polyketides with anti-inflammatory activity from the fungus Aspergillus rugulosa. Front. Pharmacol. 12, 700573 (2021).
Arulselvan, P. et al. Role of antioxidants and natural products in inflammation. Oxid. Med. Cell. Longev. 2016, 5276130 (2016).
Sangchart, P. et al. Anti-inflammatory comparison of melatonin and its Bromobenzoylamide derivatives in lipopolysaccharide (LPS)-induced RAW 264.7 cells and croton oil-induced mice ear edema. Mol. (Basel Switz.) 26, 4285 (2021).
Fernandez, D. M. & Giannarelli, C. Immune cell profiling in atherosclerosis: Role in research and precision medicine. Nat. Rev. Cardiol. 19, 43–58 (2022).
Kataoka, T. et al. Protective effects of radon inhalation on carrageenan-induced inflammatory paw edema in mice. Inflammation 35, 713–722 (2012).
Wu, H. et al. Recent advances on bioactive ingredients of Morchella esculenta. Appl. Biochem. Biotechnol. 193, 4197–4213 (2021).
Liu, C. et al. Characteristics and antitumor activity of Morchella esculenta polysaccharide extracted by pulsed electric field. Int. J. Mol. Sci. 17, 986 (2016).
Li, W. et al. Anti-inflammatory effects of Morchella esculenta polysaccharide and its derivatives in fine particulate matter-treated NR8383 cells. Int. J. Biol. Macromol. 129, 904–915 (2019).
Zhao, X. et al. Total flavones of fermentation broth by co-culture of Coprinus comatus and Morchella esculenta induces an anti-inflammatory effect on LPS-stimulated RAW264.7 macrophages cells via the MAPK signaling pathway. Microbial. Pathog. 125, 431–437 (2018).
Nitha, B., Fijesh, P. V. & Janardhanan, K. K. Hepatoprotective activity of cultured mycelium of morel mushroom, Morchella esculenta. Exp. Toxicol. Pathol. 65, 105–112 (2013).
Jones, G. et al. Antimicrobial activity of santalbic acid from the oil of santalum acuminatum (Quandong). Int. J. Pharmacogn. 33, 120–123 (1995).
Dzoyem, J. et al. In vitro and in vivo antifungal activities of selected Cameroonian dietary spices. BMC Complement. Altern. Med. 14, 1–8 (2014).
Mavundza, E. et al. Identification of compounds in Olax dissitiflora with larvacidal effect against Anopheles arabiensis. S. Afr. J. Bot. 102, 1–3 (2016).
Croft, K., Beilin, L. & Ford, G. Differential inhibition of thromboxane B2 and leukotriene 84 biosynthesis by two naturally occurring acetylenic fatty acids. Biochim. et Biophys. Acta (BBA) Lipids Lipid Metab. 921, 621–624 (1987).
Li, G., Singh, A., Liu, Y., Sunderland, B. & Li, D. Comparative effects of sandalwood seed oil on fatty acid profiles and inflammatory factors in rats. Lipids 48, 105–113 (2012).
Abo Elmaaty, A. et al. Computational insights on the potential of some NSAIDs for treating COVID-19: Priority set and lead optimization. Molecules 26, 3772 (2021).
Zhang, J. et al. A bioinformatics investigation into molecular mechanism of Yinzhihuang granules for treating hepatitis B by network pharmacology and molecular docking verification. Sci. Rep. 10, 11448 (2020).
Zhou, W. et al. Network pharmacology to unveil the mechanism of Moluodan in the treatment of chronic atrophic gastritis. Phytomed. Int. J. Phytother. Phytopharmacol. 95, 153837 (2022).
Li, X. et al. Network pharmacology prediction and molecular docking-based strategy to explore the potential mechanism of Huanglian Jiedu Decoction against sepsis. Comput. Biol. Med. 144, 105389 (2022).
Sun, P. Y., Wang, A. S., Zhang, Z. F., Zhang, Y. L. & Zheng, X. Network pharmacology-based strategy to investigate the active ingredients and molecular mechanisms of Scutellaria Barbata D. Don against radiation pneumonitis. Medicine 100, e27957 (2021).
Ma, X. et al. Integration of network pharmacology and molecular docking to explore the molecular mechanism of Cordycepin in the treatment of Alzheimer’s disease. Front. Aging Neurosci. 14, 1058780 (2022).
Patel, R. V., Hamanishi, E. T. & Provart, N. J. A human, “eFP” browser for generating gene expression anatograms. PLoS ONE 11, e0150982 (2016).
Seeliger, D. & de Groot, B. L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput. Aided Mol. Des. 24, 417–422 (2010).
Delano, W. L. The pymol molecular graphics system. Prot. Struct. Funct. Bioinf. 30, 442–454 (2002).
Hess, B., Kutzner, C., van der Spoel, D. & Lindahl, E. GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 4, 435–447 (2008).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51, D587–D592 (2023).
Bisakowski, B., Atwal, A. & Kermasha, S. Characterization of lipoxygenase activity from a partially purified enzymic extract from Morchella esculenta. Process Biochem. 36, 1–7 (2000).
Chen, C. et al. Anti-inflammatory polyketides from an alga-derived fungus Aspergillus ochraceopetaliformis SCSIO 41020. Mar. Drugs 20, 295 (2022).
Ramya, H. et al. Morel mushroom, Morchella from Kashmir Himalaya: A potential source of therapeutically useful bioactives that possess free radical scavenging, anti-inflammatory, and arthritic edema-inhibiting activities. Drug Chem. Toxicol. 45, 2014–2023 (2022).
Franceschi, C., Garagnani, P., Parini, P., Giuliani, C. & Santoro, A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 14, 576–590 (2018).
Coppé, J. et al. A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen. PLoS ONE 5, e9188 (2010).
Hishikawa, A., Hayashi, K. & Itoh, H. Transcription factors as therapeutic targets in chronic kidney disease. Molecules 23, 1123 (2018).
Liguori, I. et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 13, 757–772 (2018).
Polderman, J. et al. Adverse side effects of dexamethasone in surgical patients. Cochrane Database Syst. Rev. 11, CD011940 (2018).
Liu, Z. et al. Network pharmacology-based investigation on the mechanisms of action of Morinda officinalis How. in the treatment of osteoporosis. Comput. Biol. Med. 127, 104074 (2020).
Chen, Y. et al. Metabolites with anti-inflammatory activity from the mangrove endophytic fungus diaporthe sp. QYM12. Mar. Drugs 19, 56 (2021).
Ren, X. et al. Anti-inflammatory compounds from the mangrove endophytic fungus Amorosia sp. SCSIO 41026. Front. Microbiol. 13, 976399 (2022).
Chen, Q. et al. Anti-inflammatory effects of extracellular vesicles from Morchella on LPS-stimulated RAW264.7 cells via the ROS-mediated p38 MAPK signaling pathway. Mol. Cell. Biochem. 478, 317–327 (2023).
Zhang, Y. H. et al. Anti-inflammatory polyketides from the marine-derived fungus Eutypella scoparia. Mar. Drugs 20, 486 (2022).
Sarangi, M. K. et al. Diagnosis, prevention, and treatment of coronavirus disease: A review. Expert Rev. Anti Infect. Ther. 20, 243–266 (2022).
Hetland, G., Johnson, E., Lyberg, T. & Kvalheim, G. The Mushroom Agaricus blazei murill elicits medicinal effects on tumor, infection, allergy, and inflammation through its modulation of innate immunity and amelioration of Th1/Th2 imbalance and inflammation. Adv. Pharmacol. Sci. 2011, 157015 (2011).
Wang, B., Wu, L., Chen, J., Dong, L. & Wang, D. W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 6, 94 (2021).
Sala, A., Proschak, E., Steinhilber, D. & Rovati, G. E. Two-pronged approach to anti-inflammatory therapy through the modulation of the arachidonic acid cascade. Biochem. Pharmacol. 158, 161–173 (2018).
Jaén, R., Prieto, P., Casado, M., Martín-Sanz, P. & Boscá, L. Post-translational modifications of prostaglandin-endoperoxide synthase 2 in colorectal cancer: An update. World J. Gastroenterol. 24, 5454–5461 (2018).
Wang, Y. et al. Inhibition of PTGS1 promotes osteogenic differentiation of adipose-derived stem cells by suppressing NF-κB signaling. Stem Cell Res. Ther. 10, 57 (2019).
Ou, C. et al. Systematically investigating the pharmacological mechanism of Dazhu Hongjingtian in the prevention and treatment of acute mountain sickness by integrating UPLC/Q-TOF-MS/MS analysis and network pharmacology. J. Pharm. Biomed. Anal. 179, 113028 (2020).
Choi, S., Aid, S. & Bosetti, F. The distinct roles of cyclooxygenase-1 and -2 in neuroinflammation: Implications for translational research. Trends Pharmacol. Sci. 30, 174–181 (2009).
Mou, L., Liao, L., Zhang, Y., Ming, D. & Jiang, J. Ursolic acid ameliorates Nthy-ori 3–1 cells injury induced by IL-1β through limiting MALAT1/miR-206/PTGS1 ceRNA network and NF-κB signaling pathway. Psychopharmacology 238, 1141–1156 (2021).
Yarla, N. et al. Targeting arachidonic acid pathway by natural products for cancer prevention and therapy. Semin. Cancer Biol. 40–41, 48–81 (2016).
Funding
This project are funded by a grant from the Basic Scientific Research Business Plan Project (2021GR2920) of Liaoning Academy of Agricultural Sciences and Applied Basic Research Program (2022020382-JH2/1013) of Liaoning Province, the Discipline Construction Plan of Liaoning Province Academy of Agricultural Sciences (2022DD072311).
Author information
Authors and Affiliations
Contributions
M.X.Y. designed the research and wrote the first draft of the paper. H.Z.M. performed the data analysis, W.H. read the manuscript and approved the final version. The other authors performed the validation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xiaoying, M., Zhiming, H., Tao, Y. et al. Elucidating the molecular mechanisms underlying anti-inflammatory effects of Morchella esculenta in the arachidonic acid metabolic pathway by network pharmacology and molecular docking. Sci Rep 13, 15881 (2023). https://doi.org/10.1038/s41598-023-42658-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-42658-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.