Abstract
Ulcerative colitis (UC) is one of the two types of inflammatory bowel disease (IBDs), which have a pivotal role in weakening the quality of lives of suffering patients. According to some recent studies, significant changes in dietary patterns may have contributed to the increased prevalence of UC. Potential renal acid load (PRAL) is an index used to estimate dietary acid load of the diet. The aim of the current study is to investigate the association between PRAL and odds of UC. The current case–control study included 62 newly diagnosed cases of UC and 124 healthy controls. Dietary habits of participants in the last year were collected with a valid food frequency questionnaire (FFQ). Thereafter, PRAL score was calculated based on a formula containing the dietary intake of protein, phosphorus, potassium, calcium, and magnesium. Participants were categorized according to quartiles of PRAL. Multivariable logistic regression models were used to estimate the odds' ratio (OR) with 95% confidence intervals (CIs) of UC across quartiles of PRAL. The results of the current study indicated that in the crude model, participants in the fourth quartile of PRAL had 2.51 time higher odds of UC compared with those in the first quartile of the PRAL [(OR 2.51; 95% CI 1.03–6.14), (P = 0.043)]. After adjustment for age and biological gender, this positive association remained significant [(OR 2.99; 95% CI 1.16–7.72), (P = 0.023)]. In the final model, after further adjustment for BMI, current smoking, education, Helicobacter pylori infection, and dietary intakes of total energy, omega-3 fatty acids, trans-fatty acids, and total dietary fiber, the odds of UC in the highest quartile of PRAL was significantly higher compared to the lowest quartile [(OR 3.08; 95% CI 1.01–9.39), (P = 0.048)]. So, we observed that higher dietary acid load assessed by PRAL score is associated with greater odds of UC.
Similar content being viewed by others
Introduction
Inflammatory bowel diseases (IBDs) are chronic, relapsing inflammatory disorders of the gastrointestinal (GI) tract, characterized by abdominal pain and diarrhea. There are two main phenotypes of IBDs, namely ulcerative colitis (UC) and Crohn's disease (CD). These disorders drastically affect the quality of life of suffering patients1. The prevalence of UC is increasing all over the world2. Moreover, UC-related morbidity and mortality, health care, and societal costs are significant3. Various factors are involved in the pathophysiology of UC, including genetics, environmental factors, immune system disorders, and epithelial barrier defects4. It has been suggested that remarkable changes in dietary patterns during the past decades might play a role in the increased prevalence of UC5,6.
It has been hypothesized that dietary factors play a pivotal role in the initiation and development of UC7,8,9. Several dietary patterns, such as Western dietary regimen, dietary patterns rich in animal proteins and animal products, those with high content of saturated fats or altered ratios of omega-6 to omega-3, and those low in fruits and vegetables have been postulated to contribute to increased intestinal inflammation10,11,12,13. Therefore, it can be deduced that changing the dietary pattern in individuals with active IBDs, from an animal-based diet to a predominantly plant-based diet, might result in the inhibition of intestinal inflammation, reduction of disease severity, and maintaining the clinical remission10. In contrast, following a Western dietary approach rich in animal meat, dairy products, fat, simple sugars, processed meats, alcohol, and restricted in vegetables and fruits, has been shown to be associated with an increased risk of developing IBDs14,15. Evidence also suggests that following the Mediterranean dietary pattern is significantly related to improved clinical condition and reduction of inflammatory markers14,16,17. Furthermore, there exists abundant evidence that the individual’s dietary composition can affect the acid–base balance of the body18,19. For instance, it has been claimed that consumption of potassium and magnesium (provided mainly by the intake of fruits and vegetables) may be associated with a more alkaline environment in the human body. On the contrary, consuming a western diet is usually associated with increased acidity20,21. Based on studies, the kidneys remove the products of metabolism of some anions (chlorine, phosphorus and sulphate), organic acids and cations (sodium, potassium, calcium, magnesium). If the amount of anions exceed the cations, the urinary acid excretion mechanism (hydrogen ion H+) is stimulated22. Potential renal acid load (PRAL) is an index used to estimate dietary acid load of the diet23. In other word, PRAL is a capacity of acid or base production of any food, which includes the amount of endogenously synthesized organic acids22. The concept of PRAL calculation is physiologically based and takes under consideration the diverse rates of intestinal absorption of sulfur-containing minerals and proteins, as well as the sum of sulfate produced from metabolized proteins24. Therefore, PRAL is an index calculated from the sum of proteins and phosphorus as an anion, minus some cations such as potassium, calcium and magnesium25. A lower PRAL indicates a more alkaline diet which results in an elevated 24-h urinary pH26. If the body fails to maintain the acid–base balance, dietary acid load can cause metabolic acidosis21.
Several experimental studies have postulated that metabolic acidosis can lead to tissue damage and, consequently, initiate inflammatory responses27,28,29. Human studies have also indicated such an association between acid load and inflammatory status of the body. For instance, Wu et al. demonstrated that a higher dietary acid load (i.e. a higher PRAL) is associated with the increased levels of C-reactive protein (CRP) (an important inflammatory marker) in breast cancer survivors21. Different studies have been conducted to investigate the association between the dietary acid load and the progression of various disorders, such as non-alcoholic fatty liver disease (NAFLD)30,31, diabetes mellitus32, hyperuricemia26, albuminuria and impaired kidney function33, breast cancer21, and osteoporosis34. Nonetheless, the association between dietary acid load and the incidence/recurrence of IBDs has not been investigated. Due to this vacancy of information, the present study was designed to investigate the association between PRAL and UC.
Methods
Participants
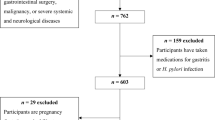
The present case–control study was conducted on newly diagnosed UC patients (< 6 months) in a referral hospital during the year 2013, Tabriz city, Iran. The details of study protocol have been published elsewhere35. Briefly, sixty two newly diagnosed patients (< 6 months) with UC were recruited as the case group. Inclusion criteria for the cases included: individuals recently diagnosed with UC, based on patients’ signs and symptoms and their colon tissue pathology report. The exclusion criteria for the case group included history of any other gastrointestinal diseases, carcinoma, and other inflammatory, infectious, and autoimmune disorders. One hundred and twenty-four age- and sex-matched healthy subjects were enrolled in the study as the control group. The control group was selected from individuals without UC visiting orthopedic clinics of the same referral hospital. The individuals suffering from gastrointestinal illnesses/symptoms (gastro-esophageal reflux disease (GERD), diarrhea, irritable bowel syndrome (IBS), and abdominal pains) or conditions which might have caused drastic changes of dietary habits (i.e. metabolic diseases, including diabetes mellitus, cardiovascular diseases (CVDs), gout, and hyperlipidemia) were excluded from the control group. The age range for the two groups was 20–80 years.
Participants completed an informed consent prior to enrollment. Information about demography, medical history, body mass index (BMI), drugs, dietary intake, current smoking, Helicobacter pylori infection, education level, and familial history of UC were obtained through completing the general information questionnaire. The study protocol was approved by the ethics committee of the Shahid Beheshti University of Medical Sciences (ethical code: IR.SBMU.RETECH.REC.1401.723. All methods were performed in accordance with the relevant guidelines and regulations.
Dietary assessment and calculation of potential renal acid load (PRAL)
Dietary habits of participants in the last year were collected by a trained interviewer with a valid and reliable 168 items food frequency questionnaire (FFQ)36. Then PRAL score was calculated based on the dietary intake of protein, phosphorus, potassium, calcium, and magnesium using the following formula25:
Statistical analysis
The data was analyzed using Statistical Package for Social Sciences software (version 20.0; SPSS, Chicago, IL, USA). Kolmogorov–Smirnov test was used to evaluate normality. The qualitative and quantitative variables were compared between groups using the chi-square test and independent t-test or one-way ANOVA test, respectively. Participants were categorized according to quartiles of PRAL (Q1: − 59.49 to − 9.87, Q2: − 9.45 to − 0.28, Q3: − 0.08 to 7.79, Q4: 7.80 to 41.51). We used multivariable logistic regression models to estimate the odds ratio (OR) of UC across quartiles of PRAL. In all models, the first quartile was determined as the reference. In logistic regression analysis, UC and PRAL were considered as the dependent and independent variables, respectively. The ORs and 95% confidence intervals (CIs) were reported. Possible confounding factors, including gender (male/female), age (years), BMI (Kg/m2), current smoking (yes/no), education (Primary, Secondary and high school or university education), Helicobacter pylori infection (yes/no), and dietary intakes of total energy (kcal/day), omega-3 fatty acids (g/day), trans-fatty acids (g/day), and total dietary fiber (g/day) were adjusted in the final model. P-values < 0.05 were considered as statistically significant.
Ethics approval and consent to participate
The study protocol was approved by the ethics committee of the Shahid Beheshti University of medical sciences (ethical code: IR.SBMU.RETECH.REC.1401.723.). Written informed consent was signed by all subjects participated.
Results
General characteristics and dietary intakes of participants across quartiles of PRAL are presented in Table 1. Sixty-two recently newly diagnosed UC patients and 124 healthy controls were included in the present study. The mean ± standard deviation (SD) of age and BMI of all subjects were 36.63 ± 12.42 years and 25.39 ± 3.82 kg/m2, respectively. The frequency of male sex was significantly different between the quartiles of PRAL (P = 0.026). Also, total calories, total proteins, total fat, saturated fatty acids (SAFA) and monounsaturated fatty acids (MUFA) were significantly different across quartiles of PRAL (P < 0.05). Regarding other general characteristics, there was no significant difference between PRAL quartiles.
Dietary intakes of participants in the case and control groups are presented in Table 2. Dietary intake of total calories, carbohydrate, fat, protein, SAFA, MUFA, polyunsaturated fatty acid (PUFA), phosphorus, potassium, and magnesium were significantly higher in cases (P < 0.05). The range and mean ± SD of cases and controls pertaining to PRAL scores were − 59.49 to 41.51 and − 0.24 ± 14.76 mEq/day, respectively.
The ORs (95% CI) of UC across quartiles of PRAL are shown in Table 3. In the crude model, participants in the fourth quartile of PRAL had 2.51 time higher odds of UC compared with those in the first quartile of the PRAL [(OR 2.51; 95% CI 1.03–6.14), (P = 0.043)]. After adjustment for age and biological gender, this positive association remained significant [(OR 2.99; 95% CI 1.16–7.72), (P = 0.023)]. In the final model, after further adjustment for BMI, current smoking, education, Helicobacter pylori infection, and dietary intakes of total energy, omega-3 fatty acids, trans-fatty acids, and total dietary fiber, the odds of UC in the highest quartile of PRAL was significantly higher compared to the lowest quartile [(OR 3.08; 95% CI 1.01–9.39), (P = 0.048)].
Discussion
In the present case–control study, we observed that there is a positive association between dietary acid load assessed by PRAL score and the development of UC. After adjusting for confounding variables, our results showed that subjects in the highest quartile of PRAL were approximately 3 times more likely to develop UC than those in the lowest quartile.
Diet is one of the main determinants of the acid load in the body, which must excreted by the kidneys37. In general, foods such as cheese, meat, eggs and grains create an acid load, whereas fruits and vegetables provide alkali in the body. Based on this, plant-based dietary patterns are considered to be mainly reducing, and on the contrary, animal-based dietary patterns are considered to be mainly increasing the acid load or PRAL in the body38.
To the best of our knowledge, there has been no study investigating the association between the dietary acid load and the risk of IBDs. Nonetheless, research indicates a positive correlation between animal-based diets and the likelihood of developing IBDs, whereas plant-based dietary approaches have demonstrated a negative association with the probability of being affected by IBDs39. In line with the findings of the current study, a large prospective cohort study involving 125,445 participants and a 14-year follow-up period revealed an 11% increased risk of developing UC, associated with a dietary pattern that includes both white and red meat varieties40. In another cohort study with even larger scales, it was observed that higher intake of total meat and red meat increases the risk of UC by 40% and 60%, respectively41. Likewise, Limketkai et al. showed that, compared to a Western diet, following a plant-based diet can reduce active symptoms of the UC by 70%42.
Some mechanisms have been proposed to justify the association between various dietary patterns and IBDs. Amongst these, higher total antioxidant capacity (TAC) provided by a diet composed mainly of plant-based foodstuff seems likely to play a role in reducing the risk of UC43. Moreover, recent studies propose that changes of the intestinal microbiota might be imperative in the pathophysiology of IBDs44. On this note, the prebiotic effects of a diet rich in insoluble fibers, leading to increased production of short-chain fatty acids (SCFAs) that can modify gut microbial composition, have also been proposed as an additional mechanism by which this dietary approach may influence the risk of IBDs45,46. On the other hand, an animal-based regimen is believed to negatively affect the diversity and composition of gut microbiota, paving the way for a more IBD-inducing environment47. Furthermore, the production of toxic metabolites by intestinal bacteria, such as hydrogen sulfide (H2S) produced from bacterial metabolization of unabsorbed sulfur-containing amino acids, can compromise the integrity of intestinal integrity, leading to impaired barrier function of colonic epithelial cells. These metabolites have also been postulated to interfere with the proliferation of these cells and, thus, hinder their regeneration. These pathways might eventually incentivize an already susceptible environment towards colonic abnormalities, including UC48,49.
The end-products obtained from metabolizing animal-based foodstuff tend to produce a net acidic content, as opposed to more alkaline metabolites produced from plant-based foodstuff23. Chronic dietary acidic conditions in the body, accompanied by changes in the body's buffering capacity, eventually lead to a low-grade metabolic acidosis, inflammation, and cell and tissue damage23,50. Studies on human subjects also indicate a link between dietary acid load and inflammation. For instance, Wu et al. showed that breast cancer survivors who are in the highest dietary acid load quartile have 30–33% higher plasma levels of C-reactive protein (CRP)21. Likewise, another study showed that PRAL had a significant correlation with TNF-α levels51. It is worthy of note that prolonged inflammatory state of the intestinal mucosa has been postulated to be one of the main factors in the pathogenesis of UC52, presenting another link between an animal-based regimen and the pathophysiology of the disease. In addition, studies have found that dietary acid load can also exert adverse metabolic effects by increasing blood levels of cortisol19; on the same note, it has been observed that cortisol levels are higher in more severe cases of IBDs53. These observations propose additional justifications for the presumed association between PRAL and UC.
To the best of our knowledge, the association between dietary acid load and the odds of UC has not been previously investigated through a well-designed case–control study. Also, the study was conducted in Iran as a developing country where cultural and socio-economic factors play major roles in creating significant inter-individual variances in dietary intake. However, this study had some limitations. Firstly, the case–control design of the study cannot ensure a causal relationship; further investigations, especially in the format of cohort and interventional studies, are needed to certify the directionality of the observed association. Secondly, although the FFQ questionnaire used in the present study has been validated, it is inherently subject to recall bias and measurement errors. Also, the limitation of the sample size did not allow us to create sex-specific quartiles. Finally, more objective measurements, including biomarkers, such as serum levels of inflammatory markers and electrolytes and the composition of intestinal microbiota are suggested to be taken into consideration in future studies.
Conclusion
In the current study, it was observed that higher dietary acid load assessed by PRAL score is associated with greater odds of UC. We recommend that future prospective studies with long-term follow-up periods be conducted to further clarify the causal relationship between dietary acid load and the risk of UC.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CD:
-
Crohn's disease
- CRP:
-
C reactive protein
- GERD:
-
Gastro-esophageal reflux disease
- GI:
-
Gastro-intestinal
- IBD:
-
Inflammatory bowel disease
- IBS:
-
Irritable bowel syndrome
- MUFA:
-
Monounsaturated fatty acid
- NAFLD:
-
Non-alcoholic fatty liver disease
- PRAL:
-
Potential renal acid load
- PUFA:
-
Polyunsaturated fatty acid
- SAFA:
-
Saturated fatty acid
- SCFAs:
-
Short-chain fatty acids
- TAC:
-
Total antioxidant capacity
- UC:
-
Ulcerative colitis
References
Knowles, S. R. et al. Quality of life in inflammatory bowel disease: A systematic review and meta-analyses-part I. Inflamm. Bowel Dis. 24(4), 742–751 (2018).
Rui, W., Zhaoqi, L., Shaojun, L. & Decai, Z. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: A systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open 13(3), e065186 (2023).
Du, L. & Ha, C. Epidemiology and pathogenesis of ulcerative colitis. Gastroenterol. Clin. 49(4), 643–654 (2020).
Yan, Y. Pathogenesis of inflammatory bowel diseases. in Inflammatory Bowel Disease-Advances in Pathogenesis and Management (IntechOpen, 2012).
Hou, J. K., Abraham, B. & El-Serag, H. Dietary intake and risk of developing inflammatory bowel disease: A systematic review of the literature. J. Am. Coll. Gastroenterol. 106(4), 563–573 (2011).
Keshteli, A. H., Madsen, K. L. & Dieleman, L. A. Diet in the pathogenesis and management of ulcerative colitis: A review of randomized controlled dietary interventions. Nutrients 11(7), 1498 (2019).
Shivappa, N., Hébert, J. R., Rashvand, S., Rashidkhani, B. & Hekmatdoost, A. Inflammatory potential of diet and risk of ulcerative colitis in a case–control study from Iran. Nutr. Cancer 68(3), 404–409 (2016).
Karimi, S. et al. The effects of two vitamin D regimens on ulcerative colitis activity index, quality of life and oxidant/anti-oxidant status. Nutr. J. 18(1), 441 (2019).
Rahmani, J. et al. Body mass index and risk of inflammatory bowel disease: A systematic review and dose-response meta-analysis of cohort studies of over a million participants. Obes. Rev. 20(9), 1312–1320 (2019).
Antoniussen, C. S., Rasmussen, H. H., Holst, M. & Lauridsen, C. Reducing disease activity of inflammatory bowel disease by consumption of plant-based foods and nutrients. Front. Nutr. 8, 433 (2021).
Thorburn, A. N., Macia, L. & Mackay, C. R. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity 40(6), 833–842 (2014).
Spooren, C. et al. The association of diet with onset and relapse in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 38(10), 1172–1187 (2013).
Hekmatdoost, A., Wu, X., Morampudi, V., Innis, S. M. & Jacobson, K. Dietary oils modify the host immune response and colonic tissue damage following Citrobacter rodentium infection in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 304(10), 917–928 (2013).
Tayyem, R. F., Qalqili, T. R., Ajeen, R. & Rayyan, Y. M. Dietary patterns and the risk of inflammatory bowel disease: Findings from a case-control study. Nutrients 13(6), 1889 (2021).
Hart, A. R. et al. Diet in the aetiology of ulcerative colitis: A European prospective cohort study. Digestion 77(1), 57–64 (2008).
Demetriou, C. A. et al. The mediterranean dietary pattern and breast cancer risk in Greek-Cypriot women: A case-control study. BMC Cancer 12(1), 1–12 (2012).
Estruch, R. Anti-inflammatory effects of the Mediterranean diet: The experience of the PREDIMED study. Proc. Nutr. Soc. 69(3), 333–340 (2010).
Williams, R. S. et al. Dietary acid load, metabolic acidosis and insulin resistance: Lessons from cross-sectional and overfeeding studies in humans. Clin. Nutr. 35(5), 1084–1090 (2016).
Williamson, M., Moustaid-Moussa, N. & Gollahon, L. The molecular effects of dietary acid load on metabolic disease (the cellular PasaDoble: The fast-paced dance of pH regulation). Front. Mol. Med. 1, 777088 (2021).
Alam, I., Alam, I., Paracha, P. I. & Pawelec, G. Higher estimates of daily dietary net endogenous acid production (NEAP) in the elderly as compared to the young in a healthy, free-living elderly population of Pakistan. Clin. Interv. Aging 7, 565 (2012).
Wu, T. et al. Associations between dietary acid load and biomarkers of inflammation and hyperglycemia in breast cancer survivors. Nutrients 11(8), 1913 (2019).
Osuna-Padilla, I., Leal-Escobar, G., Garza-García, C. & Rodríguez-Castellanos, F. Dietary acid load: Mechanisms and evidence of its health repercussions. Nefrologia 39(4), 343–354 (2019).
Osuna-Padilla, I. A., Leal-Escobar, G., Garza-García, C. A. & Rodríguez-Castellanos, F. E. Dietary acid load: Mechanisms and evidence of its health repercussions. Nefrologia. 39(4), 343–354 (2019).
Remer, T., Dimitriou, T. & Manz, F. Dietary potential renal acid load and renal net acid excretion in healthy, free-living children and adolescents. Am. J. Clin. Nutr. 77(5), 1255–1260 (2003).
Noormohammadi, M., Eslamian, G., Kazemi, S. N. & Rashidkhani, B. Dietary acid load, alternative healthy eating index score, and bacterial vaginosis: is there any association? A case-control study. BMC Infect. Dis. 22(1), 1–9 (2022).
Esche, J., Krupp, D., Mensink, G. B. & Remer, T. Dietary potential renal acid load is positively associated with serum uric acid and odds of hyperuricemia in the German adult population. J. Nutr. 148(1), 49–55 (2018).
Kellum, J. A., Song, M. & Almasri, E. Hyperchloremic acidosis increases circulating inflammatory molecules in experimental sepsis. Chest 130(4), 962–967 (2006).
Pedoto, A. et al. Acidosis stimulates nitric oxide production and lung damage in rats. Am. J. Respir. Crit. Care Med. 159(2), 397–402 (1999).
Pedoto, A. et al. Role of nitric oxide in acidosis-induced intestinal injury in anesthetized rats. J. Lab. Clin. Med. 138(4), 270–276 (2001).
Krupp, D., Johner, S. A., Kalhoff, H., Buyken, A. E. & Remer, T. Long-term dietary potential renal acid load during adolescence is prospectively associated with indices of nonalcoholic fatty liver disease in young women. J. Nutr. 142(2), 313–319 (2012).
Emamat, H. et al. The association between dietary acid load and odds of non-alcoholic fatty liver disease: A case-control study. Nutr. Health. 2022, 1088383 (2022).
Emamat, H., Tangestani, H., Bahadoran, Z., Khalili-Moghadam, S. & Mirmiran, P. The associations of dietary acid load with insulin resistance and type 2 diabetes: A systematic review of existing human studies. Recent Pat. Food Nutr. Agric. 10(1), 27–33 (2019).
Banerjee, T. et al. Dietary potential renal acid load and risk of albuminuria and reduced kidney function in the Jackson Heart Study. J. Ren. Nutr. 28(4), 251–258 (2018).
Bullo, M. et al. Mediterranean diet and high dietary acid load associated with mixed nuts: Effect on bone metabolism in elderly subjects. J. Am. Geriatr. Soc. 57(10), 1789–1798 (2009).
Rashvand, S., Somi, M. H., Rashidkhani, B. & Hekmatdoost, A. Dietary fatty acid intakes are related to the risk of ulcerative colitis: A case–control study. Int. J. Colorectal Dis. 30(9), 1255–1260 (2015).
Esfahani, F. H., Asghari, G., Mirmiran, P. & Azizi, F. Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the Tehran Lipid and Glucose Study. J. Epidemiol. 20(2), 150–158 (2010).
Gonick, H. C., Goldberg, G. & Mulcare, D. Reexamination of the acid-ash content of several diets. Am. J. Clin. Nutr. 21(9), 898–903 (1968).
Scialla, J. J. & Anderson, C. A. Dietary acid load: A novel nutritional target in chronic kidney disease?. Adv. Chronic Kidney Dis. 20(2), 141–149 (2013).
Yan, J. & Wang, L. Dietary patterns and gut microbiota changes in inflammatory bowel disease: Current insights and future challenges. Nutrients 14(19), 4003 (2022).
Peters, V. et al. Western and carnivorous dietary patterns are associated with greater likelihood of IBD development in a large prospective population-based cohort. Nutrients 16(6), 931–939 (2022).
Dong, C. et al. Meat intake is associated with a higher risk of ulcerative colitis in a large european prospective cohort studyø. Nutrients 16(8), 1187–1196 (2022).
Limketkai, B. N., Hamideh, M., Shah, R., Sauk, J. S. & Jaffe, N. dietary patterns and their association with symptoms activity in inflammatory bowel diseases. Inflamm. Bowel Dis. 28(11), 1627–1636 (2022).
Rahmani, J. et al. Dietary total antioxidant capacity and risk of ulcerative colitis: A case-control study. J. Dig. Dis. 20(12), 636–641 (2019).
Qiu, P. et al. The gut microbiota in inflammatory bowel disease. Front. Cell Infect. Microbiol. 12, 992 (2022).
Parada Venegas, D. et al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 10, 277 (2019).
Bolte, L. A. et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut 70(7), 1287–1298 (2021).
Jowett, S. et al. Influence of dietary factors on the clinical course of ulcerative colitis: A prospective cohort study. Gut 53(10), 1479–1484 (2004).
Vidal-Lletjós, S. et al. Dietary protein and amino acid supplementation in inflammatory bowel disease course: What impact on the colonic mucosa?. Nutrients 9(3), 310 (2017).
Blachier, F., Beaumont, M. & Kim, E. Cysteine-derived hydrogen sulfide and gut health: A matter of endogenous or bacterial origin. Curr. Opin. Clin. Nutr. Metab. Care 22(1), 68–75 (2019).
Storz, M. A. & Ronco, A. L. Observational and clinical evidence that plant-based nutrition reduces dietary acid load. Curr. Opin. Clin. Nutr. Metab. Care 11, e93 (2022).
Kord Varkaneh, H., Fatahi, S., Rahmani, J. & Shab-Bidar, S. Association of dietary acid load with body composition and inflammatory biomarkers in patients with type 2 diabetes. MUQ J. 12(6), 63–72 (2018).
Feuerstein, J. D. & Cheifetz, A. S. editors. Ulcerative colitis: epidemiology, diagnosis, and management. in Mayo Clinic Proceedings (Elsevier, 2014).
Cardoso, H., Nunes, A. C. R., Cruz, A., Santos, C. C. & Veloso, F. T. Importance of serum cortisol levels in inflammatory bowel disease: 1197. Am. Coll. Gastroenterol. 101, 465 (2006).
Acknowledgements
This study is related to the Project NO: 1401/59455 from Student Research Committee, Shahid Beheshti University of Medical Sciences (SBMU), Tehran, Iran. We also appreciate the “Student Research Committee” and “Research & Technology Chancellor” in Shahid Beheshti University of Medical Sciences for their financial support of this study.
Funding
This study is related to the project NO: 1401/59455 from the Student Research Committee, Shahid Beheshti University of Medical Sciences (SBMU), Tehran, Iran.
Author information
Authors and Affiliations
Contributions
A.H., H.E. and M.M. conceptualized the study. S.R. and M.H.S. collected the data. M.M., H.E. and H.T. wrote the manuscript. H.E. analyzed the data. H.G. and A.H. revised the manuscript. A.H. supervised the study. All authors approved the final version of the manuscript. Written consent for publication obtained from all participants.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Movahedian, M., Emamat, H., Tangestani, H. et al. Association between dietary acid load and the odds of ulcerative colitis: a case–control study. Sci Rep 13, 13738 (2023). https://doi.org/10.1038/s41598-023-41069-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-41069-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.