Abstract
Despite several studies on the link between dietary inflammatory potential and risk of several conditions, limited studies investigated the association between pro-inflammatory diet and ulcerative colitis (UC). The objective of the present study was to examine the link between food-based dietary inflammatory potential (FDIP) and odds of UC in Iranian adults. This case–control study was carried out among 109 cases and 218 randomly chosen healthy controls. UC was diagnosed and confirmed by a gastroenterologist. Patients with this condition were recruited from Iranian IBD registry. Age- and sex-matched controls were selected randomly from participants of a large cross-sectional study. Dietary data were obtained using a validated 106-item semi-quantitative food frequency questionnaire (FFQ). We calculated FDIP score using subjects’ dietary intakes of 28 pre-defined food groups. In total 67% of subjects were female. There was no significant difference in mean age between cases and controls (39.5 vs. 41.5y; p = 0.12). The median (interquartile range) of FDIP scores for cases and controls were − 1.36(3.25) and − 1.54(3.15), respectively. We found no significant association between FDIP score and UC in the crude model (OR 0.93; 95% CIs 0.53–1.63). Adjustment for several potential confounders in multivariate model did not change this association (OR 1.12; 95% CIs 0.46–2.71). We failed to observe any significant association between greater adherence to a pro-inflammatory diet and risk of UC in this study. Prospective cohort studies are needed to further assess this relationship.
Similar content being viewed by others
Introduction
Ulcerative colitis (UC) is a chronic inflammatory condition of the gastrointestinal tract. In patients with UC, the superficial inflammation speared in an uninterrupted pattern starting from the rectum and may affect the entire colon1,2. Although inflammatory bowel disease (IBD) including UC was initiated in Western nations, nowadays newly industrialized countries in Asia and the Middle East have reported a rapid increase in its incidence3. In a national study, in Iran, it has been estimated that UC affects 35.52 per 100,000 subjects4.
The exact etiology of UC is yet to be determined, though, it is postulated that improper immune response, arising from a complex interaction between genetic susceptibility, an altered gut microbiota5,6, and several environmental factors5 might cause this condition. Dietary factors have been consistently found to modulate inflammation7, through which they might affect the development of chronic diseases8,9, including UC. Findings from previous studies indicated a positive link between adherence to a pro-inflammatory diet, assessed by dietary inflammatory index (DII), and odds of UC10,11. DII is a nutrient-based index constructed mostly based on pro-inflammatory or anti-inflammatory properties of nutrients12. It must be noted that people do not consume isolated nutrients rather they eat a diet consisting of numerous foods, nutrients, and other bioactive components with synergistic effects or interaction with each other13,14. Therefore, dietary inflammatory potential might be better reflected through a food-based index rather than a nutrient-based index. In 2016, Tabung et al.15 developed and validated an empirically dietary inflammatory pattern (EDIP) index using inflammatory biomarkers. Such assessment of dietary inflammatory potential has been examined in relation to ovarian and colorectal cancers13,16. Regarding IBD, in a recent prospective cohort, EDIP was significantly associated with risk of Crohn's disease (CD), but not UC17. Considering the paucity of information about the association between this dietary index and risk of UC in the Middle East, we aimed to investigate the association between dietary inflammatory potential, as assessed by a food-based dietary index, and risk of UC in a population-based case–control study in Iran.
Methods
Study participants
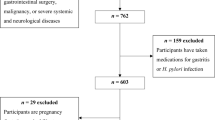
This population-based case–control study was performed among 109 patients with UC and 218 apparently healthy controls in Isfahan, Iran, between 2015 and 2019. The sample size was computed based on prior evidence indicating that approximately 60% of Iranian adults were following non-healthy dietary patterns18. According to earlier investigations in the country, we assumed that the consumption of unhealthy dietary patterns would double the risk of UC10. Therefore, with 80% study power and 5% type I error, and the ratio of controls to cases as 2, we reached the sample size of at least 101 cases and 202 controls for the current study. To enroll cases, we invited UC patients, whose information was registered in the IBD registry of Isfahan, to participate in a class intending to educate them about lifestyle modification. In that class, we mentioned the study and its aims and requested the participants to take part in the study. Out of all registered patients who attended that class (n = 140), 109 subjects agreed to take part in our study. All these patients provided written informed consent. To recruit controls, we used the study on the epidemiology of psychological, alimentary health, and nutrition (SEPAHAN) dataset. For each patient, two apparently healthy controls were randomly selected from SEPAHAN dataset on more than 8,000 people. Detailed information about the population-based cross-sectional study of SEPAHAN has been published elsewhere19. Controls were matched with cases in terms of age (± 2 years) and sex. Participants in the SEPAHAN study also provided informed written consent before enrollment19. Before random selection of controls from SEPAHAN dataset, we excluded individuals with any history of gastrointestinal disorders (including CD, UC, irritable bowel syndrome, functional dyspepsia, and gastroesophageal reflux disorder). The final sample size in the current study was 327 people (109 cases and 218 controls). This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures ethically approved by the Ethical Committee of the Tehran University of Medical Sciences, Tehran, Iran (IR.TUMS.VCR.REC.1398.497).
Assessment of dietary intakes
Subjects were requested to complete a validated self-administrated 106-item dish-based semi-quantative food frequency questionnaire (DS-FFQ). This questionnaire was designed to be used in epidemiologic studies of Iranian population. Detailed data about this questionnaire has also been given elsewhere20. Foods and dishes included in that questionnaire were in five major categories: (1) 29 items of mixed dishes such as cooked or canned foods; (2) 10 items of grain-based foods and potatoes; (3) 9 items of dairy products; (4) 22 items of fruits and vegetables; and (5) 36 items of miscellaneous foods and beverages such as sweets, fast foods, nuts, desserts, and beverages. Nine multiple-choice frequency response categories, ranging from “never or less than once a month” to “12 or more times per day.” were available for each food item, allowing participants to report their usual daily intake. At last, data obtained from the FFQ was converted to grams per day for all participants via the booklet of household measures. Total daily energy and nutrient intakes were calculated using the modified US Department of Agriculture (USDA) food consumption database.
Assessment of food-based dietary inflammatory potential
The construction of a food-based index to examine dietary inflammatory potential has been explained previously21. Briefly, data from a previous publication22 on 486 female teachers were used to identify foods, and food groups linked with serum C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α). Then using the factor loading values of foods and food groups provided by that publication22 and considering the quantity of consumption for each individual, the food-based dietary inflammatory potential (FDIP) index was constructed. In the present study at first, we computed mean daily intakes of pre-defined anti-inflammatory food groups (green leafy vegetables, cruciferous vegetables, yellow vegetables, tomatoes, other vegetables, fruits, fruit juices, poultry, fish, whole grains, legumes, and tea) and pro-inflammatory food groups (French fries, pizza, snacks, red meats, processed meats, eggs, refined grains, potatoes, dairy products, butter, hydrogenated oils, hydrogenated fats, mayonnaise, sweets and desserts, soft drinks, and coffee). And by applying the residual method23 energy-adjusted amounts of the above-mentioned food groups were calculated. Then, for each subject, we multiplied these amounts by their assigned factor loadings, obtained from that study22. Finally, we summed up the scores for each person to calculate total score of dietary inflammatory potential. To decrease the magnitude of the scores, the total scores were divided by 100 for all participants.
Assessment of UC
UC was diagnosed by an experienced gastroenterologist according to physical, colonoscopic, and histological examinations. We additionally reviewed medical records to confirm the diagnosis.
Assessment of other variables
Data on other variables including age, sex, education, smoking status, and diabetes history were collected through a pre-tested structured self-administered questionnaire. We used a pretested dietary habit questionnaire to obtain information regarding dietary habits including meal regularity, fluid consumption during meals, chewing efficiency, fried foods intake, and fatty meals intake. To assess physical activity, we applied General Practice Physical Activity Questionnaire (GPPAQ)24, and then classified study subjects into five categories: no activity, < 3 h per week, 3–5 h per week, 5–7 h per week, and \(\ge\) 7 h per week. Other required data including weight and height were collected using a self-administered questionnaire. In a recent study from our group, it was found that anthropometric data driven from self-reported questionnaires provided valid information compared with actual measured values25. Body mass index (BMI) was calculated as weight (kg) divided by height square (m2).
Statistical analysis
The normal distribution of the data was evaluated using the Kolmogorov– Smirnov normality test. We classified all study participants according to defined tertile cut-off points for FDIP score in the control group. The distribution of cases and controls by continuous and categorical variables were assessed using student’s t-test and chi-square test, respectively. To examine the differences across tertiles of FDIP score, we applied analysis of variance (ANOVA) for continuous variables and chi-square test for categorical variables. Analysis of covariance (ANCOVA) was used to compare energy, age, and sex-adjusted dietary intakes of subjects across tertiles of FDIP score. Association between adherence to a pro-inflammatory diet and UC was examined in multivariable-adjusted models, using binary logistic regression. We adjusted for age (continuous) and sex (female/male) in the first model and additionally for total energy intake (calory/day) and BMI (continuous) in the second model. Further adjustment for education (high school diploma or below/university graduated), smoking status (non-smoker/smoker), diabetes history (yes/no), and physical activity (no activity/ < 3 h per week/3–5 h per week/5–7 h per week/\(\ge\) 7 h per week) was done in the third model. We also adjusted for regular meal consumption (often or always/never or occasionally), fluid consumption during meals (< 3 glasses/ ≥ 3 glasses), chewing efficiency (a lot/not a lot), fried foods intake (< 4 per week/ ≥ 4 per week), and consumption of fatty meals (non-fatty meal/fatty meal) in the last model. The first tertile of FDIP score was considered the reference category, in all analyses. By treating tertile of FDIP score as an ordinal variable, P for trends was determined. All statistical analyses were carried out using SPSS software version 19. P values were considered significant at < 0.05.
Results
Subjects with UC were less likely to be physically active (17% vs. 32%; P = 0.02) and university graduates (38% vs. 54%; P = 0.007) than controls. No significant differences in mean age and BMI were seen between cases and controls. We failed to find any significant differences between cases and controls, comparing them in terms of sex, smoking status, and history of diabetes. Comparing cases and controls in terms of dietary habits of chewing efficiency, regular meal pattern, fried foods intake, fluid consumption during meals, and fatty meals intake, no significant differences were found.
UC cases reported higher intakes of total energy (3014 ± 101 vs. 2328 ± 69; P < 0.001), polyunsaturated fatty acid (PUFA) (43 ± 1.08 vs. 31 ± 0.72; P < 0.001), yellow vegetables (16.03 ± 1.01 vs. 6.54 ± 0.68; P < 0.001), cruciferous vegetables (8.97 ± 0.91 vs. 3.89 ± 0.61; P < 0.001), vegetable oils (52.0 ± 1.71 vs. 42.8 ± 1.16; P < 0.001), and red meat (85.6 ± 4.15 vs. 73.9 ± 2.80; P = 0.02) as well as lower intakes of protein (89 ± 1.80 vs. 95 ± 1.21; P < 0.001), dietary fiber (18 ± 0.67 vs. 24 ± 0.45; P < 0.001), monounsaturated fatty acid (MUFA) (29 ± 1.02 vs. 40 ± 0.68; P < 0.001), other vegetables (128.1 ± 7.13 vs. 146.9 ± 4.64; P = 0.03), hydrogenated fats (0.35 ± 0.44 vs. 5.34 ± 0.30; P < 0.001), and refined grain (286.4 ± 17.4 vs. 346.3 ± 11.6; P = 0.005) than controls.
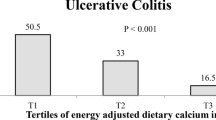
Participants in the top tertile of FDIP score were more likely to be younger and had lower BMI. No other significant differences were observed in terms of other main characteristics across tertiles of FDIP score. Comparing study participants across tertiles of FDIP score, we found no significant associations regarding dietary habits (Table 1).
Subjects with greater adherence to a pro-inflammatory diet (tertile 3), had higher intakes of total energy, carbohydrates, and refined grains and lower intakes of total fats, PUFA, MUFA, fruits, fruit juices, legumes, cruciferous vegetables, green leafy vegetables, yellow vegetables, other vegetables, tea, tomatoes, and whole grains than those with the least adherence (Table 2).
Neither in crude nor in multivariable-adjusted models, we found a significant association between FDIP score and UC. In model 1, after adjustment for sex and age, no significant association between FDIP score and UC was observed (OR 1.004; 95% CI 0.55–1.82). Further adjustments for total energy intake and BMI in model 2 (OR 0.98; 95% CI 0.51–1.89), and additionally controlling for educational status, smoking status, diabetes history, physical activity, and total fiber intake in model 3 (OR 1.07; 95% CI 0.46–2.47) did not change the association. In the last model, we also took dietary habits into account, but no significant link was found (OR 1.12; 95% CI 0.46–2.71) (Table 3).
Discussion
In the present study, we failed to find a significant association between FDIP score and odds of UC. Adjustment for a wide range of potential covariates did not change the association. This study is among the first studies investigating FDIP score in relation to UC risk.
Traditionally known as a Western disease, UC incidence is increasing worldwide, causing a major public health burden and reducing quality of life for those who suffer from this condition5. Despite growing investigations examining dietary patterns in relation to UC risk, findings are still conflicting. In 2014, Racine et al.26 found a link between the “high sugar and soft drinks” pattern and an increased risk of UC incidence only in subjects with low vegetable intake in the EPIC cohort. In another cohort study examining adherence to the Mediterranean dietary pattern and later onset of UC, no significant association was observed27. Findings of the NutriNet-Sante cohort also revealed no link between 3 dominant retained dietary patterns:” healthy”, “traditional”, and “western” with UC incidence28.
In this study, we found no significant association between adherence to a pro-inflammatory dietary pattern and UC risk. In agreement with our findings, in a recent analysis of 3 prospective cohort studies, dietary patterns with high inflammatory potential were not significantly linked with UC development17. On the contrary, our previous analysis of this group of individuals revealed a positive link between nutrient-based dietary inflammatory potential and risk of UC11. Shivappa et al.10 also observed a positive association between higher DII score and odds of UC in the framework of a hospital-based case–control study in Iran. The reason behind these conflicting findings might be due to different approaches used to assess the inflammatory potential of the diet in different studies. In this study, unlike previous ones10,11, we used a food-based dietary index to examine inflammatory potential of diet because nutrient-based DII cannot capture complex interactions within the whole diet29,30,31. In the past, undernutrition and nutritional deficiencies were among leading diet-induced diseases, this shifted epidemiological studies to focus on nutrients and their contribution to health outcomes. Nowadays, this approach failed considering the rising incidence of chronic diseases32,33, therefore focusing only on nutrient-disease links might lead to finding biased associations. Additional studies in this field are needed to reach definite findings.
Though we did not observe any significant findings, there are plausible mechanisms explaining how adherence to a pro-inflammatory diet might contribute to the etiology of IBD. For instance, it is known that cytokines are directly involved in the development, expansion, and maintenance of UC34,35,36. Therefore, a pro-inflammatory diet resulting in increased cytokine production might be involved in UC pathogenesis. Considering the critical role of gut microbiota in the healthy intestine, a diet rich in food items with inflammatory properties may promote intestinal inflammation by altering gut microbiota composition37,38,39.
Lack of finding any association in this study might be attributed to the nature of IBD. Interestingly, in several previous publications, investigators found diet and its components to be associated with CD risk and not UC40,41. It seems that diet is a more significant risk factor for CD than UC42, due to the more significant role of gut microbiome in CD pathogenesis and the ability of dietary components to modulate the composition of gut microbiota43. Lack of finding any association in regression models might also be due to lack of power, given the low number of study participants.
Our study has several strengths as well as limitations. Being among the first studies in this field, applying a validated FFQ for dietary assessment, and adjustment for several potential covariates are among the strengths. Moreover, in this study, we used a food-based index to determine the inflammatory potential of the diet, which can capture complex interactions within the whole diet. However, some limitations should be also noted. First, due to the case–control design of this study, we were not able to establish casualty. Second, recall and selection biases are common in case–control studies and might affect our findings. Third, despite adjustment for several potential covariates, the effect of residual confounders such as history of medication (e.g., non-steroidal anti-inflammatory drugs (NSAIDs)) intake cannot be ignored. Forth is the small sample size of the present study. Finally, the possibility of diet alteration in UC patients due to their condition should also be kept in mind.
In conclusion, we failed to observe any significant link between adherence to a pro-inflammatory diet, measured by FDIP, and odds of UC. Additional adjustments for several potential covariates did not change the result. Prospective cohort studies are needed to confirm these findings.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- UC:
-
Ulcerative colitis
- IBD:
-
Inflammatory bowel disease
- DII:
-
Dietary inflammatory index
- EDIP:
-
Empirically dietary inflammatory pattern
- CD:
-
Crohn's disease
- SEPAHAN:
-
Study on the epidemiology of psychological, alimentary health, and nutrition
- DS-FFQ:
-
Dish-based semi-quantitative food frequency questionnaire
- USDA:
-
US department of agriculture
- CRP:
-
C-reactive protein
- IL-6:
-
Interleukin-6
- TNF-α:
-
Tumor necrosis factor-α
- FDIP:
-
Food-based dietary inflammatory potential
- GPPAQ:
-
General practice physical activity questionnaire
- BMI:
-
Body mass index
- ANOVA:
-
One-way analysis of variance
- ANCOVA:
-
Analysis of covariance
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
References
Abraham, C. & Cho, J. H. Inflammatory bowel disease. N. Engl. J. Med. 361(21), 2066–2078 (2009).
Meckel, K. et al. Serum 25-hydroxyvitamin D concentration is inversely associated with mucosal inflammation in patients with ulcerative colitis. Am. J. Clin. Nutr. 104(1), 113–120 (2016).
Nishida, A. et al. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 11(1), 1–10 (2018).
Malekzadeh, M. M. et al. Emerging epidemic of inflammatory bowel disease in a middle income country: A nation-wide study from Iran. Arch. Iran. Med. 19(1), 2–15 (2016).
Ananthakrishnan, A. N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 12(4), 205–217 (2015).
Cabre, E. & Domenech, E. Impact of environmental and dietary factors on the course of inflammatory bowel disease. World J. Gastroenterol. 18(29), 3814–3822 (2012).
Corley, J., Shivappa, N., Hebert, J. R., Starr, J. M. & Deary, I. J. Associations between dietary inflammatory index scores and inflammatory biomarkers among older adults in the Lothian birth cohort 1936 study. J. Nutr. Health Aging 23(7), 628–636 (2019).
Godos, J. et al. Dietary inflammatory index and sleep quality in southern Italian adults. Nutrients 11(6), 1324 (2019).
Vahid, F. et al. Validation of a dietary inflammatory index (DII) and association with risk of gastric cancer: A case–control study. Asian Pac. J. Cancer Prevent. APJCP. 19(6), 1471–1477 (2018).
Shivappa, N., Hébert, J. R., Rashvand, S., Rashidkhani, B. & Hekmatdoost, A. Inflammatory potential of diet and risk of ulcerative colitis in a case–control study from Iran. Nutr. Cancer 68(3), 404–409 (2016).
Khademi, Z. et al. Association between inflammatory potential of the diet and ulcerative colitis: A case–control study. Front. Nutr. 7(358), 602090 (2021).
Shivappa, N., Steck, S. E., Hurley, T. G., Hussey, J. R. & Hébert, J. R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 17(8), 1689–1696 (2014).
Tabung, F. K. et al. Association of dietary inflammatory potential with colorectal cancer risk in men and women. JAMA Oncol. 4(3), 366–373 (2018).
Hu, F. B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 13(1), 3–9 (2002).
Tabung, F. K. et al. Development and validation of an empirical dietary inflammatory index. J. Nutr. 146(8), 1560–1570 (2016).
Tabung, F. K. et al. The inflammatory potential of diet and ovarian cancer risk: Results from two prospective cohort studies. Br. J. Cancer 117(6), 907–911 (2017).
Lo, C.-H. et al. Dietary inflammatory potential and risk of Crohn’s disease and ulcerative colitis. Gastroenterology 159, 873–883 (2020).
Kimiagar, S. M., Ghaffarpour, M., Houshiar Rad, A., Hormozdyari, H. & Zellipour, L. Food consumption pattern in the Islamic Republic of Iran and its relation to coronary heart disease. EMHJ-Eastern Mediterr. Health J. 4(3), 539–547 (1998).
Adibi, P. et al. The study on the epidemiology of psychological, alimentary health and nutrition (SEPAHAN): Overview of methodology. J. Res. Med. Sci. 17(5), S292–S298 (2012).
Keshteli, A. et al. A Dish-based semi-quantitative food frequency questionnaire for assessment of dietary intakes in epidemiologic studies in Iran: Design and development. Int. J. Prev. Med. 5(1), 29–36 (2014).
Salari-Moghaddam, A., Keshteli, A. H., Esmaillzadeh, A. & Adibi, P. Empirically derived food-based inflammatory potential of the diet, irritable bowel syndrome, and its severity. Nutrition 63, 141–147 (2019).
Esmaillzadeh, A. et al. Dietary patterns, insulin resistance, and prevalence of the metabolic syndrome in women. Am. J. Clin. Nutr. 85(3), 910–918 (2007).
Willett, W. C., Howe, G. R. & Kushi, L. H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 65(4), 1220S-S1228 (1997).
Do, H. The General Practice Physical Activity Questionnaire. Department of Health London (2006).
Aminianfar, A. et al. Validity of self-reported height, weight, body mass index, and waist circumference in Iranian adults. Int. J. Prev. Med. 12, 75 (2021).
Racine, A. et al. Dietary patterns and risk of inflammatory bowel disease in Europe: Results from the EPIC study. Inflamm. Bowel Dis. 22(2), 345–354 (2016).
Khalili, H. et al. Adherence to a Mediterranean diet is associated with a lower risk of later-onset Crohn’s disease: Results from two large prospective cohort studies. Gut 69, 1637–1644 (2020).
Vasseur, P. et al. Dietary patterns, ultra-processed food, and the risk of inflammatory bowel diseases in the Nutrinet-Santé cohort. Inflamm. Bowel Dis. 27(1), 65–73 (2021).
Kant, A. K. Dietary patterns and health outcomes. J. Am. Diet. Assoc. 104(4), 615–635 (2004).
Ocké, M. C. Evaluation of methodologies for assessing the overall diet: Dietary quality scores and dietary pattern analysis. Proc. Nutr. Soc. 72(2), 191–199 (2013).
Jacobs, D. R. & Tapsell, L. C. Food synergy: The key to a healthy diet. Proc. Nutr. Soc. 72(2), 200–206 (2013).
Tapsell, L. C. & Neale, E. P. Nutrients, and dietary patterns: Interconnections and implications for dietary guidelines. Adv. Nutr. 7(3), 445–454 (2016).
Echouffo-Tcheugui, J. B. & Ahima, R. S. Does diet quality or nutrient quantity contribute more to health?. J. Clin. Investig. 129(10), 3969–3970 (2019).
Neurath, M. F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 14(5), 329–342 (2014).
Papadakis, K. A. & Targan, S. R. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu. Rev. Med. 51(1), 289–298 (2000).
Marafini, I., Sedda, S., Dinallo, V. & Monteleone, G. Inflammatory cytokines: from discoveries to therapies in IBD. Expert Opin. Biol. Ther. 19(11), 1207–1217 (2019).
Rapozo, D. C., Bernardazzi, C. & de Souza, H. S. Diet and microbiota in inflammatory bowel disease: The gut in disharmony. World J. Gastroenterol. 23(12), 2124–2140 (2017).
Rogler, G., Biedermann, L. & Scharl, M. New insights into the pathophysiology of inflammatory bowel disease: Microbiota, epigenetics and common signalling pathways. Swiss Med. Wkly. 148, w14599 (2018).
Rizzello, F. et al. Implications of the westernized diet in the onset and progression of IBD. Nutrients 11(5), 1033 (2019).
Khalili, H. et al. No association between consumption of sweetened beverages and risk of later-onset Crohn’s disease or ulcerative colitis. Clin. Gastroenterol. Hepatol. 17(1), 123–129 (2019).
Khalili, H. et al. Dietary Iron and Heme Iron consumption, genetic susceptibility, and risk of Crohn’s disease and ulcerative colitis. Inflamm. Bowel Dis. 23(7), 1088–1095 (2017).
Lewis, J. D. & Abreu, M. T. Diet as a trigger or therapy for inflammatory bowel diseases. Gastroenterology 152(2), 398-414.e6 (2017).
Morgan, X. C. et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 13(9), R79 (2012).
Funding
The study was financially supported by Research Council of School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran.
Author information
Authors and Affiliations
Contributions
Z.K.H. and P.S. contributed to the conception, design, search, statistical analyses, data interpretation, and manuscript drafting. A.H.K. and H.D. contributed to the design and data interpretation. H.T. and P.A. contributed to the conception, design, statistical analyses, data interpretation, and manuscript drafting. AE supervised the study. All authors approved the final manuscript for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khademi, Z., Saneei, P., Hassanzadeh-Keshteli, A. et al. Association between food-based dietary inflammatory potential and ulcerative colitis: a case–control study. Sci Rep 13, 8464 (2023). https://doi.org/10.1038/s41598-023-33138-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-33138-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.